寬帶隙p區(qū)金屬氧化物/氫氧化物對苯的光催化降解
李朝暉 劉 平 付賢智
(福州大學(xué)光催化研究所,省部共建國家重點(diǎn)實(shí)驗(yàn)室培育基地,福州 350002)
Benzene has severe health and environmental consequences due to its high toxicity and confirmed carcinogenicity[1-3].A recent study has shown that a long-term exposure to a very low levels (volume fraction less than 1×10-6)of benzene can reduce blood cell counts in human being.Workers exposed to benzene fumes, runintoanincreasedriskofleukemiaandbone-marrowtoxicity[4]. Benzene is also ubiquitous as an air pollutant from cigarette smoke,gasoline vapors,paint,industrial exhaust gases,and automobile emissions.The benzene pollution has already become one of the main environmental problems facing humanity. Therefore,the development of an efficient,cost-effective and environmentally sustainable technology in treating benzene and its derivatives is indispensable.
Semiconductor photocatalysis for environmental remediation has received increasingly interest since it is environmentally friendly,capable of performing at room temperature and can treat organic pollutants at extremely low concentrations[5-13].The photocatalytic reactions occur over the semiconductor photocatalysts are initiated by the photo-generated electrons and holes that are captured subsequently by the surface adsorbed species. The capture of photo-generated charge carriers induce the formation of very reactive radicals such as O-·20,O·2,HO·2,HO·and so on,leading to the final decomposition of organic pollutants[6].
Due to its high stability,non-toxicity,cheapness as well as its appropriate electronic band positions capable of oxidizing most organic pollutants,titanium dioxide(TiO2)has become the most widely used semiconductor photocatalyst in heterogeneous photocatalysis.Although TiO2-based photocatalytic oxidation(PCO) has been established to be one of the most promising technologies for the environment remediation and has been successful in treating a wide variety of volatile organic compounds(VOCs), PCO meets with limited success in the treatment of aromatic compounds like benzene due to the deactivation of TiO2resulted from the accumulation of the stable reaction intermediates on the surface[14-15].Loading of noble metals like Pt,Pd,or Rh over TiO2has been used to enhance its performance for photocatalytic oxidation of benzene in gas phase.However,these noble-metalloaded TiO2photocatalysts suffer from the problem of stability due to the oxidation of the noble metal nanoparticles on the surface of TiO2[16-18].Adding sufficient amount of H2O in the reaction feed gas can improve the efficiency of TiO2photocatalyst toward the complete oxidization of benzene to a certain degree[19-20].Our recent studies have also demonstrated that the introduction of magnetic field[21]or H2[22-23]into the photochemical reaction system can greatly improve the efficiency of Pt/TiO2for the photodecomposition of benzene at room temperature.However,it is not easy to realize such a complicated hybrid system for photocatalytic air purification.Therefore the development of photocatalysts with high performance for benzene degradation is indispensable in view of the application of photocatalysis for benzene treatment,yet it remains a great challenge till now.
Recently,a series of wide band gap p-block metal oxides/hydroxides with superior performance for photocatalytic degradation of benzene have been developed in our institute[24-29].These wide band gap p-block metal oxides/hydroxides are a series of promising photocatalysts for benzene degradation.The preparations of these p-block metal oxides/hydroxides,their photocatalytic activity and mechanism for benzene degradation as well as the structure-activity relationship are summarized in this review.
1 Preparations and photocatalytic activity for benzene degradation
1.1 Binary p-block metal oxides/hydroxides β-Ga2O3, In(OH)3and InOOH
Porous binary gallium oxide β-Ga2O3can be prepared via the hydrolysis of gallium nitrate in ammonia solution followed by a heat treatment at 600℃[24].The XRD pattern of the as-prepared β-Ga2O3is shown in Fig.1.The as-prepared β-Ga2O3exhibits stepwise adsorption and desorption(type IV isotherm)in the N2-sorption isotherm,indicative of a porous solid.The BET specific surface area is 80 m2·g-1and the average pore size is 7.3 nm with a narrow distribution of pore size for the as-prepared β-Ga2O3.With a band gap of 4.7 eV,β-Ga2O3can be excited with 254 nm UV irradiation.The as-prepared β-Ga2O3is highly photoactive for mineralizing benzene and its derivatives like toluene and ethylbenzene to CO2under 254 nm UV irradiation.For an initial benzene concentration of 450 μL·L-1,β-Ga2O3shows a high conversion rate of 42%and can maintain its reactivity during the prolonged operation of 80 h.In the meantime about 1070 μL·L-1of CO2can be produced over β-Ga2O3,indicating that 95%of benzene converted has been mineralized to CO2over β-Ga2O3(Fig.2)[24].
Binary p-block metal hydroxide In(OH)3also shows high photocatalytic performance for benzene degradation under 254 nm UV irradiation.Porous In(OH)3can be prepared by hydrolysis of In(NO3)3in an aqueous solution of ammonium followed by a thermal treatment at 120℃.The BET specific surface area for the as-prepared In(OH)3is 120 m2·g-1.For an initial benzene concentration of 1300 μL·L-1,the conversion of benzene and the mineralization rate over In(OH)3can maintain at 25%and 40%,respectively,even after 30 h photocatalytic reaction(Fig.3)[25].
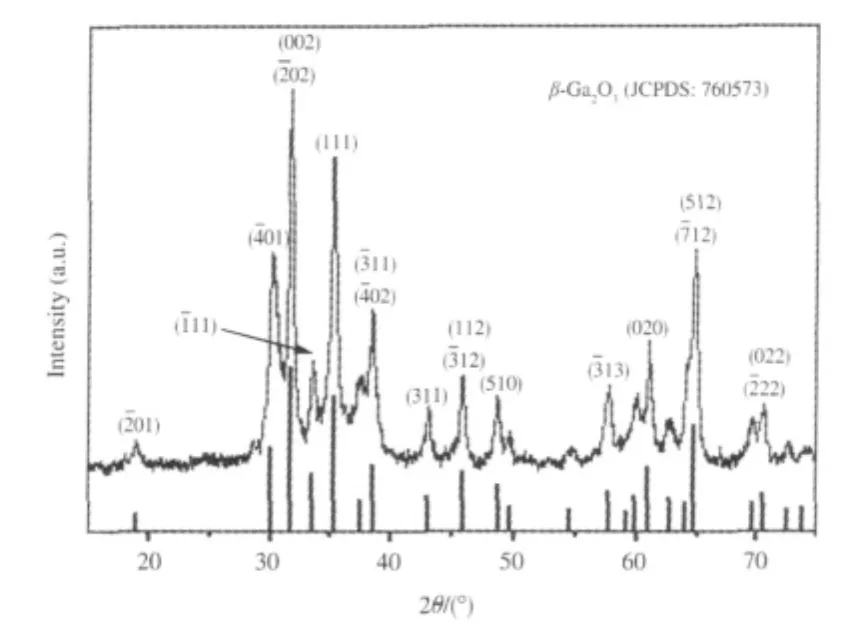
Fig.1 X-ray diffraction(XRD)pattern of the as-synthesized Ga2O3sample[24]
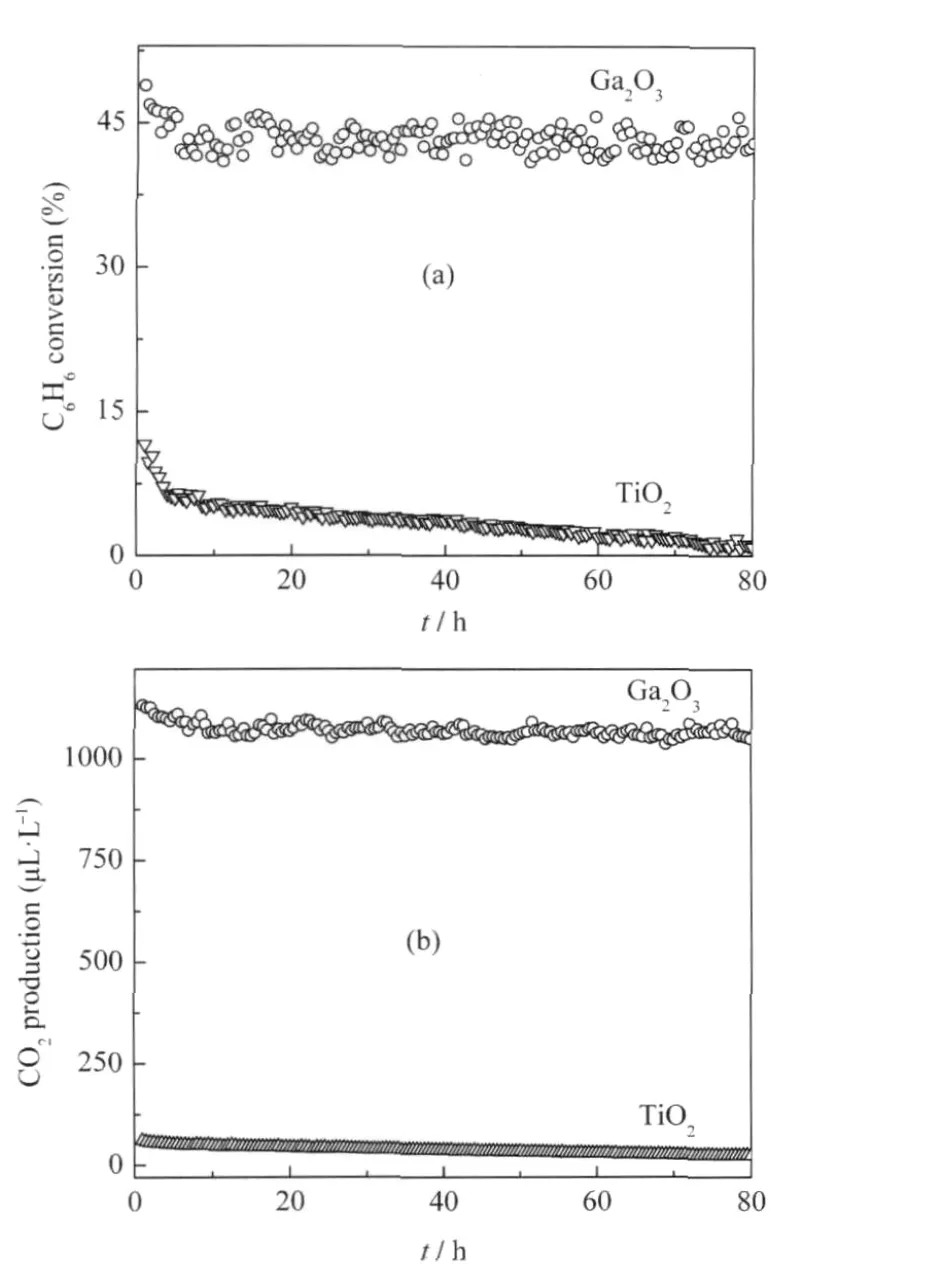
Fig.2 (a)Conversion of C6H6and(b)the amount of produced CO2over the Ga2O3as a function of reaction time(t)with TiO2(P25)as a reference[24]
Oxyhydroxide InOOH can be prepared via a facile solvothermal process from In(NO3)3in a water/ethylenediamine(1∶6)solution.The XRD pattern of the as-prepared InOOH is shown in Fig.4.The average particle size is about 20 nm and the BET specific surface area is about 55 m2·g-1for the as-prepared InOOH. With a band gap energy of 3.7 eV,InOOH can be excited by 300 nm UV irradiation.For an initial benzene concentration of 260 μL·L-1,the conversion of benzene over InOOH can reach 7.5%.In addition,the amount of the produced CO2is about 50 μL·L-1,corresponding to a benzene mineralization ratio of more than 50%.The photocatalytic activity can be maintained for more than 30 h,during which no noticeable deactivation is observed(Fig.5)[26].
1.2 Ternary p-block metal semiconductors Sr2Sb2O7, ZnGa2O4and Zn2GeO4
Besides the binary p-block metal oxides/hydroxides,the photocatalytic activity for benzene degradation can also be found over ternary p-block metal oxides with more diversified composition and structure.
Nanocrystalline Sr2Sb2O7prepared via a facile hydrothermal method is found to show high activity for benzene degradation. AlthoughSr2Sb2O7preparedviaaconventionalsolidstatereaction method(Sr2Sb2O7(SSR))has been reported to be a photocatalyst for water splitting[30]and the degradation of organic dyes[31],it shows very low activity for benzene degradation due to its low specific surface area.To extend its application for photocatalytic degradation of benzene,we developed for the first time a facile method in the preparation of nanocrystalline Sr2Sb2O7directly from commercial Sb2O5.The XRD patterns of the obtained products under different pH values are shown in Fig.6.It is found that the reaction pH plays an important role in the final product and only under highly basic condition can nanocrystalline Sr2Sb2O7be obtained.The hydrothermal prepared nanocrystalline Sr2Sb2O7consists entirely of small particles with average size at around 6 nm and has a relatively large BET specific surface area of about 24.8 m2·g-1,much higher than Sr2Sb2O7prepared via the solid state reaction(1.4 m2·g-1).The N2-sorption isotherm indicates that the as-prepared Sr2Sb2O7is a mesoporous solid with a narrow distribution of pore size at ca 4.0 nm.The as-prepared nanocrystalline Sr2Sb2O7show high photocatalytic performance for the degradation of benzene.For an initial ben-zene concentration of 220 μL·L-1,the conversion of benzene is about 24%and more than 160 μL·L-1of CO2is produced in the meantime,corresponding to a high mineralization ratio of about 50%.Both the conversion ratio and the mineral-ization ratio are higher than those over solid state prepared Sr2Sb2O7(4%,30 μL· L-1).The high conversion and mineralization ratio can be maintained for more than 40 h,during which no obvious deactivation is observed(Fig.7)[27].
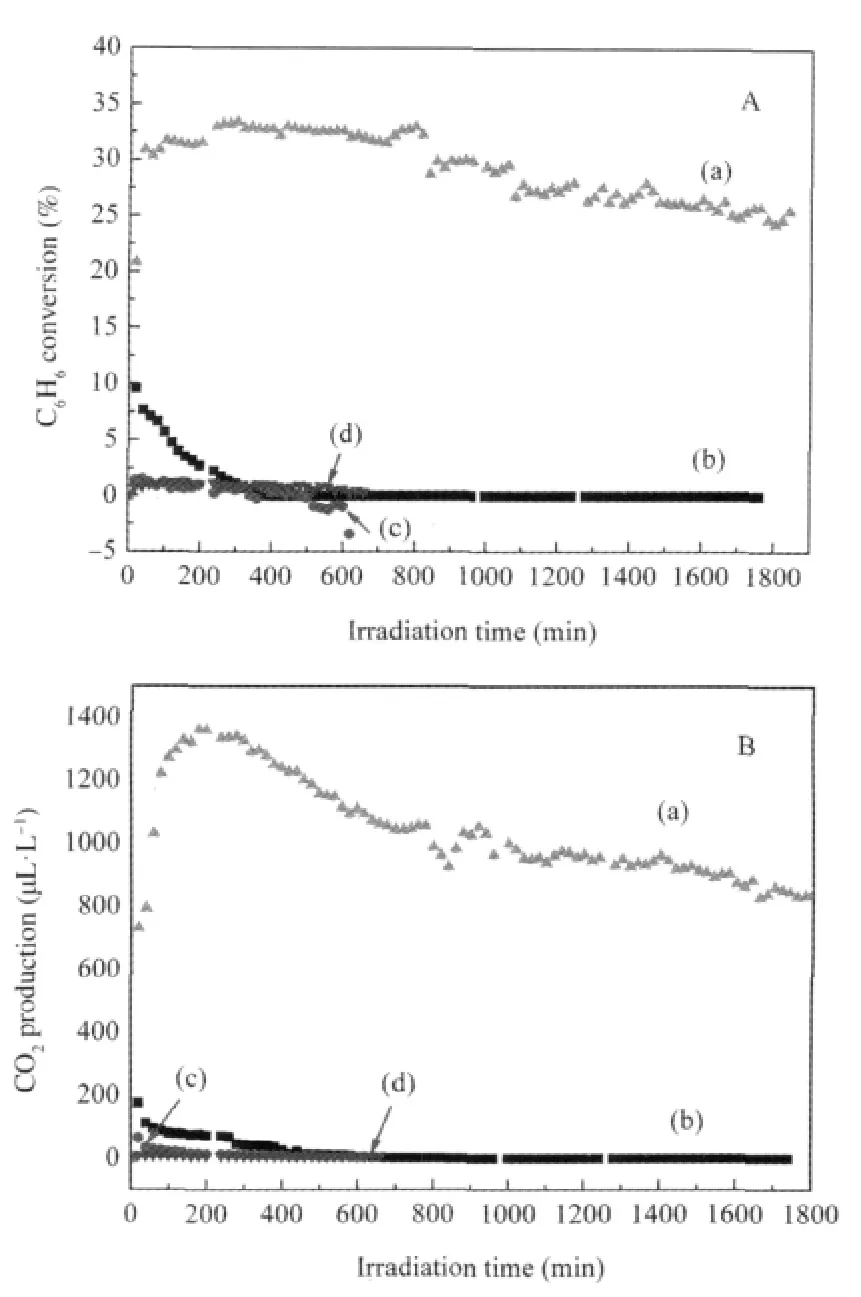
Fig.3 Photocatalytic oxidation conversion of henzene(A) and production of CO2(B)over the catalyst in dry O2[25](a)In(OH)3,(b)TiO2;curve(c)was a control experiment with In(OH)3without irradiation,and(d)was a control experiment without In(OH)3under 254 nm irradiation;the initial benzene volume fraction:1300 μL·L-1
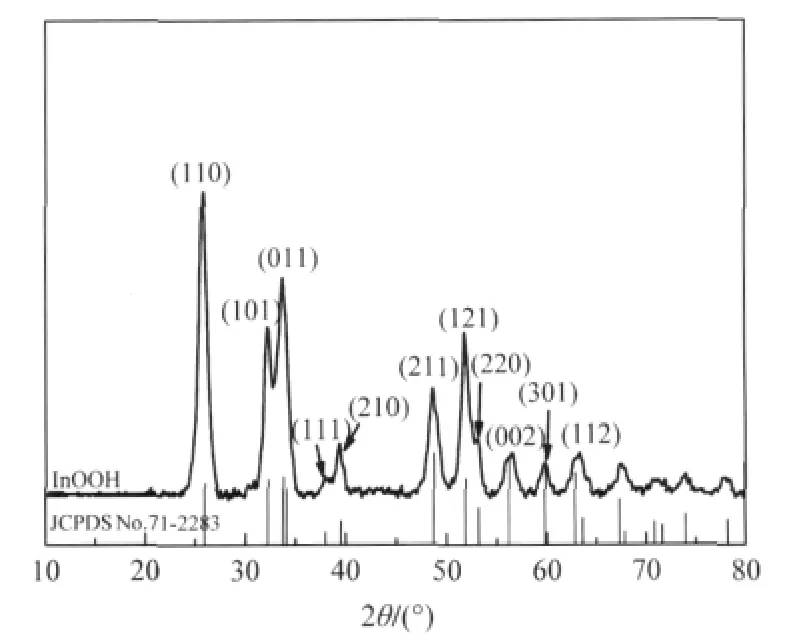
Fig.4 XRD pattern of the as-prepared InOOH[26]
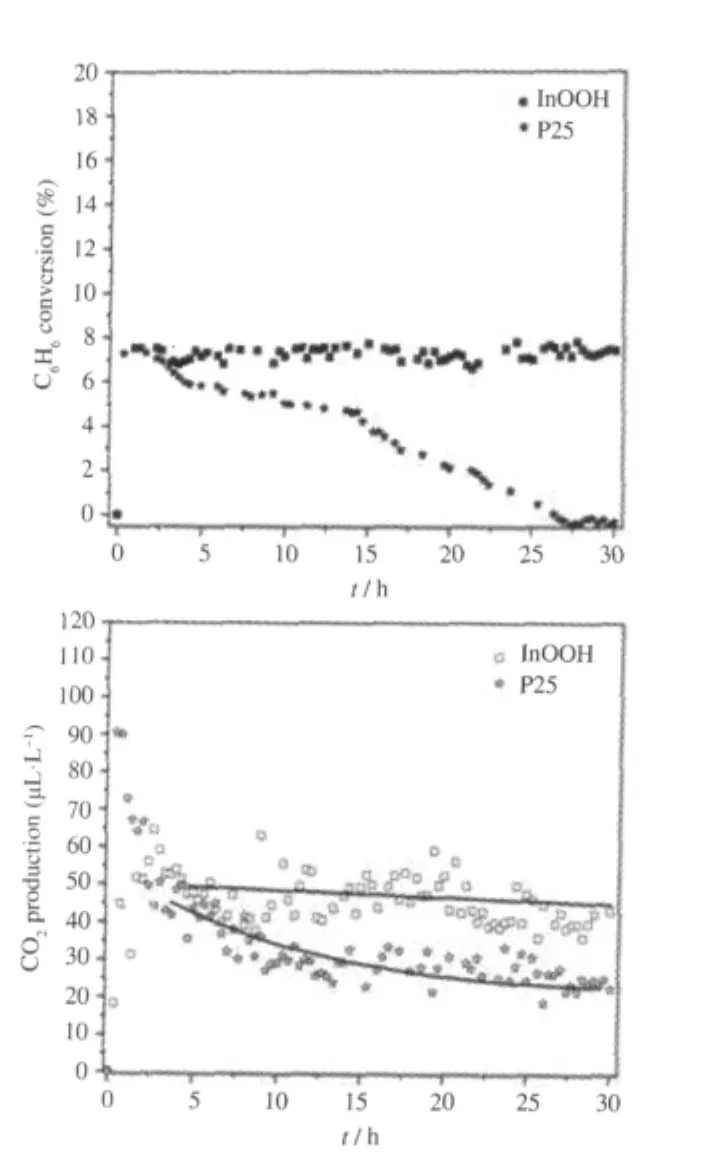
Fig.5 (A)Conversion of benzene and(B)the amount of produced CO2over InOOH and P25 for decomposing benzene as a function of reaction time under UV illumination(λ=300 nm)[26]
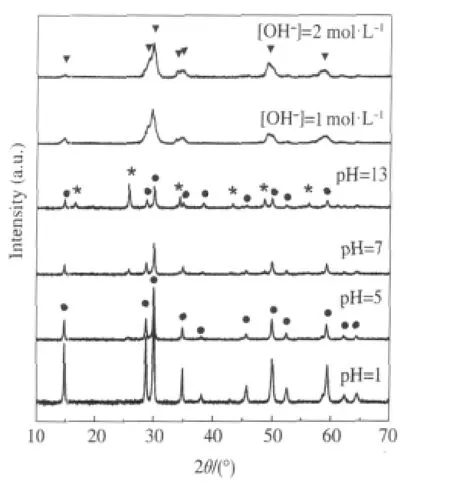
Fig.6 XRD pattern of the samples prepared at 180℃for 48 h with different pH values[27](●)Sr1.36Sb2O6,(*)SrSb2O6,(▼)Sr2Sb2O7
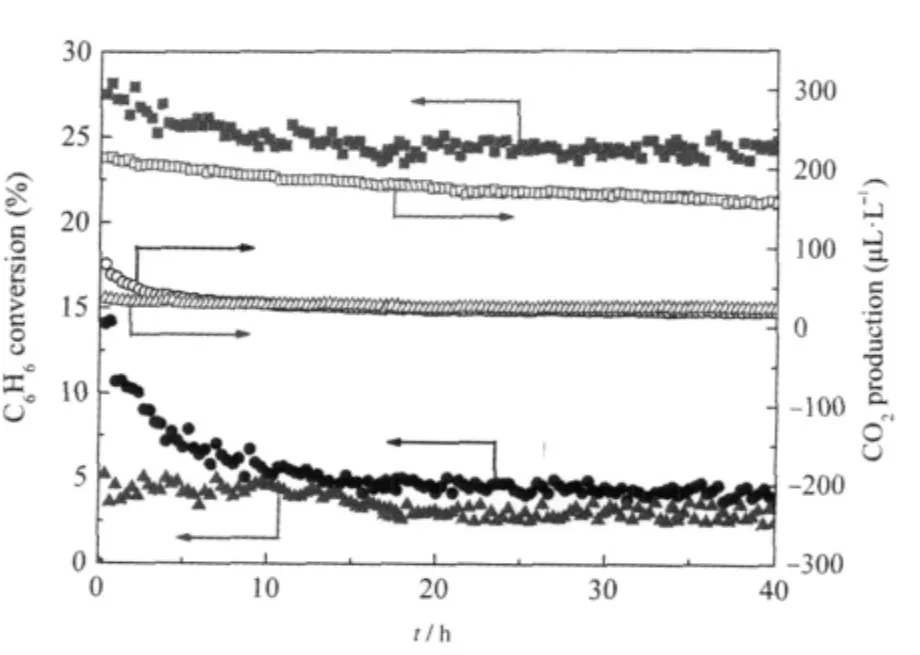
Fig.7 Conversion of C6H6and the amount of produced CO2 over the Sr2Sb2O7(180℃,48 h,[OH-]=2 mol·L-1)as a function of reaction time,with TiO2(P25)and Sr2Sb2O7(SSR)as references[27](■)(●)(▲)the conversion of C6H6over the Sr2Sb2O7,TiO2,and Sr2Sb2O7(SSR) respectively;(□)(○)(Δ)the amount of produced CO2over the Sr2Sb2O7,TiO2, and Sr2Sb2O7(SSR)respectively;Sr2Sb2O7(SSR)refers to the sample prepared via a solid state reaction.
Nanocrystalline ZnGa2O4with a specific surface area of about 36.7 m2·g-1can be prepared via a co-precipitation method from Zn(NO3)2and Ga(NO3)3followed by a heat treatment at 600℃. The XRD pattern of the as-prepared ZnGa2O4is shown Fig.8. For an initial benzene concentration of 220 μL·L-1,the conversion of benzene over the thus-prepared ZnGa2O4is about 12.0% and more than 100 μL·L-1of CO2can be produced,corresponding to a mineralization ratio of about 63%(Fig.9)[28].With an aim at enhancing its activity,ZnGa2O4with an extremely high specific surface area of 201 m2·g-1has been prepared from Zn(NO3)2and Ga(NO3)3via a hydrothermal treatment at 80℃.The increase of the specific surface area leads to a significant enhancement of the photocatalytic activity for benzene degradation.The conversion ratio of benzene can be increased to as high as 41%and the produced CO2can reach 500 μL·L-1for an initial benzene concentration of 300 μL·L-1,which is much higher than Pt/TiO2(Fig.10)[32].
Another ternary p-block metal oxides with high photocatalytic performance for benzene degradation is Zn2GeO4.Nanorods of Zn2GeO4can be prepared from GeO2and Zn(Ac)2under the assistance of surfactant cetyltrimethylammonium bromide(CTAB) via a facile hydrothermal method and the XRD pattern is shown in Fig.11.The SEM images reveal that the as-prepared sample contains a large quantity of nanorods 20-50 nm in width and 150-600nminlength(Fig.12).Under254nmUVirradiations,for an initial benzene concentration of 300 μL·L-1,the benzene conversion and CO2concentration over the as-prepared Zn2GeO4nanorods can be maintained steady at ca 21%and ca 280 μL·L-1, respectively,which corresponding to a high mineralization ratio of ca 75%(Fig.13)[29].
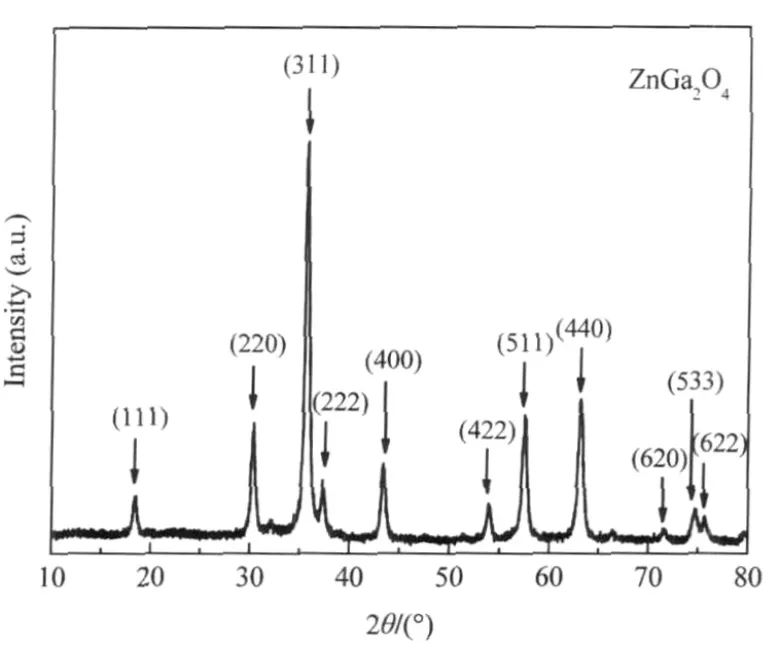
Fig.8 XRD pattern of the nanocrystalline ZnGa2O4[28]
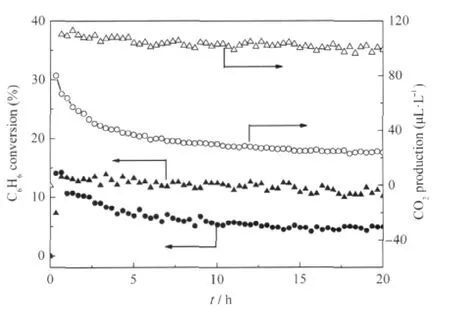
Fig.9 Conversion of C6H6and the amount of produced CO2 over the nanocrystalline ZnGa2O4as a function of reaction time,with TiO2(P25)as references[28](▲)(●)the conversion of C6H6over the ZnGa2O4and TiO2respectively, (Δ)(○)the amount of produced CO2over the ZnGa2O4and TiO2respectively
2 Structure-activity relationship
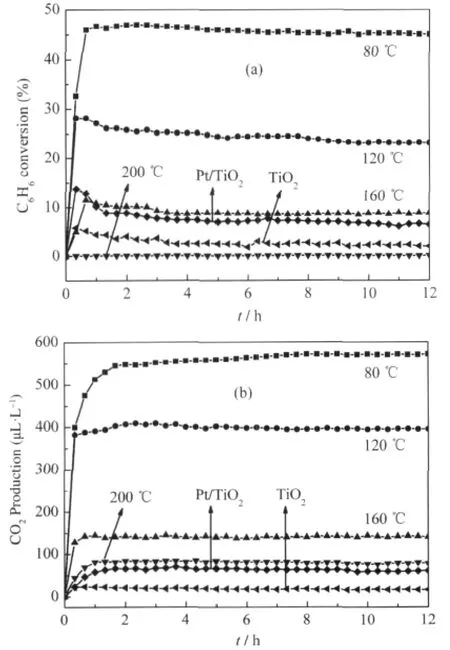
Fig.10 Photocatalytic conversion of benzene(a)and the amount of produced CO2(b)over ZnGa2O4hydrothermal prepared under 80,120,160,200℃,TiO2and Pt/TiO2[32]
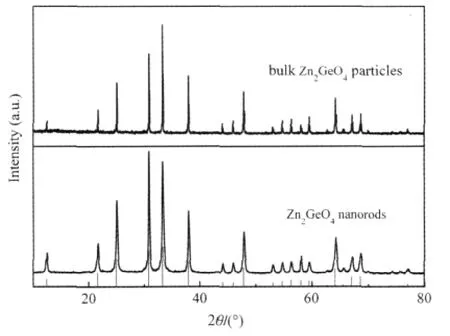
Fig.11 XRD patterns of Zn2GeO4nanorods and bulk Zn2GeO4particles[29]
The band gap,crystallinity,and the specific surface area are important factors that can influence the photocatalytic activity of the semiconductor photocatalysts.However,the activity of some p-block metal oxide photocatalysts can not be simply explained in terms of the above factors.A study on three crystalline phase of Ga2O3reveals that the intrinsic crystallographic structure,especially the geometric structure of the M—O polyhedron(M=pblock metal)can influence the photocatalytic activity of these wide band gap semiconductors as well[33].The crystal structure of α-Ga2O3and β-Ga2O3(Fig.14)and the calculations using the crystallographic data regarding the atom positions reveal that α-Ga2O3is constituted only by distorted octahedron GaO6with a dipole moment of 14.0×10-30C·m,while β-Ga2O3contains both distorted GaO6octahedron(7.3×10-30C·m)and GaO4tetrahedron(2.3×10-30C·m).It is believed that the dipole moment induced by the distorted polyhedron can create a local electric field inside the distorted polyhedron,which can promote the separation of the photo-generated electron-hole pairs[34].Although both α-Ga2O3and β-Ga2O3contain distorted polyhedron in their structure,α-Ga2O3contains only distorted octahedron,while β-Ga2O3has both heavily distorted octahedron and tetrahedron in its structure.The coexistence of two different kinds of electric fields might have synergic effects in promoting the separation of photoexcited electron-hole pairs.Therefore,α-Ga2O3shows a lower photocatalytic activity than β-Ga2O3(Table 1).This promo-moting effect is confirmed by the time-resolved photoluminescence(PL)measurements.The PL measurements reveal that the lifetime of the photogenerated electron-hole pairs on β-Ga2O3is longer than that on α-Ga2O3(Fig.15 and Table 2).The existence of the relationship between the geometric structure and the photocatalytic activity among these semiconductor photocatalysts provides some guideline in our development of new wide band gap p-block metal semiconductor photocatalysts.
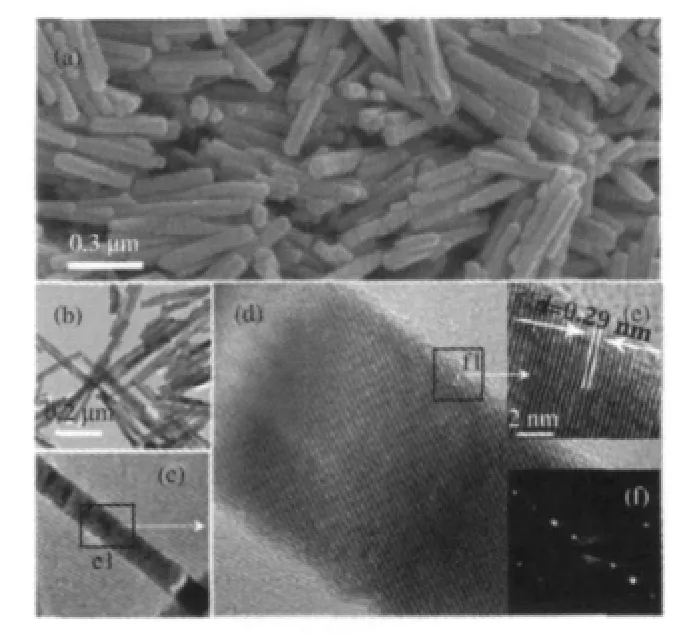
Fig.12 Structural characterization and general morphologies of Zn2GeO4nanorods[29](a)SEM image,(b)TEM image,(c)TEM image of a Zn2GeO4nanorod, (d)HRTEM image of area e1 in(c),(e)enlarged image of area f1 in(d), (f)SAED pattern recorded along the zone axis[110]of the Zn2GeO4nanorods
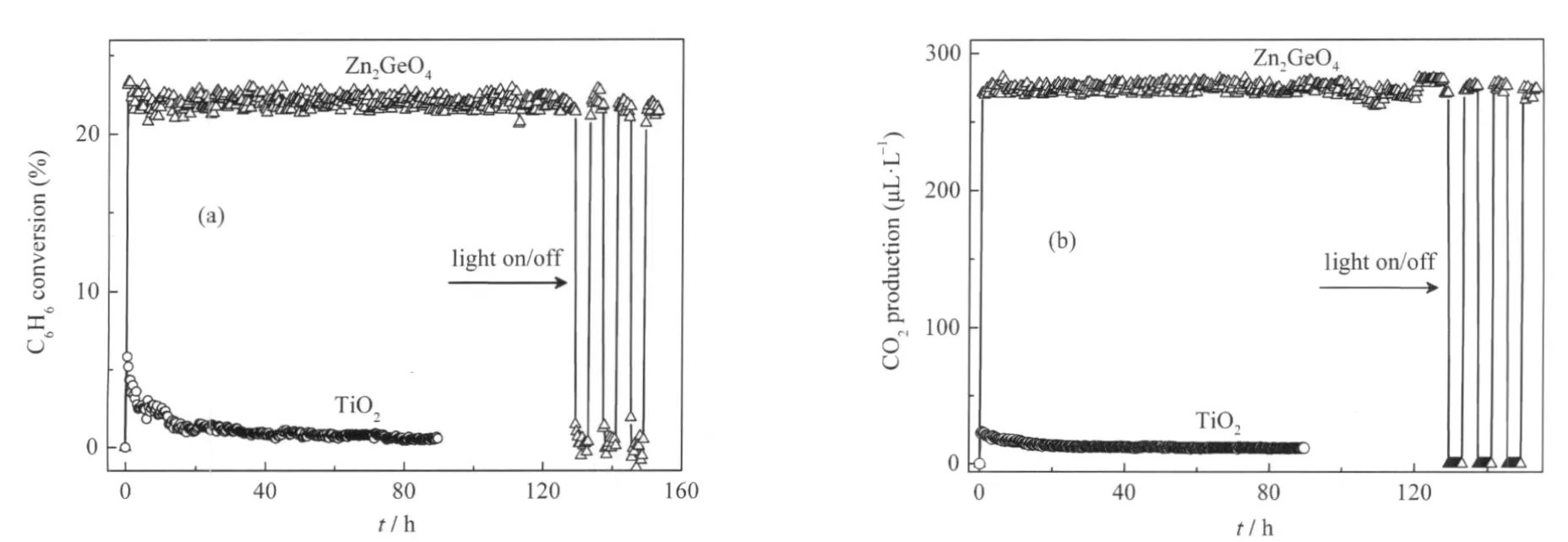
Fig.13 (a)Photocatalytic conversion of benzene and(b)amount of produced CO2in the stream over the Zn2GeO4nanorods against the reaction time,with TiO2(Degussa P25)as a reference catalyst[29]
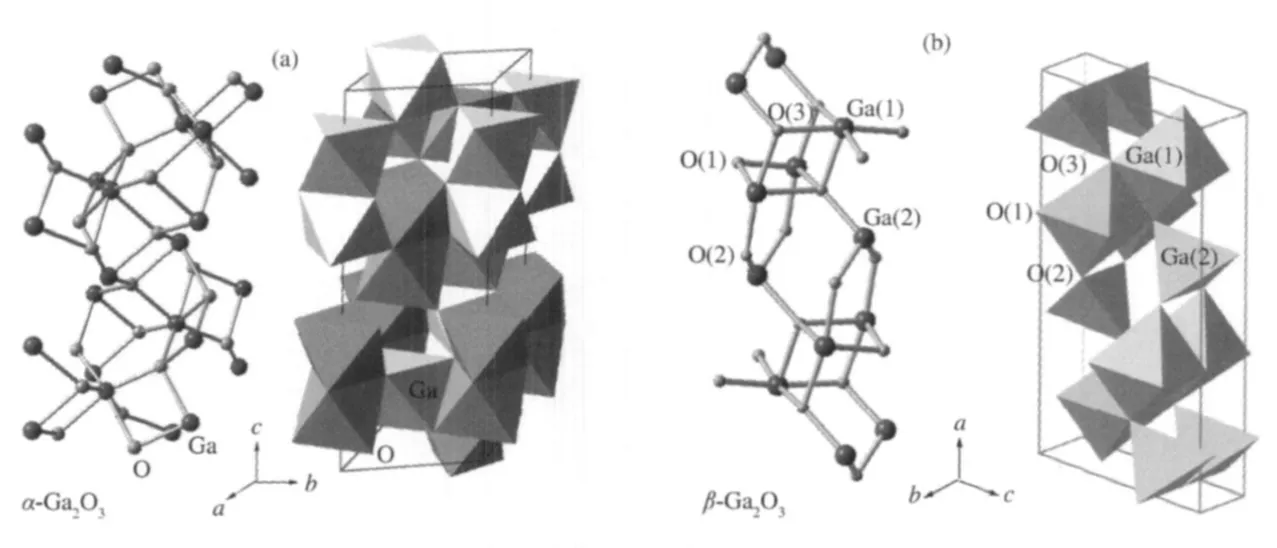
Fig.14 Three-dimensional crystal structures of α-Ga2O3and β-Ga2O3with a unit cell[33]
3 Mechanism for benzene degradation
All the above mentioned wide band gap p-block metal oxides/ hydroxides show high stability for benzene degradation,while TiO2deactivate very quickly.Their obvious different behavior implies that these p-block metal oxides/hydroxides and TiO2may have different routes in the photocatalytic degradation of benzene.
Generally,it is believed that the degradation of benzene over TiO2under dry air proceeds preferentially via a direct-hole-oxidation route.Such a direct-hole-oxidation process would pro-duce benzene cationic radical,which react further with an incoming benzene molecule,leading to the polymerization of benzene on the catalyst surface and the deactivation of TiO2during the degradation of benzene[35].The deposition of the stable intermediates can be confirmed by the color change of TiO2from the original white to dark brown after the photocatalytic reaction.In addition to this,the FT-IR spectrum of used TiO2indicates the formation of the stable intermediates by showing three new peaks at 1483,1686,and 1711 cm-1(Fig.16).On the contrary,no color change has been observed after photocatalytic benzene degradation for the p-block metal oxides/hydroxides, like InOOH.Besides this,no new peaks appear on the FT-IR spectrum over these p-block metal oxides/hydroxides,indicating that no stable intermediates have been deposited on the surface of these photocatalysts(Fig.16).All these phenomena imply that the degradation of benzene over these p-block metal oxides/hydroxides may proceed preferentially via the HO·radical route.Photocatalysts proceed via the HO·radical degradation route may have long term stability since the HO·radical route could significantly suppress the polymerization of benzene on the catalyst surface.
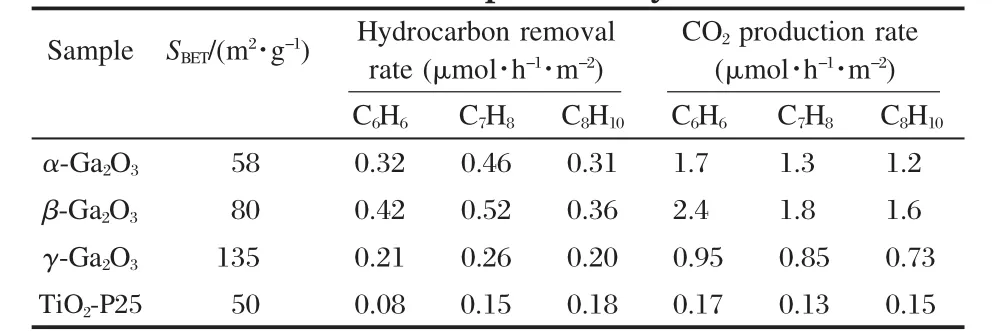
Table 1 Hydrocarbon removal rate and CO2production rate over different photocatalysts[33]
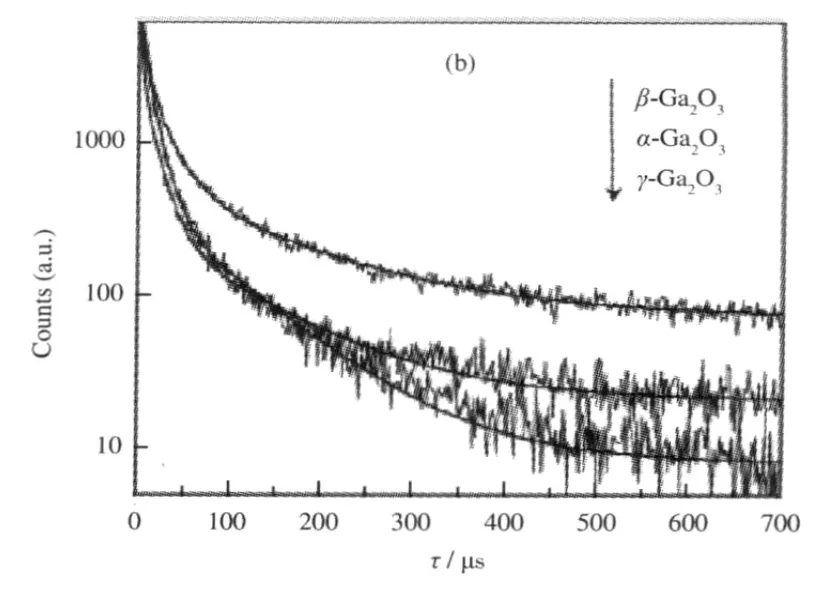
Fig.15 Photoluminescence decay curves of the gallium oxide catalysts[33]
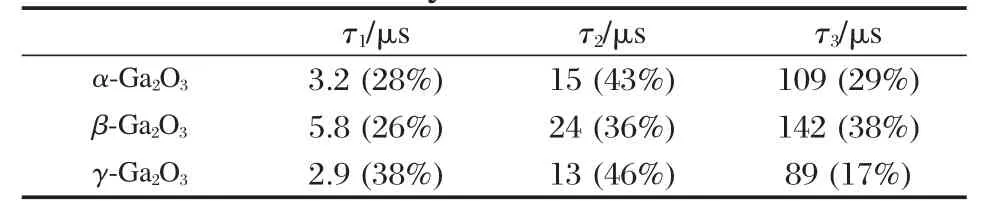
Table 2 Lifetimes(τ)and relative amplitudes of timeresolved photoluminescence of the gallium oxide catalysts at 77 K[33]
The generation of HO·radicals over these p-block metal oxides/hydroxides is credible since they all have proper band structure.The calculations of their band position based on the following equation:ECB=X-Ec-0.5Egis-0.4 V(vs NHE)[36]reveal that the edge of their conduction band are all negative than that of E°(O2/O-·20)(-0.33 V vs NHE),while the edge of their valence band(EVB)are positive than that of E°(HO·/OH-)(2.38 V vs NHE)[37].These suggest that the photogenerated electrons on these p-block metal oxides/hydroxides can reduce O2to give O-·20, while the photogenerated holes can oxidize OH-to give HO· when illuminated.The ESR spin-trap with DMPO technique confirmed the production of both O-·20and HO·over all these pblock metal oxides/hydroxides.In addition to this,it is observed that the intensities of the signal corresponding to the DMPOHO·radical produced over the p-block metal oxides/hydroxides (for example,InOOH)are much stronger than those over P25 (Fig.17).This indicates that under similar condition,more HO· radicals can be produced over irradiated p-block metal oxides/ hydroxides than over P25[26].This again confirms the above assumption that the degradation of benzene over p-block metal oxides/hydroxides may proceeds preferentially via the HO·radical route,while that over P25 may proceed via the direct-hole oxidation route.
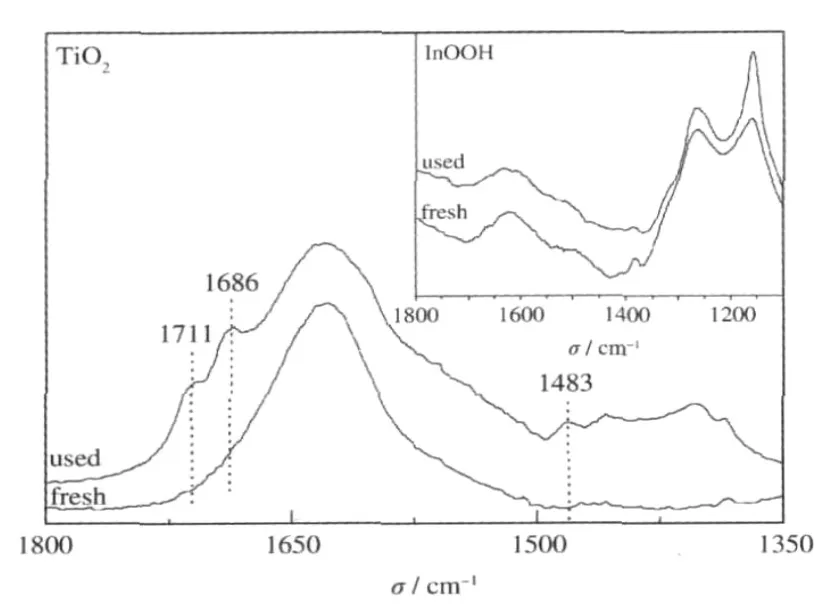
Fig.16 FT-IR spectra of used and fresh P25 and InOOH(inset)[26]
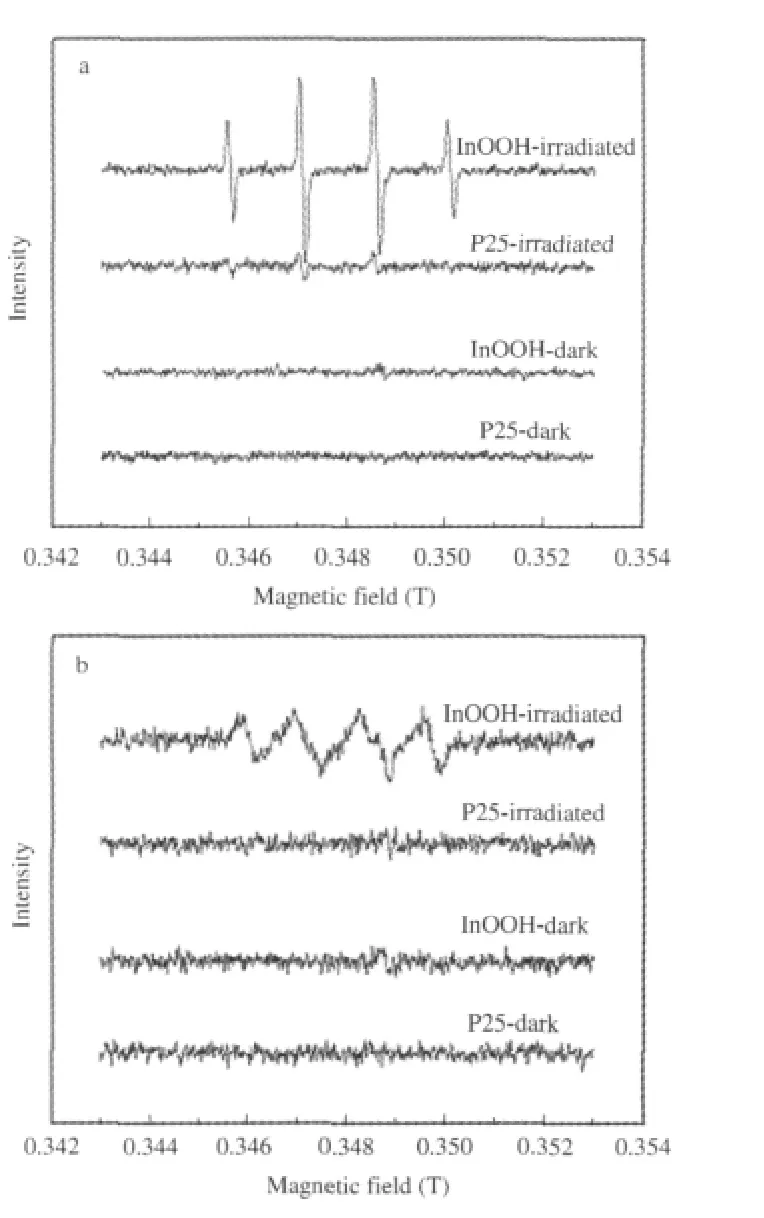
Fig.17 DMPO spin-trapping ESR spectra(a)in aqueous dispersion for DMPO-·OH and(b)in methanol dispersion for DMPO-O-·20[26]
The degradation preferentially via the HO·radical route over these p-block metal oxides/hydroxides can be attributed to their peculiar structure.The intrinsic wide band gap of these p-block metal oxides/hydroxides endow the photogenerated holes with strong oxidation ability and make them thermodynamically more favorable to react with chemi-adsorbed H2O or the surface hydroxyl group to produce HO·radicals.On the other hand,the highly dispersive conduction band due to the hybridizations of the orbitals usually observed over these p-block metal oxides/ hydroxides can promote the mobility of the photoexcited electrons,leading to enhanced charge separation.All these characteristics are favorable for the generation of the HO·radicals over these p-block metal oxides/hydroxides when illuminated. Therefore the degradation of benzene over these p-block metal oxides/hydroxides can proceed preferentially via HO·radical route and hence a high stability is observed.
The possible benzene degradation mechanism over these wide band gap p-block metal oxides/hydroxides is illustrated in Scheme 1.When illuminated,these p-block metal oxides/hydroxides can be efficiently excited to create electron-hole pairs. The photogenerated electrons and holes are long-lived enough to react with adsorbed H2O or surface hydroxyl group to produce HO·radicals.Since the water content in the feed gas is maintained at such a low level(<5 μL·L-1),H2O involved in the genera tion of the HO·radicals must come from the photocatalytic reaction itself.The photocatalytic degradation of benzene over these p-block metal oxides/hydroxides can proceed preferentially via the HO·radical.In this way,no polymerized intermedistes can be deposited and these p-block metal oxides/ hydroxides can maintain a clean surface and a higher stability in the photodegradation of benzene.
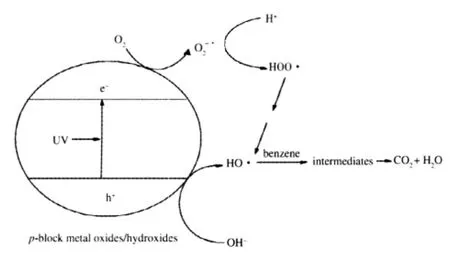
Scheme 1 Possible mechanism of the photocatalytic degradation of benzene over wide band gap p-block metal oxides/hydroxides
4 Conclusions and outlook
Wide band gap p-block metal oxides/hydroxides can be a promising new generation of photocatalysts for benzene degradation.Their superior photocatalytic performance for benzene degradation may be attributed to their peculiar structures and their mechanism different from TiO2in the degradation of benzene.A limitation for the application of these wide band gap pblock metal oxides/hydroxides in the practical environmental remediation is their wide band gap since they can only adsorb the UV energy,which account for only ca 4%of the solar energy. Since the ultimate goal of photocatalysis is to use the solar light, the application of the photocatalysis of these wide band gap semiconductors in the visible light region is important.The extension of the adsorption of these wide band gap semiconductors to the visible light region is still going on in our laboratory.
1 Hudak,A.;Ungvary,G.Toxicology,1978,11:55
2 Caprino,L.;Togna,G.I.Environ.Health Perspect.,1998,106: 115
3 Bird,M.G.;Greim,H.;Snyder,R.;Rice,J.M.Chem.-Biol. Interact.,2005,153:1
4 Lan,Q.;Zhang,L.;Li,G.;Vermeulen,R.V.;Weinberg,R.S.; Dosemeci,M.;Rappaport,S.M.;Shen,M.;Alter,B.P.;Wu,Y.; Kopp,W.;Waidyanatha,S.;Rabkin,C.;Guo,W.;Chanock,S.; Hayes,R.B.;Linet,M.;Kim,S.;Yin,S.;Rothman,N.;Smith,M. T.Science,2004,306:1774
5 Mills,A.;Davies,R.H.;Worsley,D.Chem.Soc.Rev.,1993,22: 417
6 Hoffman,M.R.;Martin,S.T.;Choi,W.;Bahnemann,D.W. Chem.Rev.,1995,95:69
7 Fujishima,A.;Rao,T.N.;Tryk,D.A.J.Photochem.Photobiol.C, 2000,1:1
8 Linsebigler,L.;Lu,G.;Yates Jr.,J.T.Chem.Rev.,1995,95:735
9 Fujishima,A.;Hashimoto,K.;Watanabe,T.Photocatalysis fundamentals and applications.1st ed.Tokyo:BKC,1999
10 Kaneko,M.;Okura,I.Photocatalysis,science and technology, Berlin:Springer,2002
11 Serpone,N.;Pelizzetti,E.Photocatalysis:fundamentals and applications.New York:Wiley,1989
12 Ollis,D.F.;Al-Ekabi,H.Photocatalytic purification and treatment of water and air.Amsterdam:Elsevier,1993
13 Fox,M.A.;Dulay,M.T.Chem.Rev.,1993,93:341
14 Mendez-Roman,R.;Cardona-Martinez,N.Catal.Today,1998, 40:353
15 Martra,G.;Coluccia,S.;Marchese,L.;Augugliaro,V.;Loddo,V.; Palmisano,L.;Schiavello,M.Catal.Today,1999,53:695
16 Fu,X.Z.;Zeltner,W.A.;Anderson,M.C.Appl.Catal.B: Environ.,1995,6:209
17 Einaga,H.;Futamura,S.;Ibusuki,T.Environ.Sci.Technol.,2001, 35:1880
18 Einaga,H.;Futamura,S.;Ibusuki,T.Environ.Sci.Technol.,2004, 38:285
19 Sitkiewitz,S.;Heller,A.New J.Chem.,1996,20:233
20 Einaga,H.;Futamura,S.;Ibusuki,T.Phys.Chem.Chem.Phys., 1999,1:4903
21 Zhang,W.;Wang,X.X.;Fu,X.Z.Chem.Commun.,2003:2196
22 Chen,Y.L.;Li,D.Z.;Wang,X.C.;Wang,X.X.;Fu,X.Z.Chem. Commun.,2004:2304
23 Chen,Y.L.;Li,D.Z.;Wang,X.C.;Wang,X.X.;Fu,X.Z.New J. Chem.,2005,29:1514
24 Hou,Y.D.;Wang,X.C.;Wu,L.;Ding,Z.X.;Fu,X.Z.Environ. Sci.Technol.,2006,40:5799
25 Yan,T.J.;Long,J.L.;Chen,Y.S.;Wang,X.X.;Li,D.Z.;Fu,X. Z.C.R.Chim.,2008,11:101
26 Li,Z.H.;Xie,Z.P.;Zhang,Y.F.;Wu,L.;Wang,X.X.;Fu,X.Z. J.Phys.Chem.C,2007,111:18348
27 Xue,H.;Li,Z.H.;Wu,L.;Ding,Z.X;Wang,X.X.;Fu,X.Z. J.Phys.Chem.C,2008,112:5850
28 Chen,X.;Xue,H.;Li,Z.H.;Wu,L.;Wang,X.X.;Fu,X.Z. J.Phys.Chem.C,2008,112:20393
29 Huang,J.H.;Wang,X.C.;Hou,Y.D.;Chen,X.F.;Wu,L.;Fu,X. Z.Environ.Sci.Technol.,2008,42:7387
30 Sato,J.;Saito,N.;Nishiyama,H.;Inoue,Y.J.Photochem. Photobio.A:Chem.,2002,148:85
31 Lin,X.P.;Huang,F.Q.;Wang,W.D.;Wang Y.M.;Xia,Y.J.; Shi,J.L.Appl.Catal.A:Gen.,2006,313:218
32 Zhang,X.;Huang,J.;Ding,K.;Hou,Y.;Wang,X.;Fu,X.Environ. Sci.Technol.,2009,43:5947
33 Hou,Y.;Wu,L.;Wang,X.;Ding,Z.;Li,Z.;Fu,X.J.Catal., 2007,250:12
34 Sato,J.;Kobayashi,H.;Inoue,Y.J.Phys.Chem.B,2003,107: 7970
35 d′Hennezel,O.;Pichat,P.;Ollis,D.F.J.Photochem.Photobiol.A: Chem,1998,118:197
36 Butler,M.A.;Ginley,D.S.J.Electrochem.Soc.,1978,125:228
37 Bard,A.J.;Parsons,R.;Jordan,J.Standard potentials in aqueous solution.New York:Marcel Dekker,1985

