脂肪酰胺水解酶催化三聯(lián)體膦酰化失活的機(jī)理:一個(gè)模型體系的理論研究
李強(qiáng)根 薛 英 郭 勇 鄢國(guó)森
(四川大學(xué)化學(xué)學(xué)院,教育部綠色化學(xué)與技術(shù)重點(diǎn)實(shí)驗(yàn)室,成都 610064)
Fatty acid amide hydrolase(FAAH)[1-3]is a mammalian amidase signature enzyme belonging to the amidase signature(AS) family[4-5].Meanwhile,FAAH is an integral membrane enzyme that degrades members of the fatty acid amide class of neural signaling lipids,including the endogenous cannabinoid N-arachidonyl ethanolamine(anandamide)[6],the sleep-inducing substance 9(Z)-octadecenamide(oleamide)[7],the anti-inflammatory factor N-palmitoyl ethanolamine(PEA)[8],and the satiating signal N-oleoyl ethanolamine(OEA)[9].Early experimental results[10-21]indicated that FAAH may represent an attractive therapeutic target for treatment of pain,inflammation,and other central nervous system(CNS)disorders.The recent determination of the threedimensional structures of FAAH[22]and the two distantly related bacterial amidase signature enzymes[23]suggest that the catalytic machinery of this enzyme is a serine-serine-lysine catalytic triad,in contrast to the classical serine-histidine-aspartate triad found in most serine hydrolases.This unusual triad is assumed to be responsible for the ability of FAAH to hydrolyse amides and esters at equivalent rates through a mechanism in which acylation is rate-limiting step.Afterwards,the detail catalytic mechanism of FAAH was studied by mutagenesis and affinity labeling[24],where both Ser217 and Lys142 contribute to the basecatalyzed activation of the nucleophile Ser241 of FAAH and cooperate to deprotonate Ser241,and Ser217 serves as a bridge between Lys142 and Ser241,Lys142 appears to play a uniquely important role in the acid-catalyzed protonation of the substrateleaving group.
Since the discovery of FAAH and the awareness of its therapeutic potential,much attention has been paid to design the selective and/or potent FAAH inhibitors[25].It is known that organophosphorus acid anhydrides(OPs)are rapid,stoichiometric,and essentially irreversible inhibitors of serine hydrolases,which can act as“hemisubstrate”to trap the enzyme[26-29].As one of the representative of OP-type inhibitors,methyl arachidonyl fluorophosphonate(MAFP)interacts with FAAH by blocking the active center of FAAH through the formation of a phosphonate ester bond with the active site on Ser241(Scheme 1),and subsequentlyleads to the loss ofenzyme activity of FAAH[30].Although the inhibition reaction of FAAH by MAFP has been known greatly,the detailed reaction mechanism of MAFP with FAAH still remains considerably elusive,and no related theoretical work has been reported to our best knowledge.In addition,the reaction mechanism of MAFP with FAAH may be different from hydrolysis of amides or esters due to the five covalence bonds of phosphor as well as its larger atom radius than carbon. Through a combination of site-directed mutagenesis,enzyme kinetics,and chemical labeling experiments,McKinney et al.[24]found that the reaction rate constants of FAAH with fluorophosphonate(FP)-rhodamine are quite different from oleamide or oleoyl methyl ester(about 104times faster),indicating that the phosphonylation reaction of FP-rhodamine with FAAH may proceedviaaspecialpathway,differentfromhydrolysisofamides or esters.
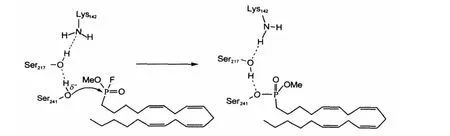
Scheme 1 Inhibition of FAAH by MAFP
Interestingly,Bracey and coworkers[22]have found that the active site of FAAH appears to simultaneously access both the aqueous environment of the cytoplasm and the lipid milieu of the bilayer.This architecture may provide an exit route to the cytosol for the polar amine substituents and could also provide entries for water molecules.Furthermore,McKinney et al.[24]also concluded that the Lys142 residue of the triad is too far away (>0.45 nm)to make direct contacts with either the serine nucleophile or the predicted position of the substrate-leaving group.Therefore,we think that there may be some solvent water molecules filling in the“huge hole”of the active site of FAAH and forming a water-chain bridge to connect Lys142 residue and the substrate-leaving group in the reaction process.In this study, we will propose a new phosphonylation reaction mechanism of MAFP with the active site of the catalytic triad of FAAH using density functional theory B3LYP/6-311G(d,p)method.As described in Scheme 2,two possible reaction pathways(Path A and Path B)have been considered.For the computational model, in order to use less time,more importantly to focus our main attention on the active site of FAAH,the Ser241-Ser217-Lys142 catalytic triad is simplified to CH3OH-CH3OH-CH3NH2system, labeled as Ser-m241-Ser-m217-Lys-m142.MAFP is modeled by O-methyl methylphosphonofluridate(OMPF).Moreover,the polarizable continuum model(PCM)is used to evaluate the solvent influence of water on the studied reactions.This study might be useful for design of new inhibitors of FAAH.
1 Computational details
The geometric optimizations of all species,including the reactant complex(RC),the intermediate(INT),the transition state (TS),and the product complex(PC),were carried out using the density functional theory(DFT)with Becke′s three-parameter (B3)[31]exchange functional along with the Lee-Yang-Parr(LYP) nonlocal correlation functional(B3LYP)[32-33]and using the standard valence triple-ζ basis set,expanded with d-type polarization functions for heavy elements and p-type polarization functions for H,6-311G(d,p)[34].This approach has recently been successfully applied to model the phosphonylation mechanisms of sarin and acetylcholinesterase[35-38].The nature of all optimized structures was determined using harmonic frequency analysis as true minimum with no imaginary frequency or transition state with only one imaginary frequency.The frequency calculations at the B3LYP/6-311G(d,p)level without scaling also provided thermodynamic quantities such as the zero-point vibrational energy, thermal correction,enthalpies,Gibbs free energies,and entropies at 298.15 K and 101.325 kPa.Single-point MP2 calculations,MP2/6-311G(d,p)//B3LYP/6-311G(d,p),were also carried out to assess the accuracy of the B3LYP energies.
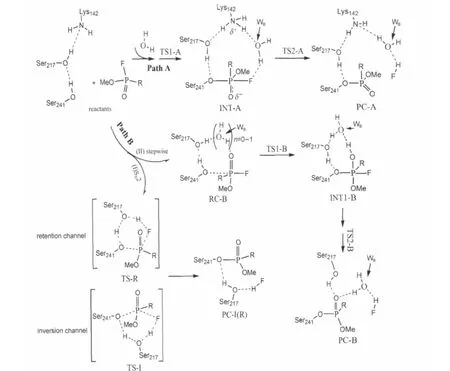
Scheme 2 Possible reaction pathways for MAFP and the active site of FAAHPath A:the unaffected catalytic triad of FAAH;Path B:mutagenesis of Lys142
The polarizable continuum model(PCM)[39]by performing SCRF calculations at the MP2/PCM/6-311G(d,p)//B3LYP/6-311G(d,p)level with a relative dielectric constant ε=78.41(water)was applied for all gas-phase-optimized structures to evaluate the solvent effects of water on the reactions.Charge distribu-tions were obtained employing natural population analysis (NPA)[40-41]from the wave functions calculated at the MP2/6-311G(d,p)//B3LYP/6-311G(d,p)level.Throughout this study, allcalculationswereperformedusingtheGaussian03program[42]. The relative free energy to the reactant complex in the gas-phase (denoted as ΔG298Kin kJ·mol-1)is computed using the Gibbs free energy value at 298.15 K and 101.325 kPa,which is obtained by adding the Gibbs free energy correction at the B3LYP/6-311G (d,p)level to the electronic energy at the MP2/6-311G(d,p)// B3LYP/6-311G(d,p)level.In water,the relative free energy is described by the Gibbs free energy change(ΔGsolin kJ·mol-1) with PCM treatment.
2 Results and discussion
As mentioned above,we investigated the phosphonylation reaction mechanism of OMPF with two CH3OH and one CH3NH2molecules,the simplified active center model between MAFP and FAAH,by use of B3LYP/6-311G(d,p)method(see Scheme 2).Path A is a two-step addition-elimination process,involving a zwitterionic trigonal bipyramidal intermediate.One of the solvent water molecules(labeled as WB)acts as a“hydrogen bridge”connecting Lys-m142 residue and OMPF in the reaction process. At the same time,to illustrate the catalytic power and the detailed phosphonylation mechanism of serine-serine-lysine triad of FAAH,we took into account the other possible reaction pathway,Path B,considered as the case of Lys-m142 residue mutagenesis to alanine(denoted as K142A in Ref.[24])in the catalytic triad of FAAH.The energy data,including zero-point energy corrections and Gibbs free energy barriers,are summarized, while atomic charges and bond order(BO)of selected bonds are provided,respectively.In this section,we will discuss in detail this phosphonylation mechanism and the influences of mutagenesis of catalytic triad residues on it.
2.1 Path A
2.1.1 Geometries and the relative energies
Path A represents the phosphonylation reaction process of OMPF with the active site of FAAH,which is a typical stepwise mechanism.As shown in Fig.1,the configuration of the reaction complex(RC-A)obtained from the DFT optimization has the WBpositioned between Lys-m142 and OMPF,which forms two hydrogen bonds,respectively,with hydrogen atom of the amino group in Lys-m142(r(O10…H9)=0.2093 nm)and the fluorine atom of OMPF(r(F2…H11)=0.2048 nm).Ser-m217 serves as the bridge connecting Lys-m142 and Ser-m241 in the catalytic triad of FAAH,through the hydrogen bonds between the N8 atom and the H7 atom with the distances of 0.1762 nm as well as between the O6 atom and the H5 atom with the distance of 0.1746 nm,respectively.Ser-m241 is close to the methyl and methoxyl hydrogen of OMPF with the hydrogen bond lengths of 0.2348 and 0.2217 nm,respectively,and will act as a nucleophile to attack the phosphorus atom of OMPF in the next step. Lys-m142 is distant from Ser-m241 and OMPF by 0.4342 and 0.4220 nm,respectively,which is reasonable compared with the experimental observation[24].
In the transition state TS1-A,the single imaginary frequency amounts to 929.1i cm-1,whose vibrational mode corresponds to the nucleophilic attack of oxygen atom(O4)of Ser-m241 on the phosphorus atom of OMPF from the backside relative to the fluorine atom.The TS1-A presents a ten-membered ring structure, formed by the Ser-m241-Ser-m217-Lys-m142 catalytic triad of FAAH,the connecting water molecule WB,and OMPF(Fig.1). The nucleophile Ser-m241 attacks the phosphor atom of OMPF and the distance between the hydroxyl oxygen of Ser-m241 and the phosphor atom of OMPF is 0.1939 nm,indicating the formation of a partial O—P single bond.Also at the same time,the proton transfers from Ser-m241 to nitrogen atom(N8)of Lys-m142 through the“bridge”of Ser-m217.The O4—H5 and O6—H7 single bonds increase by 0.0235 and 0.0274 nm from RC-A to TS1-A,respectively,implying that both Ser-m217 and Lysm142 residues contribute to the base-catalyzed activation of the nucleophile Ser-m241.During this process,the O4—P1—F2 moiety in the TS1-A assumes a linear conformation approximately(θ(O4—P1—F2)=161.8°).Meanwhile,due to the attack of the nucleophile Ser-m241 at the phosphor atom of OMPF,the P—F single bond and the P=O double bond distances increase by 0.0159 and 0.0013 nm,respectively.The θ(C13—P1—O12) bond angle increases by 10.8°from RC-A to TS1-A.Those larger deformations may result in higher reactive energy barrier.
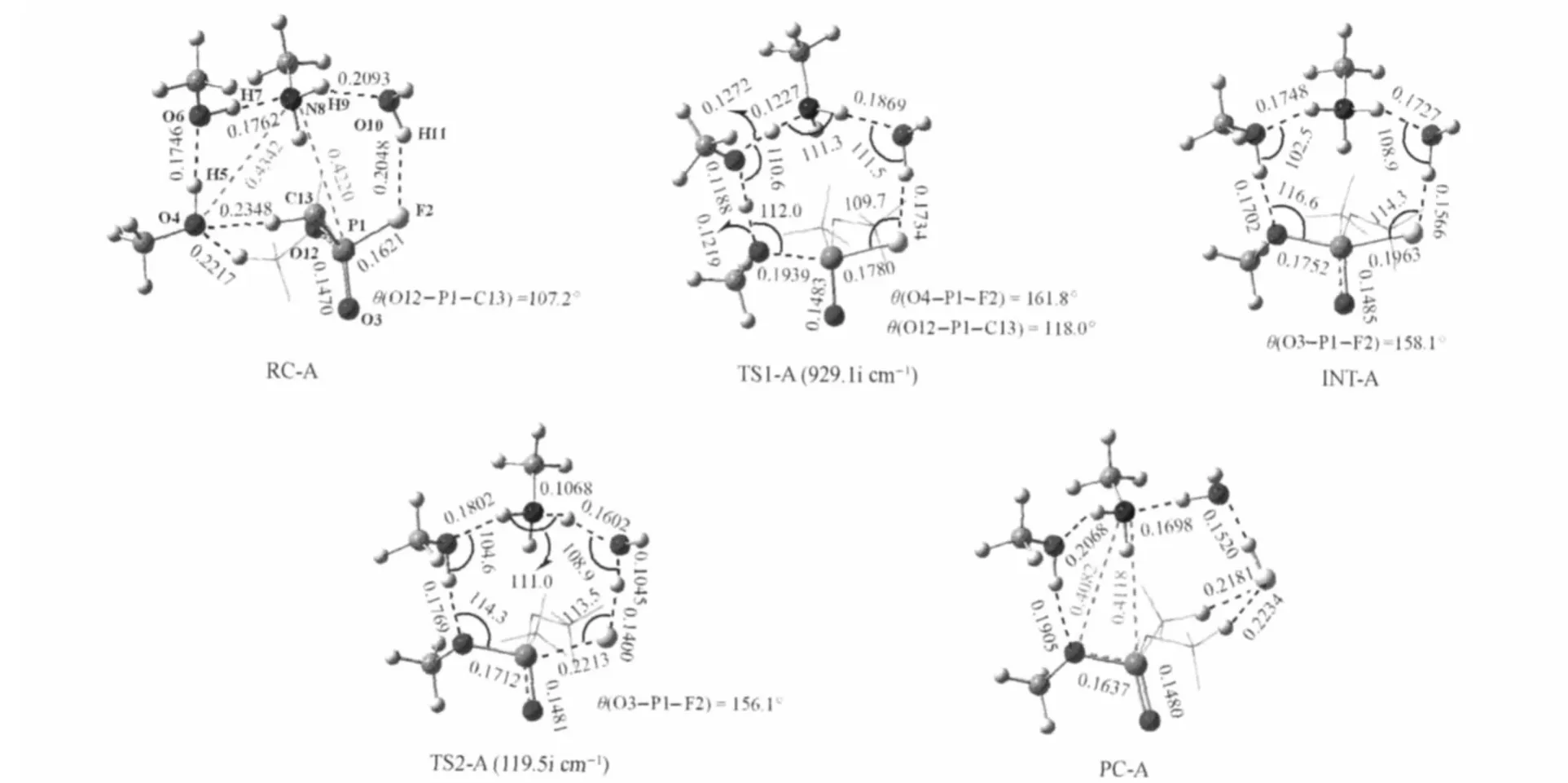
Fig.1 B3LYP/6-311G(d,p)optimized structures of all the complexes,intermediates and transition states in the Path Abong length or distance in nm,bond angle in degree
The first step(addition process)leads to a zwitterionic trigonal bipyramidal intermediate INT-A(Fig.1).Fig.1 shows that the INTA presents a ten-membered ring conformation similar to TS1-A. While the N8 atom of Lys-m142 has captured the proton(H7)of Ser-m217 and the N8—H7 bond distance is 0.1044 nm.The O3=P1 double bond distance increases by 0.0002 nm compared with that in TS1-A and 0.0015 nm compared with that in RC-A, respectively.The P1—O4 single bond length and the P1—F2 single bond length decreases by 0.0187 nm and increases by 0.0183 nm from TS1-A to INT-A,respectively.Those geometry changes reveal the less stability of this zwitterionic trigonal bipyramidal intermediate compared with RC-A.
In the TS2-A,the single imaginary frequency amounts to 119.5i cm-1,whose vibrational mode corresponds to the broken of the P1—F2 single bond and simultaneously the proton transfer from N8 to F2 assisted with WBmolecule.The P1—F2 bond distance is 0.2213 nm and increases by 0.0250 nm compared with that in INT-A and 0.0592 nm compared with that in RC-A, respectively,implying that the P1—F2 single bond has almost broken.The P1—O4 single bond and the O3=P1 double bond distances are 0.1712 and 0.1481 nm,respectively,and very close to those in PC-A.On the other hand,the N8—H9 and O10—H11 bond distances increase only by 0.0021 and 0.0042 nm from INT-A to TS2-A,respectively,indicating that the proton transfer may be difficult to occur in this step.In the PC-A,Lysm142 is distant from Ser-m241 and OMPF by 0.4082 and 0.4118 nm,re-spectively,which is close to 0.45 nm attained from experimental study[24].
The relative energies of all the species to the reactant complex at the MP2/6-311G(d,p)//B3LYP/6-311G(d,p)level of theory were presented in Table 1.The energy values show that the phosphonylation reaction process of OMPF with the active site of FAAH is moderately exothermic,with ΔE0Kof-25.1 kJ·mol-1(-28.0 kJ·mol-1after zero-point correction)in the gas phase. The reactive energy barrierof the rate-determining step is 69.0kJ·mol-1(79.1kJ·mol-1afterzero-pointcorrection)inthegasphase.After the solvent effects of water have been taken into account,the energy barrier is greatly decreased.The activation en-ergy barrierand activation free energy barrierof this phosphonylation reaction is 52.3 and 64.9 kJ·mol-1,respectively.Meanwhile,the solvent water molecules further increase the stability of PC-A and the energetic separation between RC-A and PC-A is-34.3 kJ·mol-1for ΔEsol.

Table 1 Energy data of all the reaction structures at the MP2/6-311G(d,p)//B3LYP/6-311G(d,p)and B3LYP/6-311G(d,p)levels(in parentheses)

Table 2 Selected NPA charge distributions(e)of the stationary points in Path A at the MP2/6-311G(d,p)//B3LYP/6-311G(d,p)level
It is very interesting that INT-A is energetically located at 66.9 kJ·mol-1(ΔE0K)above RC-A in the gas phase,and very close to TS2-A at 66.1 kJ·mol-1.In water,although the solvent water molecules increase the stabilities of INT-A and TS2-A greatly, the INT-A is less stable than TS2-A and the energetic separation between INT-A and TS2-A is-1.6 kJ·mol-1for ΔEsol,and-4.2 kJ·mol-1for ΔGsol,indicating the short-life of this zwitterionic trigonal bipyramidal intermediate in this phosphonylation reaction process.Furthermore,our calculation results indicate that the dipole moments of RC-A,TS1-A,and PC-A are 1.396×10-29, 3.210×10-29,and 2.116×10-29C·m,respectively.The dipole moments of INT-A and TS2-A are 3.555×10-29and 3.475×10-29C· m,respectively.Such larger dipole moments may be responsible for their higher energies of solvation.
2.1.2 NPA charge and molecular orbital analysis
The phosphonylation reaction process of OMPF with the active site of FAAH can be further analyzed by the NPA charge distribution from natural bond orbital(NBO)method and molecular orbital analysis.The selected atom charges presented in Table 2 reveal that the charge of N8 atom decreases by 0.108e from RC-A to TS1-A,implying that the N8 atom has been partially protonated in the transition state TS1-A and activates the nucleophile greatly.The charge of O4 atom increases by 0.073e from RC-A to TS1-A.So,it has been activated by the Lys-m142 and Ser-m217 and will be more facile to nucleophilicly attack the phosphorus atom of OMPF.The O3 and F2 atoms also get 0.041e and 0.086e from RC-A to TS1-A,respectively.The HOMO orbital of TS1-A,as shown in Fig.2,illustrates that the p-orbital of O6 atom is involved in the interaction with O4 atom and assists the O4 atom to attack the phosphorus atom of OMPF. Meantime,the p-orbitals of O4 and F2 atoms are both involved in the interaction with O3 atom and result in the partial P1=O3 double bond cleavage.From RC-A to INT-A,the O4,O3,and F2 atoms gain 0.125e,0.046e,and 0.134e,respectively.On the other hand,the N8 atom loses 0.143e from RC-A to INT-A.It is clearly that the INT-A presents a zwitterionic character,and the negative charges mostly concentrate on the O4,O3,and F2 moieties,while the positive charges on the NH3moiety.The HOMO orbital of INT-A(Fig.2)also shows that,the p-orbitals of O4 and F2 atoms both interact with O3 atom containing partial negative charge,and keep NH3moiety alone possessing partial positive charge.From RC-A to TS2-A,the charge of F2 atom increases by 0.170e,which can be deduced that the P1—F2 single bond has been partial cleavage.The N8 and H9 atoms still possess -0.761e and 0.480e in the TS2-A,respectively,revealing that the interaction between N8 and H9 atoms is very strong and Lye-m142 is not a good“bridge”for proton transfer.While the O10 and H11 atom charges are a little affected,which can explain the difficult occurrence of the proton transfer in the TS2-A.
2.2 Path B
In Path B,Ser-m241 still serves as a nucleophile attacking OMPF molecule,while Ser-m217 acts as a“bridge”connecting Ser-m241 and OMPF,and Alal42 is not included in the model reaction,which stands for the case of K142A in the serine-serinelysine catalytic triad of FAAH.Furthermore,we studied two types of the phosphonylation reaction mechanisms on Path B: concerted(SN2)and stepwise processes(see Scheme 2).In the stepwise process,there are also two pathways considered,with one water molecule assisted and without water molecule assisted. The structures of all RC,INT,TS,and PC in Path B are shown in Figs.3-5.The energy properties of all related structures are summarized in Table 1.
2.2.1 The concerted SN2 process in Path B
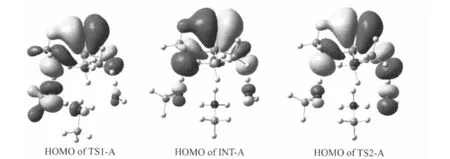
Fig.2 HOMOs of the TS1-A,INT-A,and TS2-A in the Path A
For the SN2 displacement process of Path B,two reaction channels(inversion and retention)have been calculated(Fig.3). In the inversion reaction channel,the RC-I underwent a“Walden invert”to yield the product(PC-I)via a six-membered ring tran sition state(TS-I)with the single imaginary frequency 742.9i cm-1.In this six-membered ring transition state,the largest bond angle θ(O4—P1—F2)and the least bond angle θ(P1—F2—H7)is 106.8°and 92.5°,respectively.On going from RC-I to TS-I, great changes in the geometric conformation of OMPF take place.The P1—F2 single bond and P1=O3 double bond distances increase by 0.0422 and 0.0018 nm,respectively.The bond angle θ(C8—P1—O9)expands by 48.8°,which may result in higher active energy barrier for this reaction process.Furthermore,the O6—H7 single bond distance only increases by 0.0059 nm,indicating that the proton transfer is also not easy in this reaction process and may lag behind the nucleophilic substitute process.
The MP2 relative energies in Table 1 show that this inversion SN2 displacement process is less exothermic either in the gas phase or in water.The reactive energy barrierof the inversion reaction channel of Path B is 218.4 kJ·mol-1(226.4 kJ·mol-1after zero-point correction)in the gas phase.If the continuum solvent effects are taken into account,it is found that the reactive energy barrier is almost unaffected with the solvent molecules.Therefore,in view of the thermodynamics and kinetics,the inversion reaction channel of Path B is difficult to take place both in the gas phase and in water.
In the retention reaction channel,the attack of the nucleophile Ser-m241 at the OMPF molecule also leads to a six-membered ring transition state(TS-R)with the single imaginary frequency 158.5i cm-1.In TS-R,the least bond angle θ(O4—P1—F2)is only 71.1°.From RC-R to TS-R,the P1—F2 single bond and P1= O3 double bond distances increase by 0.0508 and 0.0017 nm, respectively.While the change of the θ(C8—P1—O9)bond angle is smaller than that in the inversion path,which may result in a smaller active energy barrier contrasted with the latter.NPA charge analysis presented in Table 3 shows that O4 atom bears 0.816e in RC-R and more than by about 0.006e in RC-I,indicating that Ser-m24 in RC-R possesses more nucleophilic ability than that in RC-I.
2.2.2 The stepwise process in Path B
Different from the SN2 mechanism of Path B,the stepwise process in Path B reveals a two-step addition-elimination reaction mechanism(Fig.4).In this path,Ser-m241 also acts as the nucleophile binding to the phosphorus of OMPF,while the proton simultaneously transfers to the oxygen of the phosphono group of OMPF via the“hydrogen-bridge”of Ser-m217(addition step).In the following reaction,the transferred proton helps to break the P1—F2 bond of the OMPF serine complex,finally the fluorine is departed as fluorine hydride(elimination step).
When the separated reactants(Ser-m241,Ser-m217 and OMPF)are mixed,the Ser-m241 and Ser-m217 first form a reactant complex(RC-B)with OMPF through a hydrogen bond chain.The bond distances of the H5…O6 and H7…O3 are predicted to be 0.1828 and 0.1776 nm,respectively.Ser-m241 bonds to the methyl hydrogen of OMPF with a hydrogen bond of 0.2279 nm,and will act as a nucleophile to attack the phosphorus atom of OMPF in the next step.
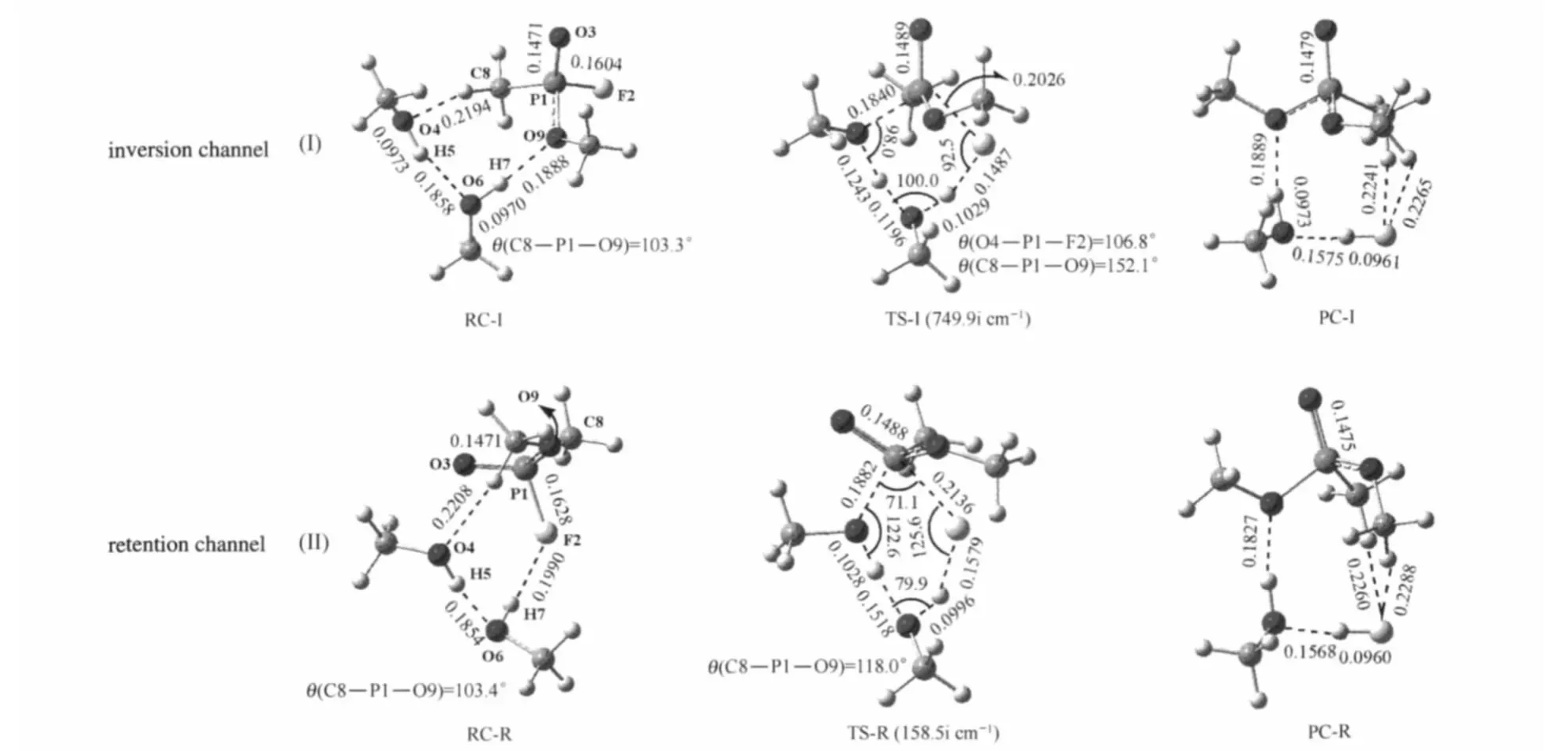
Fig.3 B3LYP/6-311G(d,p)optimized structures of all the complexes and transition states in the SN2 mechanism of Path Bbong length or distance in nm,bond angle in degree
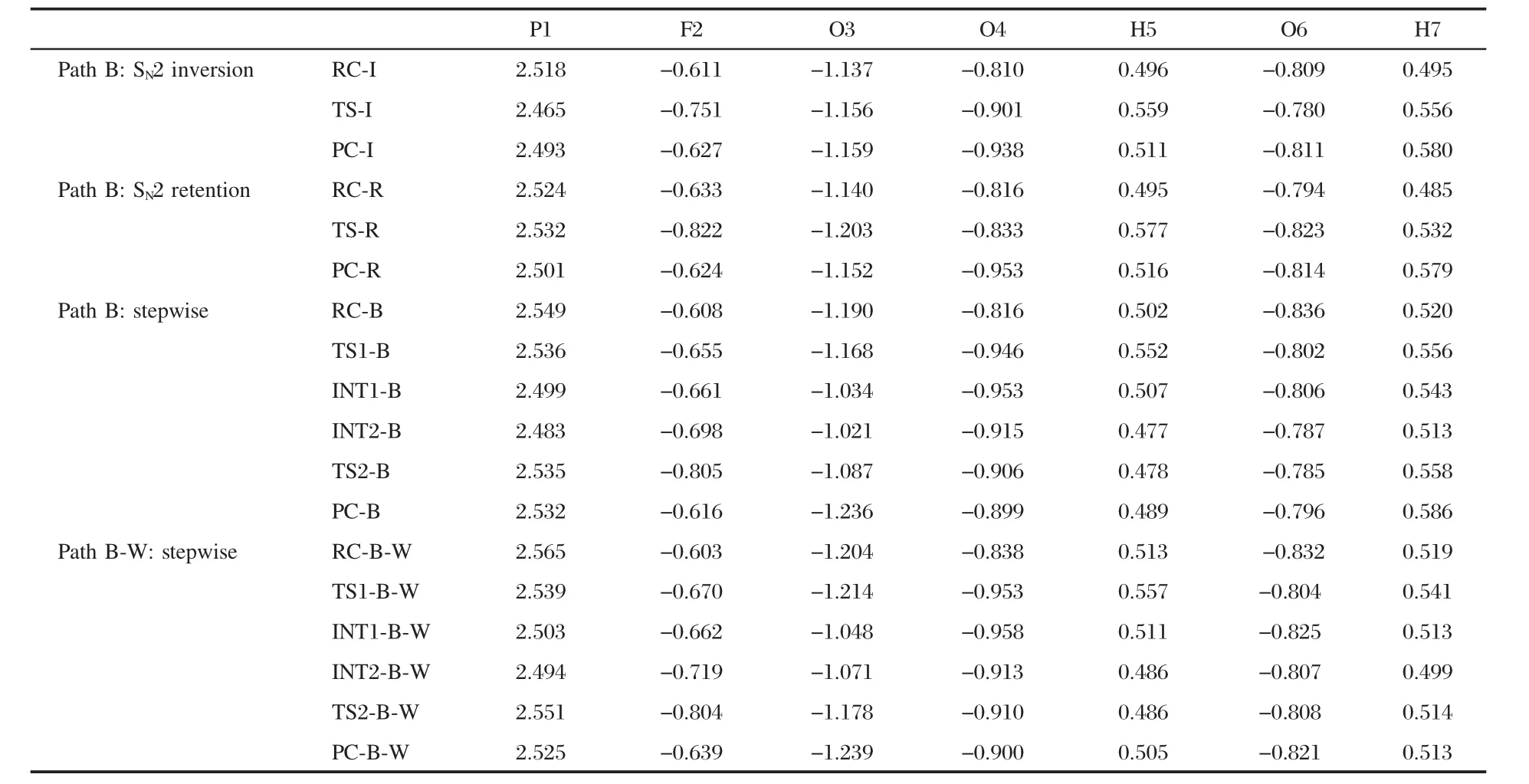
Table 3 Selected NPA charge distributions(e)of the stationary points in Path B at the MP2/6-311G(d,p)//B3LYP/6-311G(d,p)level
The transition state TS1-B is found to be characterized by a pentacoordinated phosphorus compound with a tetrahedral structure,and the Ser-m241,Ser-m217 and OMPF present a sixmembered ring configuration via a hydrogen chain.The single imaginary frequency of TS1-B is predicted to be 1075.8i cm-1, corresponding to the vibrational mode of the proton(H5)transferring from O4 of Ser-m241 to O6 of Ser-m217,the proton (H7)transferring from O6 of Ser-m217 to O3 of OMPF;and simultaneously the nucleophile Ser-m241 O4 oxygen attacking to the phosphorus.In this six-membered ring transition state, the largest bond angle θ(P1—O3—H7)and the least bond angle θ(H5—O6—H7)is 118.7°and 84.9°,respectively.From RC-B to TS1-B,the O4—H5 and O6—H7 single bond lengths are estimated to increase by 0.0304 and 0.0168 nm,respectively.While the H5—O6 and H7—O3 distances decrease by 0.0689 and 0.0507 nm,respectively,indicating that the proton transfer is more facile than that in the SN2 process of Path B.Meantime,the O4—P1 distance is 0.1973 nm suggesting partial O4—P1 single bond formation.
The MP2 single-point energy data in Table 1 show that the reactive energy barrierof the addition step of the stepwise processofPath B is 90.0 kJ·mol-1(102.9 kJ·mol-1after zero-point correction)in the gas phase,which is lower by 128.4 and 95.4 kJ·mol-1than those in the inversion channel and retention channel of concerted process,respectively.While the INT1-B is energetic above the RC-B 59.0 kJ·mol-1(54.8 kJ·mol-1after zeropoint correction)in the gas phase.In water,if the continuum solvent effects are taken into account,it is found that the activation free energy barrieris 99.6 kJ·mol-1in water,and higher by 34.7 kJ·mol-1than that in the Path A.Meanwhile,the solvent water molecules decrease the stability greatly of INT1-B and the energetic separation(ΔGsol)between INT1-B and RC-B is -68.6 kJ·mol-1.
From addition step to elimination step of the stepwise process, INT1-B should undergo the O3—H7 single bond rotation about 180°fromSer-m241sidetoF2atomside(Fig.4).Fig.4alsoreveals that the elimination step of stepwise process involves a fourmembered ring transition state(TS2-B)with the single imaginary frequency 399.8i cm-1,corresponding to the vibrational mode of the proton(H7)transfer from O3 to F2 and the F2—P1 single bond cleavage.The Ser-m217 connects the O3 atom of OMPF directly by a hydrogen bond(r(O6H5…O3)=0.2086 nm), no longer acting as a“hydrogen bridge”in this process due to the steric restriction between them.In this four-membered ring transition state,compared with INT2-B,the P1—F2 distance is 0.2253 nm with increase of 0.0502 nm,meanwhile,the F2—H7 distance is 0.1380 nm with decrease of 0.0622 nm,indicating that the P1—F2 single bond is almost broken and the F2—H7 single bond is partial formed.While the H7—O3 and P1—O3 single bonds only increase by 0.0095 nm and reduce by 0.0062 nm from INT2-B to TS2-B,respectively.Therefore,the proton (H7)is also not easy to transfer from O3 to F2 in the elimination step.
The relative energy data displayed in Table 1 indicate that the reactive energy barrier(relative to RC-B)of the elimination step is 106.3 kJ·mol-1(112.5 kJ·mol-1after zero-point correction)in the gas phase,which is a little higher than that in the addition step.Moreover,the PC-B is energetic above the RC-B 3.8 kJ·mol-1(6.3 kJ·mol-1after zero-point correction)in the gas phase.In water,the reactive energy barrier(relative to RCB)decreases to 103.3 kJ·mol-1.Meanwhile,the solvent water molecules increase the stability of PC-B and the energetic separation(ΔGsol)between PC-B and RC-B is 6.7 kJ·mol-1.
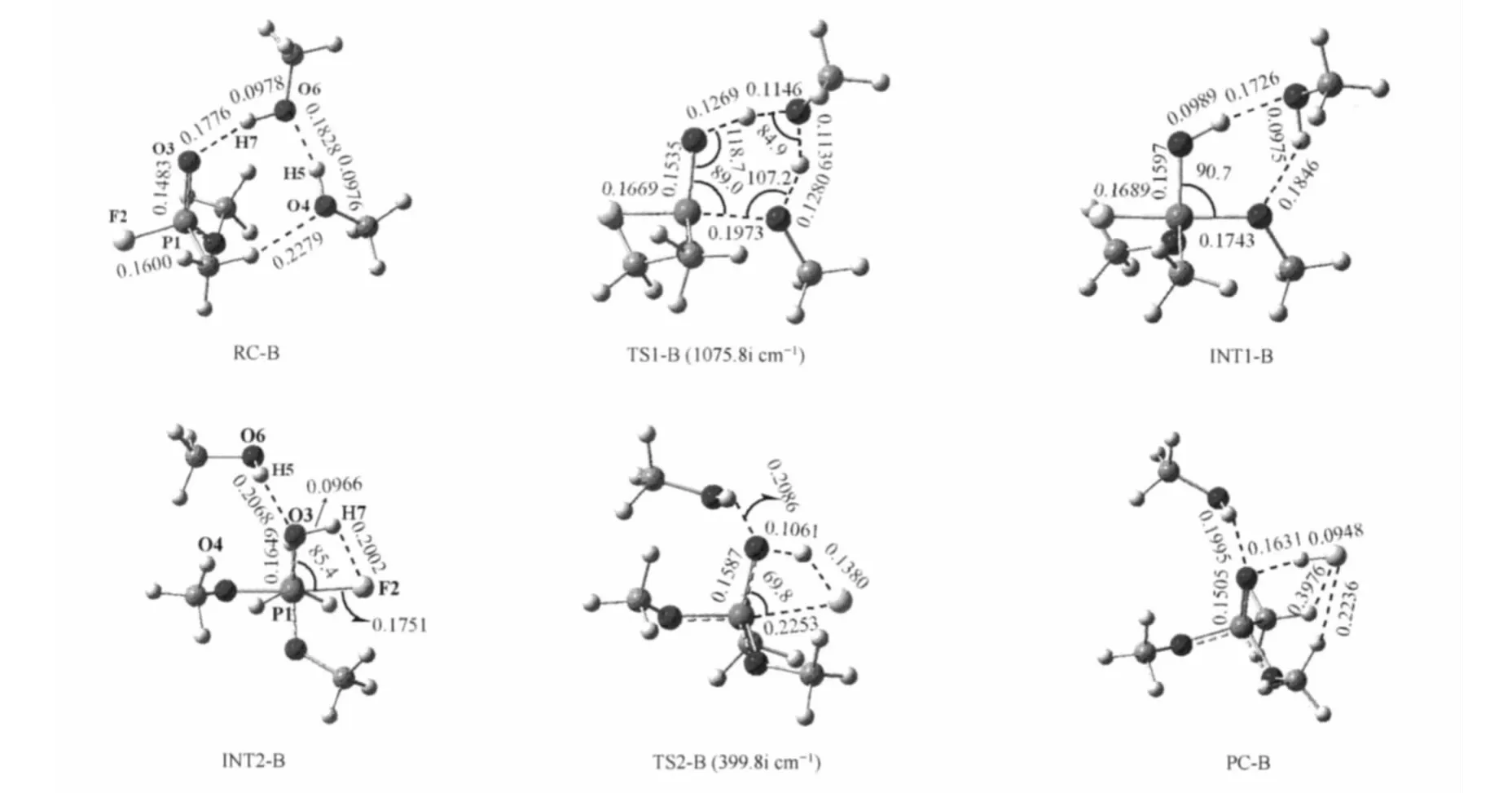
Fig.4 B3LYP/6-311G(d,p)optimized structures of all the complexes,intermediates(INT)and transition states in the stepwise mechanism of Path Bbong length or distance in nm,bond angle in degree
In summary,mutagenesis of the Lys142 residue in the catalytic triad of FAAH will decrease the nucleophilic ability of Serm241 and greatly increase the reactive energy barrier of the phosphonylation reaction of OMPF with the active site of FAAH.In view of the kinetics,the phosphonylation reaction of OMPF with the active site of FAAH may proceed through stepwise mechanism of Path B when the Lys142 residue is mutated in the catalytic triad of FAAH.
2.2.3 One water molecule assisted stepwise process of Path B
On the other hand,when the Lys142 residue is mutated in the catalytic triad of FAAH,the reactive energy barrierin the stepwise process of Path B is high to 99.6 kJ·mol-1at the MP2/6-311G(d,p)//B3LYP/6-311G(d,p)level of theory,which is higher than the experimental results attained by McKinney and Cravatt[24].We speculate that the solvent water molecule(s)may substitute for the Lys142 residue and participate in the reaction. However,the participating probability of water molecules will be limited because of the steric hindrance of the corresponding mutation residue.Thus,in this study,only one water molecule is considered in the phosphonylation reaction process of OMPF with the active site of FAAH when the Lys142 residue is mutated in the catalytic triad.Furthermore,the stepwise mechanism is chosen in this situation because the activation energy barrier is too high to proceed both for the inversion and retention SN2 displacement pathways.
The reaction pathway of one water molecule assisted stepwise process is similar to the stepwise process of Path B,which is also a two-step addition-elimination reaction mechanism(Fig.5). When the Lys142 residue is mutated in the catalytic triad,one solvent water molecule substitutes for the Lys142 residue and forms a reactant complex(RC-B-W)with Ser-m241,Ser-m217 and OMPF through a hydrogen bond chain.The bond distances of the H5…O6,H7…O8,and H9…O3 are predicted to be 0.1744,0.1721,and 0.1745 nm,respectively.Ser-m241 bonds to the methyl and O-methyl hydrogens of OMPF with hydrogen bonds of 0.2235 and 0.2369 nm,respectively,and will act as a nucleophile to attack the phosphorus atom of OMPF in the next step.
The transition state TS1-B-W is also characterized by a pentacoordinated phosphorus compound with a tetrahedral structure, and the substitutional water molecule,Ser-m241,Ser-m217 and OMPF present a eight-membered ring configuration via a hydrogen chain.The single imaginary frequency of TS1-B-W is predicted to be 804.4i cm-1,whose vibrational mode corresponds to the nucleophilic attack of oxygen atom(O4)of Ser-m241 on the phosphorus atom of OMPF from the front relative to the fluorine atom.At the same time,the proton transfers from Ser-m241 to O3 atom of OMPF through the“bridge”of Ser-m217 and the substitutional water molecule.In this eight-membered ring transition state,the largest bond angle θ(P1—O3—H9)and the least bond angle θ(H9—O8—H7)are 136.5°and 97.4°,respectively, which are larger than those(17.8°and 12.5°)in the six-membered ring transition state TS1-B,respectively.Moreover,the O4—P1 distance is 0.1893 nm,and 0.0080 nm shorter than that in the TS1-B,indicating that the proton transfer with a water molecule assisted is more facile when the Lys142 residue is mutated in the catalytic triad and expected for a lower activation energy barrier.The MP2 single-point energy data in Table 1 also show,that the reactive energy barrierand the activation free energy barrierof the addition step of one water molecule assisted stepwise process of Path B are 90.8 and 85.4 kJ· mol-1,respectively,which are lower than that in the case without water molecule assisted by 12.1 and 14.2 kJ·mol-1,respectively.

Fig.5 B3LYP/6-311G(d,p)optimized structures of all the complexes,intermediates and transition states in the one water molecule assisted stepwise process of Path Bbong length or distance in nm,bond angle in degree
The elimination step of one water molecule assisted stepwise process of Path B involves a six-membered ring transition state (TS2-B-W)with the single imaginary frequency 660.4i cm-1,in which the substitutional water molecule acts as a“hydrogen bridge”to promote the hydrogen transfer from O3 to F2.In this six-membered ring transition state,the F2—H7 and F2—P1 distance are 0.1401 and 0.2212 nm,respectively,which are 0.0021 nm longer and 0.0041 nm shorter than that in the case without water molecule assisted,respectively.This suggests that the conformation of the four-membered ring transition state in the stepwise process of Path B is closer to the product complex.The relative energy data displayed in Table 1 indicate that the activation free energy barrierof the elimination step of one water molecule assisted stepwise process of Path B is 102.9 kJ·mol-1and the same as that in the case without water molecule assisted.
From the discussion above we can see that,when the Lys142 residue is mutated in the catalytic triad of FAAH,the first step of the stepwise mechanism is a rate-controlling one with the activation free energy barrierof 85.4 kJ·mol-1.Compared with the unaffected catalytic triad of FAAH,this free energy barrier increases by 20.5 kJ·mol-1,very close to the difference of 21.6 kJ·mol-1between K142A and wild type FAAH from the experiment investigation[24],in which the K142A mutant exhibited 6100-fold reductions in reactivity rates relative to wild type FAAH.Although the solvent water molecule substituting for the Lys142 residue can decrease the reactive energy barrier through acting as a“hydrogen bridge”to promote the proton transfer,its participating probability will be limited because of the steric hindrance of the corresponding mutation residue.Hence,it results in the smaller reaction rate than that in the case of the unaffected catalytic triad of FAAH.
3 Conclusions
The density functional theory,B3LYP method with 6-311G(d, p)basis set has been used to theoretically probe the mechanism of the phosphonylation reaction of MAFP and the active site of FAAH through a simplified model.A new phosphonylation reaction mechanism(Path A)has been proposed,and the results manifest that this phosphonylation reaction of the catalytic triad of FAAH is a two-step addition-elimination process,with the first step(addition step)being the rate-determining step,and involving a zwitterionic trigonal bipyramidal intermediate.One of the solvent water molecules performs a key role in the reaction process,and acts as a“hydrogen bridge”connecting Lys142 residue and MAFP through giving and accepting protons to promote the long-range proton transfer.The Ser241 serves as the nucleophile attacking the phosphor atom of MAFP in initial.Both Ser217 and Lys142 contribute to the base-catalyzed activation of the nucleophile Ser241,while Ser217 serves as a bridge between Lys142 and Ser241.Subsequently,OMPF breaks the P—F bond and simultaneously the proton transfers from N8 atom to F2 atom via the“hydrogen bridge”of WBto generate the products. The other possible reaction pathway(Path B)has been considered to investigate the effect of the residue Lys142 mutation on the phosphonylation reaction process.Our results indicate that, when the Lys142 residue is mutated,the phosphonylation reaction is still a two-step addition-elimination process.Moreover, mutagenesis of the Lys142 residue(Path-B-W:stepwise)in the catalytic triad of FAAH will decrease the nucleophilic strength of Ser241,and increase the reactive energy barrierby more than 20.5 kJ·mol-1compared with Path A,indicating that the Lys142 catalytic residues may act as an important role in the phosphonylation reaction of MAFP with active site of FAAH.It well reproduced the earlier experimental observations that the mutation of the catalytic triad of FAAH decreases the rate of hydrolysis for FP-rhodamine.
Supporting Information Available: The optimized Cartesian coordinates and geometrical structures of all stationary points along the potential energy profiles have been included. This information is available free of charge via the internet at http://www.whxb.pku.edu.cn.
1 Cravatt,B.F.;Giang,D.K.;Mayfield,S.P.;Boger,D.L.;Lerner, R.A.;Gilula,N.B.Nature,1996,384:83
2 Giang,D.K.;Cravatt,B.F.Proc.Natl.Acad.Sci.U.S.A.,1997, 94:2238
3 Patricelli,M.P.;Cravatt,B.F.Vitamins and hormones-advances in research and applications.Vol.62.San Diego:Academic Press, 2001:95
4 Mayaux,J.F.;Cerbelaud,E.;Soubrier,F.;Faucher,D.;Petre,D. J.Bacteriol.,1990,172:6764
5 Chebrou,H.;Bigey,F.;Arnaud,A.;Galzy,P.Biochim.Biophys. Acta,1996,1298:285
6 Devane,W.A.;Hanus,L.;Breuer,A.;Pertwee,R.G.;Stevenson, L.A.;Griffin,G.;Gibson,D.;Mandelbaum,A.;Etinger,A.; Mechoulam,R.Science,1992,258:1946
7 Cravatt,B.F.;Prospero-Garcia,O.;Siuzdak,G.;Gilula,N.B.; Henriksen,S.J.;Boger,D.L.;Lerner,R.A.Science,1995,268: 1506
8 Lambert,D.M.;Vandevoorde,S.;Jonsson,K.O.;Fowler,C.J. Curr.Med.Chem.,2002,9:663
9 de Fonseca,F.R.;Navarro,M.;Gomez,R.;Escuredo,L.;Nava,F.; Fu,J.;Murillo-Rodriguez,E.;Giuffrida,A.;LoVerme,J.;Gaetani, S.;Kathuria,S.;Gall,C.;Piomelli,D.Nature,2001,414:209
10 Cravatt,B.F.;Demarest,K.;Patricelli,M.P.;Bracey,M.H.; Giang,D.K.;Martin,B.R.;Lichtman,A.H.Proc.Natl.Acad.Sci. U.S.A.,2001,98:9371
11 Kathuria,S.;Gaetani,S.;Fegley,D.;Valino,F.;Duranti,A.; Tontini,A.;Mor,M.;Tarzia,G.;La Rana,G.;Calignano,A.; Giustino,A.;Tattoli,M.;Palmery,M.;Cuomo,V.;Piomelli,D. Nat.Med.,2003,9:76
12 Lichtman,A.H.;Shelton,C.C.;Advani,T.;Cravatt,B.F.Pain, 2004,109:319
13 Hohmann,A.G.;Suplita,R.L.;Bolton,N.M.;Neely,M.H.; Fegley,D.;Mangieri,R.;Krey,J.F.;Walker,J.M.;Holmes,P.V.; Crystal,J.D.;Duranti,A.;Tontini,A.;Mor,M.;Tarzia,G.; Piomelli,D.Nature,2005,435:1108
14 Chang,L.;Luo,L.;Palmer,J.A.;Sutton,S.;Wilson,S.J.;Barbier, A.J.;Breitenbucher,J.G.;Chaplan,S.R.;Webb,M.Br.J. Pharmacol.,2006,148:102
15 Jayamanne,A.;Greenwood,R.;Mitchell,V.A.;Aslan,S.; Piomelli,D.;Vaughan,C.W.Br.J.Pharmacol.,2006,147:281
16 Russo,R.;LoVerme,J.;La Rana,G.;Compton,T.;Parrot,J.; Duranti,A.;Tontini,A.;Mor,M.;Tarzia,G.;Calignano,A.; Piomelli,D.J.Pharmacol.Exp.Ther.,2007,322:236
17 Gobbi,G.;Bambico,F.R.;Mangieri,R.;Bortolato,M.; Campolongo,P.;Solinas,M.;Cassano,T.;Morgese,M.G.; Debonnel,G.;Duranti,A.;Tontini,A.;Tarzia,G.;Mor,M.;Trezza, V.;Goldberg,S.R.;Cuomo,V.;Piomelli,D.Proc.Natl.Acad.Sci. U.S.A.,2005,102:18620
18 Naiu,P.S.;Varvel,S.A.;Ahn,K.;Cravatt,B.F.;Martin,B.R.; Lichtman,A.H.Psychopharmacology,2006,192:61
19 Huitron-Resendiz,S.;Sanchez-Alavez,M.;Wills,D.N.;Cravatt, B.F.;Henriksen,S.J.Sleep,2004,27:857
20 Cravatt,B.F.;Saghatelian,A.;Hawkins,E.G.;Clement,A.B.; Bracey,M.H.;Lichtman,A.H.Proc.Natl.Acad.Sci.U.S.A., 2004,101:10821
21 Holt,S.;Comelli,F.;Costa,B.;Fowler,C.J.Br.J.Pharmacol., 2005,146:467
22 Bracey,M.H.;Hanson,M.A.;Masuda,K.R.;Stevens,R.C.; Cravatt,B.F.Science,2002,298:1793
23 Shin,S.;Lee,T.H.;Ha,N.C.;Koo,H.M.;Kim,S.Y.;Lee,H.S.; Kim,Y.S.;Oh,B.H.Eur.Mol.Biol.Org.,2002,21:2509
24 McKinney,M.K.;Cravatt,B.F.J.Biol.Chem.,2003,278:37393
25 Labara,G.;Michaux,C.Chemistry&Biodiversity,2007,4:1882
26 Barak,D.;Ordentlich,A.;Kaplan,D.;Barak,R.;Mizrahi,D.; Kronman,C.;Segall,Y.;Velan,B.;Shafferman,A.Biochemistry, 2000,39:1156
27 Millard,C.B.;Koellner,G.;Ordentlich,A.;Shafferman,A.; Silman,I.;Sussman,J.L.J.Am.Chem.Soc.,1999,121:9883
28 Millard,C.B.;Kryger,G.;Ordentlich,A.;Greenblatt,H.M.;Harel, M.;Raves,M.L.;Segall,Y.;Barak,D.;Shafferman,A.;Silman,I.; Sussman,J.L.Biochemistry,1999,38:7032
29 Bencsura,A.;Enyedy,I.Y.;Kovach,I.M.Biochemistry,1995, 34:8989
30 Deutsch,D.G.;Omeir,R.;Arreaza,G.;Salehani,D.;Prestwich,G. D.;Huang,Z.;Howlett,A.Biochem.Pharmacol.,1997,53:255
31 Becke,A.D.J.Chem.Phys.,1993,98:5648
32 Lee,C.;Yang,W.;Parr,R.G.Phys.Rev.B,1988,37:785
33 Miehlich,B.;Savin,A.;Stoll,H.;Preuss,H.Chem.Phys.Lett., 1989,157:200
34 Hehre,W.J.;Radom,L.;Schleyer,P.R.;Pople,J.A.Ab initio molecular orbital theory.New York:Wiley,1986
35 Wang,J.;Gu,J.;Leszczynski,J.J.Phys.Chem.B,2005,109:
13761
36 Wang,J.;Roszak,S.;Gu,J.;Leszczynski,J.J.Phys.Chem.B, 2005,109:1006
37 Wang,J.;Gu,J.;Leszczynski,J.J.Phys.Chem.B,2006,110:7567
38 Wang,J.;Gu,J.;Leszczynski,J.J.Phys.Chem.B,2008,112:3485
39 Tomasi,J.;Persico,M.Chem.Rev.,1994,94:2027
40 Reed,A.E.;Weinstock,R.B.;Weinhold,F.J.Chem.Phys.,1985, 83:735
41 Reed,A.E.;Curtiss,L.A.;Weinhold,F.Chem.Rev.,1988,88: 899
42 Frisch,M.J.;Trucks,G.W.;Schlegel,H.B.;et al.Gaussian 03. Revision D.01.Pittsburgh,PA:Gaussian Inc.,2005
- 物理化學(xué)學(xué)報(bào)的其它文章
- 陽(yáng)極氧化鋁模板法可控制備金屬納米線和納米管陣列的生長(zhǎng)機(jī)制
- 蛋白質(zhì)與線性DNA片斷結(jié)合過(guò)程中的末端效應(yīng)
- 混合量子-經(jīng)典方法計(jì)算電荷轉(zhuǎn)移速率及其在實(shí)際體系中的應(yīng)用
- 旋涂水溶液前驅(qū)體制備氧化鋅薄膜及其性質(zhì)
- 通過(guò)構(gòu)象誘導(dǎo)電感耦合機(jī)制光可逆調(diào)控有機(jī)場(chǎng)效應(yīng)晶體管的性能
- 利用給體-π-受體分子模板在Au(111)電極表面構(gòu)筑富勒烯帶狀結(jié)構(gòu)

