MnO2的晶相結(jié)構(gòu)和表面性質(zhì)對低溫NH3-SCR反應(yīng)的影響
戴 韻 李俊華,* 彭 悅 唐幸福
(1清華大學(xué)環(huán)境學(xué)院,環(huán)境模擬與污染控制國家重點聯(lián)合實驗室,北京100084;2復(fù)旦大學(xué)環(huán)境科學(xué)與工程系,上海200433)
MnO2的晶相結(jié)構(gòu)和表面性質(zhì)對低溫NH3-SCR反應(yīng)的影響
戴 韻1李俊華1,*彭 悅1唐幸福2,*
(1清華大學(xué)環(huán)境學(xué)院,環(huán)境模擬與污染控制國家重點聯(lián)合實驗室,北京100084;2復(fù)旦大學(xué)環(huán)境科學(xué)與工程系,上海200433)
采用水熱法合成了兩種具有相同形貌但是不同物相結(jié)構(gòu)的MnO2納米棒,分別為隧道狀和層狀結(jié)構(gòu),考察其低溫NH3選擇性催化還原NOx(NH3-SCR)的性能.結(jié)果表明MnO2納米棒的比表面積不是影響活性的主要因素,催化劑的晶相結(jié)構(gòu)和表面性質(zhì)對催化活性有很大影響,隧道狀α-MnO2納米棒的低溫NH3-SCR活性明顯高于層狀δ-MnO2納米棒.結(jié)構(gòu)分析和NH3程序升溫脫附(NH3-TPD)實驗表明,α-MnO2納米棒的暴露晶面(110)面存在大量的配位不飽和Mn離子,形成較多的Lewis酸性位點,而且α-MnO2較弱的Mn―O鍵和隧道結(jié)構(gòu)都有利于NH3的吸附;而δ-MnO2納米棒的暴露晶面(001)面上的Mn離子已達到配位飽和,所以其表面Lewis酸性位點較少.X射線光電子能譜(XPS)和熱重(TG)分析表明α-MnO2納米棒的表面更有利于NH3和NOx的活化.具有有利于吸附NH3和活化NH3和NOx的表面性質(zhì)和晶型結(jié)構(gòu),是α-MnO2納米棒活性高的主要原因.
α-MnO2;δ-MnO2;低溫;NH3-選擇性催化還原NOx;晶相結(jié)構(gòu);表面性質(zhì).
1 Introduction
Nitrogen oxides(NOx)emitted from stationary and mobile sources are major air pollutants,contributing to acid rain,photochemical smog,ozone depletion,and fine particle pollution.1Over the past years,many methods have been used to abate NOx,and selective catalytic reduction of NOxwith NH3(NH3-SCR)has been approved to be the most efficient one.2,3The commercial catalyst for NH3-SCR is V2O5-WO3(MoO3)/ TiO2.3The catalyst has to be located upstream the electric precipitator and desulfurizing unit to meet the optimum operating temperature of 350-400°C.2,4Thus the catalyst is subjected to be blocked and poisoned by the particles and sulfates(resulting from SO2)in the flue gas.A better alternative is locating the catalysts downstream the electric precipitator and desulfurizing unit,where the temperature of the flue gas is lower than 200°C.4Therefore,it is significant to develop the low-temperature catalysts that are active below 200°C.
Various transition metal oxides have been studied as the catalysts for low-temperature NH3-SCR,and Mn-based catalysts are considered to be the most active ones.3,5Supported MnOxcatalysts such as MnOx/Al2O36and MnOx/TiO27,8and unsupported MnOxcatalysts such as MnOx-CeO24have attracted much interest due to their high SCR activities.Kapteijn et al.9studied the NH3-SCR activities over pure manganese oxides of different valences and concluded that MnO2(Mn4+)had the best activity for its highest valence.In the recent studies,Tian et al.10prepared MnO2with different morphologies and found out that the activities decreased in the order:nanorod>nanoparticle>nanotube.Wang et al.11analyzed the NH3-SCR activities over α-MnO2and β-MnO2with tunnel structures,and deduced that α-MnO2showed higher activity than β-MnO2because α-MnO2had semitunnel structured external surface and more surface lattice oxygen.
MnO2exists with various structures,and among them,the tunnel and layer structures have been paid considerable attention for their applications on electrochemical and catalytic fields.12,13However,few investigations have been done on lowtemperature NH3-SCR over MnO2with different structures. Therefore,in the present study,tunneled α-MnO2nanorods and layered δ-MnO2nanorods were prepared and investigated for low-temperature NH3-SCR.Analysis on the exposed crystal planes and characterizations,such as X-ray diffraction(XRD), temperature-programmed desorption of NH3(NH3-TPD),X-ray photoelectron spectroscopy(XPS),and thermal gravimetric (TG)analysis,were carried out to investigate the relationship between surface structure and catalytic activity.
2 Experimental
2.1 Catalyst synthesis
α-and δ-MnO2were synthesized by hydrothermal methods.14For the preparation of α-MnO2,KMnO4(2.5 g,AR)and MnSO4·H2O(1.05 g,AR)were mixed in distilled water and then hydrothermally treated in a Teflon-lined autoclave at 160°C for 12 h.δ-MnO2was obtained by hydrothermally heating the mixture of KMnO4(6.0 g,AR)and MnSO4·H2O(1.1 g,AR)at 240°C for 24 h.Before used,α-and δ-MnO2were calcinated at 400 and 350°C for 4 h,respectively.
2.2 Catalytic performance
The activity measurements were carried out in a fixed-bed quartz reactor(inner diameter 9 mm)using 0.4 g catalyst (40-60 mesh).The feed gas mixture contained 0.1%(volume fraction,φ)NO,0.1%(φ)NH3,2%(φ)O2and N2as the balance gas.The total flow rate of the feed gas was 200 mL· min-1,corresponding to a space velocity of about 38000 h-1. The concentrations of NO,NO2,and NH3in the inlet and outlet gases were measured by Fourier transform infrared(FT-IR) spectroscopy gas analyzer Gasmet Dx-4000(Gasmet Technologies,Finland).
2.3 Catalyst characterization
X-ray diffraction measurements were performed on a D/ MAX-RB system with Cu Kαradiation(PIGAKV,Japan). Brunauer-Emmett-Teller(BET)surface areas were measured by nitrogen adsorption at liquid nitrogen temperature(77 K)on a Micromeritics ASAP 2010 micropore size analyzer(Quantachrome,America).JSM 7401 scanning electron microscope (SEM)instrument(JEOL,Japan)was used to characterize the morphology and the particle size of the catalysts.X-ray photoelectron spectroscopy data were obtained with an ESCAL-ab220i-XL electron spectrometer using 300 W Mg Kαradiations(VG scientific,England).The binding energies(EB)were referenced to the C 1s line at 284.8 eV from adventitious carbon.Thermal gravimetric analyses were performed on a Perkin-Elmer Pyric Diamond TG Analyzer(Perkin-Elmer,America)at a heating rate of 10°C·min-1.Temperature programmed desorption(TPD)experiments of NH3were carried out in a fixed-bed quartz reactor.The experiments consisted of four stages:(1)purge the sample in N2at 300°C for 1 h,(2)adsorb 0.1%(φ)NH3at 100°C for 1 h,(3)isothermal desorption in N2at room temperature until no NOxor NH3was detected,and(4) temperature-programmed desorption in N2(TPD stage)at a rate of 10°C·min-1up to 800°C.
3 Results and discussion
3.1 SCR catalytic activity

Fig.1 NOxconversion(a)and NH3conversion(b)over α-and δ-MnO2for low-temperature NH3-SCR
Thelow-temperatureNH3-SCR activitiesoverα-and δ-MnO2catalysts are shown in Fig.1.α-MnO2with a tunnel structure showed much higher activity than δ-MnO2with a layer structure.As shown in Fig.1a,the NOxconversion for α-MnO2was higher than 90%during the temperature range of 120-200°C,while that for δ-MnO2was less than 40%.The activities reached to maximum at 150°C.Fig.1b shows that the NH3conversion increases as the temperature rises.Comparing the NOxconversion in Fig.1a with the NH3conversion in Fig.1b,it could be inferred that the SCR reaction carried out between NH3and NO at a ratio of 1:1,and the NH3oxidation reaction occurred above 150°C.The BET surface areas of αand δ-MnO2were 28.0 and 40.5 m2·g-1,respectively.In general,the catalytic activity is greatly influenced by the surface area.However,δ-MnO2nanorods with a larger surface area showed much lower activity than α-MnO2nanorods.Consequently,the catalytic activities of the MnO2catalyst were not predominately controlled by the surface area.
3.2 Structure analysis
Fig.2a shows the XRD patterns of the two synthetic manganese oxides.The manganese oxide prepared at 160°C was attributed to a cryptomelane-type α-MnO2(JCPDS 44-0141,tetragonal,I4/m,a=b=0.978 nm,c=0.286 nm).14The pattern of MnO2prepared at 240°C showed planes(001),(002),(111)at 2θ=12.3°,24.9°,36.9°,indexed to δ-MnO2(JCPDS 80-1098, monoclinic,C2/m,a=0.515 nm,b=0.284 nm,c=0.717 nm).14,15

Fig.2 XRD patterns(a),schematic structures(b,b?), model structures of the exposed planes(c,c?),andSEM images(d,d?)of α-and δ-MnO2
α-MnO2and δ-MnO2are both constructed by chains of MnO6octahedra linking in different ways.Fig.2(b,b?)shows the schematic structures of the α-MnO2and δ-MnO2.The structure of α-MnO2consists of double chains of edge-sharing MnO6octahedra to form[2×2]tunnels of ca 0.46 nm×0.46 nm.16The morphology of α-MnO2was nanorod-shaped as shown in Fig.2d.Wang et al.11proposed that α-MnO2nanorods grew along a(001)axis direction and exposed the most stable (110)crystal planes(Fig.2(c,c?)).The crystal structure of δ-MnO2is built up from layers of edge-sharing MnO6octahedra with a certain number of water molecules and foreign cations between them.The spacing between the two layers is about 0.713 nm,16larger than the tunnel size of α-MnO2,so δ-MnO2needs more H2O or other foreign cations to stabilize the structure.According to Bragg?s equation,the interplanar spacing for(001)plane of δ-MnO2was calculated to be 0.719 nm,close to the interlayer spacing of 0.713 nm,which was in agreement with the schematic structures of δ-MnO2(Fig.2b?). Fig.2d?shows that the δ-MnO2prepared also had nanorod morphology.On the basis of the XRD pattern,the(001)plane(parallel to(002)plane)of δ-MnO2had much higher diffraction in-tensities than(111)plane,indicating that δ-MnO2might expose the(001)plane as shown in Fig.2c?,and the conclusion was proved by Xiao et al.15.
3.3 Acidity
NH3-TPD experiments were taken to demonstrate the acidities of the two MnO2nanorods and the results are shown in Fig.3 and Table 1.NH3,together with N2O and NO were desorbed as the temperature increased.The NH3desorption temperature for α-MnO2was higher than that for δ-MnO2,revealing the higher acidity of α-MnO2.The total NH3adsorption amounts(NH3+2N2O+NO)for α-MnO2were much higher than that for δ-MnO2as shown in Table 1.Therefore,α-MnO2nanorods had much more acid sites than δ-MnO2nanorods on the surface.NH3was adsorbed on the catalyst as the form of NH4+ions(Br?nsted acid sites)or coordinated NH3(Lewis acid sites).Since the surface hydroxyl groups can act as Br?nsted acid sites,17,18NH3adsorption as the form of NH4+on δ-MnO2nanorods was greatly enhanced by the large number of H2O between the layers.In spite of many Br?nsted acid sites,δ-MnO2nanorods exhibited less acidity than α-MnO2,probably because of few Lewis acid sites existed on the exposed plane.The Lewis acid sites on the surface of MnO2nanorods should be octahedral Mn sites in coordinatively unsaturated environment.11As seen in Fig.2c?,all of the Mn cations on the exposed(001) plane of δ-MnO2are at the center of oxygenic octahedra,6 fold coordinated to oxygen in a coordinatively saturated environment.The octahedral coordination model for δ-MnO2is considerably stable,19indicative of few Lewis acid sites on the plane. For α-MnO2,the Mn cations on the exposed(110)plane are 3 or 5 fold coordinated to oxygen in coordinately unsaturated environment,which indicates that α-MnO2nanorods possesses many Lewis acid sites.11The average Mn―O bond lengths of α-and δ-MnO2are 0.198 and 0.194 nm,respectively.16It suggests that the Mn cations of α-MnO2are more active than those of δ-MnO2,beneficial to the NH3adsorption on Mn sites.Furthermore,α-MnO2owns the[2×2]tunnels as shown in Fig.2b, of which the effective pore opening for gas adsorption is close to 0.265 nm,so that NH3and H2O molecules with diameters below 0.265 nm can be inserted into the tunnels.20,21Therefore, α-MnO2has much higher NH3adsorption than δ-MnO2,due to more Lewis acid sites on the surface,weaker Mn―O bonds, and effective[2×2]tunnels.Remarkably,α-MnO2had a N2O desorption peak centered at ca 280°C,while δ-MnO2had a high-temperature NO desorption peak but no low-temperature one.It could be seen that the adsorbed NH4+ions between the layers of δ-MnO2were easy to desorb as the form of NH3and difficult to be oxidized at low temperature.
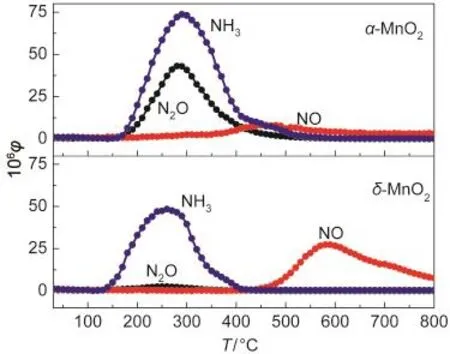
Fig.3 NH3-TPD profiles of α-and δ-MnO2nanorods
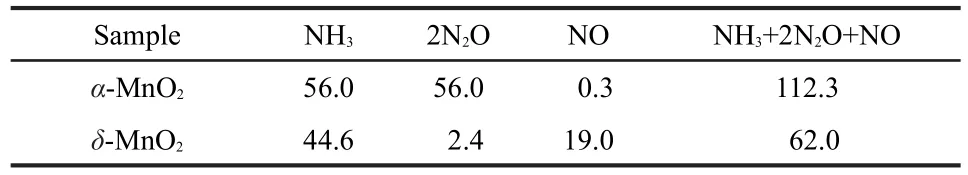
Table 1 NH3,N2O,and NO desorption amounts(unit in μmol·g-1) of α-and δ-MnO2nanorods during the NH3-TPD experiments
3.4 Redox property
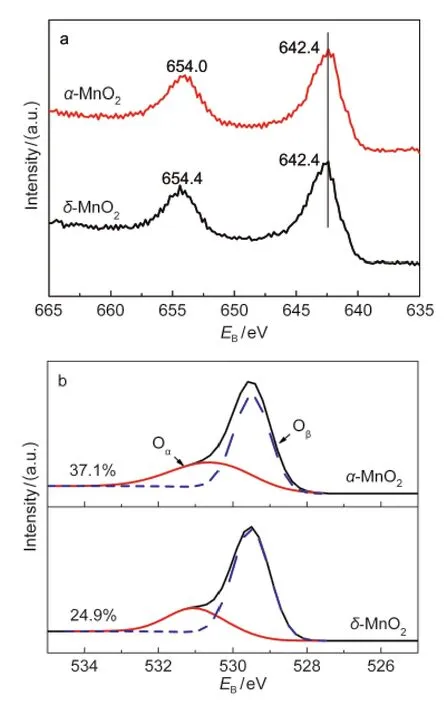
Fig.4 Mn 2p(a)and O 1s(b)XPS spectra of α-and δ-MnO2nanorods
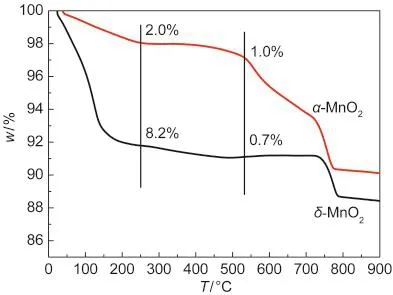
Fig.5 TG curves of α-and δ-MnO2nanorods
In general,for low-temperature NH3-SCR over Mn-based catalysts,the surface redox property is more important than the bulk redox property.XPS experiments were done to present the surface electronic state of the catalysts,and the results are shown in Fig.4.As seen in Fig.4a,two main peaks corresponding to Mn 2p1/2and Mn 2p3/2were observed for Mn 2p XPS spectra.The Mn 2p3/2peaks for the two MnO2nanorods were both located at 642.4 eV,indicating that the surfaces were presented dominantly as Mn4+.22Fig.4b shows the O 1s XPS spectra.The O 1s peak is generally composed of two surface oxygen species.The binding energy range of 531.0-532.0 eV is assigned to surface chemisorbed oxygen such as defect oxides or oxygen ions with low coordination(donated as Oα),and the binding energy range of 529.5-530.0 eV is characteristic of lattice oxygen(donated as Oβ).23Oαwas reported to be highly active in the oxidation reaction due to its higher mobility than lattice oxygen Oβ.24Therefore,Oαcould promote the oxidation of NO to NO2and H-abstraction of the adsorbed NH3,which were both supposed to be very important in low-temperature NH3-SCR.17,25The corresponding concentrations of Oαwere calculated from the relative areas of the sub-peaks and the results were 37.1%and 24.9%for α-and δ-MnO2,respectively.It indicated that α-MnO2nanorods had a higher capability to activate NH3and NO,which were also proved by NH3-TPD and NO-TPD (Fig.S1(see Supporting Information))results.The results of H2-TPR are shown in Fig.S2(see Supporting Information). δ-MnO2nanorods exhibited slightly higher bulk reducibility than α-MnO2nanorods,which implied that the bulk reducibility was not the main factor to affect the activity in the present study.
The profiles of TG are shown in Fig.5.The initial mass loss below 250°C is generally attributed to the loss of physically and chemically adsorbed water,including the loosely bound and tightly bound(interlayer)H2O molecules;26,27the mass loss in the temperature range of 250-540°C is believed to be the loss of chemical oxygen,which was considered to be highly active in the oxidation reactions;11the following evident mass losses are due to the transformation of MnO2to Mn2O3,then to Mn3O4.18The water losses for α-and δ-MnO2were 2.0%and 8.2%,respectively.The water losses of δ-MnO2were much larger than that of α-MnO2,in accordance with the existence of abundant water molecules between the layers even after calcination.The chemical oxygen losses for α-and δ-MnO2were 1.0%and 0.7%,respectively.α-MnO2had more chemical oxygen loss than δ-MnO2,which probably promoted the activation of NO and NH3,consistent with the XPS results.
4 Conclusions
α-MnO2nanorods with a[2×2]tunnel structure obtained much better low-temperature NH3-SCR performance than δ-MnO2nanorods with a layer structure.The BET surface area is not the main factor to affect the catalytic activity.The SCR activity over the MnO2nanorods is structure sensitive.The exposed(110)plane of α-MnO2possesses many manganese cations in coordinatively unsaturated environment while all the manganese cations on the exposed(001)plane of δ-MnO2are in coordinatively saturated environment,which suggests that α-MnO2has more Lewis acid sites.With more Lewis acid sites,weaker Mn―O bonds,efficient tunnel structure,and higher capacity to activate NO and NH3,α-MnO2catalyst is more adaptable for low-temperature NH3-SCR.
Supporting Information: available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1) Schneider,H.;Tschudin,S.;Schneider,M.;Wokaun,A.;Baiker, A.J.Catal.1994,147,5.doi:10.1006/jcat.1994.1109
(2) Busca,G.;Lietti,L.;Ramis,G.;Berti,F.Appl.Catal.B: Environ.1998,18,1.doi:10.1016/S0926-3373(98)00040-X
(3) Marban,G.;Valdes-Solis,T.;Fuertes,A.B.J.Catal.2004,226, 138.doi:10.1016/j.jcat.2004.05.022
(4) Qi,G.S.;Yang,R.T.J.Catal.2003,217,434.
(5) Tang,X.F.;Li,J.H.;Sun,L.;Hao,J.M.Appl.Catal.B: Environ.2010,99,156.doi:10.1016/j.apcatb.2010.06.012
(6) Kijlstra,W.S.;Brands,D.S.;Smit,H.I.;Poels,E.K.;Bliek,A. J.Catal.1997,171,219.doi:10.1006/jcat.1997.1789
(7) Li,J.H.;Chen,J.J.;Ke,R.;Luo,C.K.;Hao,J.M.Catal. Commun.2007,8,1896.doi:10.1016/j.catcom.2007.03.007
(8) Lin,T.;Zhang,Q.L.;Li,W.;Gong,M.C.;Xing,Y.X.;Chen, Y.Q.Acta Phys.-Chim.Sin.2008,24,1127.[林 濤,張秋林,李 偉,龔茂初,幸怡汛,陳耀強.物理化學(xué)學(xué)報,2008,24, 1127.]doi:10.1016/S1872-1508(08)60046-7
(9) Kapteijn,F.;Singoredjo,L.;Andreini,A.;Moulijn,J.A.Appl. Catal.B:Environ.1994,3,173.doi:10.1016/0926-3373(93) E0034-9
(10)Tian,W.;Yang,H.S.;Fan,X.Y.;Zhang,X.B.J.Hazard. Mater.2011,188,105.doi:10.1016/j.jhazmat.2011.01.078
(11)Wang,C.;Sun,L.;Cao,Q.Q.;Hu,B.B.;Huang,Z.W.;Tang, X.F.Appl.Catal.B:Environ.2011,101,598.doi:10.1016/j. apcatb.2010.10.034
(12) Brock,S.L.;Duan,N.G.;Tian,Z.R.;Giraldo,O.;Zhou,H.; Suib,S.L.Chem.Mater.1998,10,2619.doi:10.1021/ cm980227h
(13) Suib,S.L.Accounts Chem.Res.2008,41,479.doi:10.1021/ ar7001667
(14) Liang,S.H.;Teng,F.;Bulgan,G.;Zong,R.L.;Zhu,Y.F. J.Phys.Chem.C 2008,112,5307.doi:10.1021/jp0774995
(15)Xiao,W.;Wang,D.L.;Lou,X.W.J.Phys.Chem.C 2010,114, 1694.doi:10.1021/jp909386d
(16) Albering,J.H.Structural Chemistry of Manganese Dioxide and Related Compounds.In Handbook of Battery Materials;Daniel, C.,Besenhard,J.O.Eds.;McGraw-Hill:New York,1997;pp 85-107.
(17) Liu,F.D.;He,H.;Ding,Y.;Zhang,C.B.Appl.Catal.B: Environ.2009,93,194.doi:10.1016/j.apcatb.2009.09.029
(18) Wang,Z.M.;Kanoh,H.Thermochim.Acta 2001,379,7.doi: 10.1016/S0040-6031(01)00596-2
(19) Chen,S.H.;Niu,J.Z.;Liu,J.X.;Li,S.B.Chin.J.Chem.Phys. 1999,12,176.[陳善宏,牛建中,劉新建,李樹本.化學(xué)物理學(xué)報,1999,12,176.]
(20)Wang,Z.M.;Tezuka,S.;Kanoh,H.Chem.Mater.2001,13, 530.doi:10.1021/cm0007609
(21) Wang,Z.M.;Tezuka,S.;Kanoh,H.Chem.Lett.2000,29,560.
(22) Lee,S.J.;Gavriilidis,A.;Pankhurst,Q.A.;Kyek,A.;Wagner, F.E.;Wong,P.C.L.;Yeung,K.L.J.Catal.2001,200,298.doi: 10.1006/jcat.2001.3209
(23) Larachi,F.;Pierre,J.;Adnot,A.;Bernis,A.Appl.Surf.Sci. 2002,195,236.doi:10.1016/S0169-4332(02)00559-7
(24)Wu,Z.B.;Jin,R.B.;Liu,Y.;Wang,H.Q.Catal.Commun. 2008,9,2217.doi:10.1016/j.catcom.2008.05.001
(25) Kang,M.;Park,E.D.;Kim,J.M.;Yie,J.E.Appl.Catal.A: Gen.2007,327,261.doi:10.1016/j.apcata.2007.05.024
(26) Giovanoli,R.Thermochim.Acta 1994,234,303.doi:10.1016/ 0040-6031(94)85154-9
(27) Lemus,M.A.;Lopez,T.;Recillas,S.;Frias,D.M.;Montes,M.; Delgado,J.J.;Centeno,M.A.;Odriozola,J.A.J.Mol.Catal. A:Chem.2008,281,107.doi:10.1016/j.molcata.2007.10.037
March 6,2012;Revised:April 17,2012;Published on Web:April 17,2012.
Effects of MnO2Crystal Structure and Surface Property on the NH3-SCR Reaction at Low Temperature
DAI Yun1LI Jun-Hua1,*PENG Yue1TANG Xing-Fu2,*
(1State Key Joint Laboratory of Environment Simulation and Pollution Control,School of Environment,Tsinghua University,Beijing 100084,P.R.China;2Department of Environmental Science and Engineering,Fudan University,Shanghai 200433,P.R.China)
Two manganese oxides with the same nanorod-shaped morphology but different crystal structures,tunnel and layer structures,were synthesized and investigated for selective catalytic reduction of NOxwith NH3(NH3-SCR)at low temperature.Tunneled α-MnO2had much higher catalytic activity than layered δ-MnO2under the same reaction conditions.Experiment results revealed that the surface area was not the main factor to affect the NH3-SCR activities over the MnO2nanorods and that the activities were structure sensitive.Structure analysis and temperature-programmed desorption experiments of NH3(NH3-TPD)suggested that the exposed(110)plane of α-MnO2had many Mn cations in coordinatively unsaturated environment,while all of the Mn cations on the exposed(001)plane of δ-MnO2were in coordinatively saturated environment.Thus,α-MnO2possessed many more Lewis acid sites.Furthermore, α-MnO2has weaker Mn―O bonds and an efficient tunnel structure,which are favorable characteristics for NH3adsorption.Moreover,X-ray photoelectron spectroscopy(XPS)and thermal gravimetric(TG)analysis indicated that α-MnO2obtained a higher capability for NH3and NOxactivation than δ-MnO2.The crystal structure and surface properties of α-MnO2are more suitable to the adsorption of NH3and activation of NH3and NOx,which accounts for the higher catalytic activity of the α-MnO2nanorods.
α-MnO2;δ-MnO2;Low-temperature;Selective catalytic reduction of NOxwith NH3; Crystal structure; Surface property
10.3866/PKU.WHXB201204175
O643
?Corresponding authors.LI Jun-Hua,Email:lijunhua@tsinghua.edu.cn;Tel:+86-10-62771093.TANG Xing-Fu,Email:tangxf@fudan.edu.cn; Tel:+86-21-55664880.
The project was supported by the National Natural Science Fundation of China(51078203)and National High-Tech Research and Development Program of China(863)(2010AA065002,2009AA06Z301).
國家自然科學(xué)基金(51078203)及國家高技術(shù)研究發(fā)展計劃項目(863)(2010AA065002,2009AA06Z301)資助

