Proton-Exchange Sulfonated Poly (ether ether ketone) (SPEEK)/SiOx-S Composite Membranes in Direct Methanol Fuel Cells*
GAO Qijun (高啟君), WANG Yuxin (王宇新), XU Li (許莉), WEI Guoqiang (衛(wèi)國(guó)強(qiáng)) and WANG Zhitao (王志濤)
?
Proton-Exchange Sulfonated Poly (ether ether ketone) (SPEEK)/SiO-S Composite Membranes in Direct Methanol Fuel Cells*
GAO Qijun (高啟君)1,2, WANG Yuxin (王宇新)1,2, XU Li (許莉)1,**, WEI Guoqiang (衛(wèi)國(guó)強(qiáng))1,2and WANG Zhitao (王志濤)1,2
1School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China2State Key Laboratory of Chemical Engineering, Tianjin 300072, China
sulfonated poly(ether ether ketone), functionalized silica, composite membrane, direct methanol fuel cell
1 INTRODUCTION
Development and research on direct methanol fuel cells (DMFCs) have been an area of active interest since the 1990s [1]. DMFC technology has made significant progress over the years, but two obstacles still need to be surmounted before DMFC commercialization [2, 3]. First, the anode catalyst is inactive and unstable enough causing a high overpotential loss of the anode. Second, severe methanol crossover of the commercially available perfluorosulfonic acid (PFSA) proton-exchange membranes (PEM) (.., Nafionò) from anode to cathode reduces fuel efficiency and increases the mixed electrode potential of the cathode, resulting in low cell performance [2, 3]. Production of the commercially available PFSA polymer membranes is costly and time-consuming. Therefore, there is an urgent need to develop PEM with improved properties, including high proton conductivity, low methanol permeability, and low cost.
Much effort has been made in recent years to develop an alternative fluorine-free polymer membranes [4-8] and to modify PFSA polymer membranes [9]. It is widely recognized that sulfonated poly (ether ether ketone) (SPEEK) polymers are very promising materials for membranes in DMFCs [4]. SPEEK polymers can, in theory, have higher ion-exchange capacity (IEC) than PFSA polymers, which may compensate for the demerit of weaker acidity of their own sulfonic groups (SO3H). SPEEK membranes also exhibit lower methanol crossover and are less costly to produce than PFSA membranes [5]. The demand for high proton conductivity calls for SPEEK membranes to have a high degree of sulfonation (DS) and to function at high temperatures. However, highly sulfonated SPEEK membranes tend to swell excessively or even dissolve at high temperatures. There have been several attempts to overcome the excessive swelling while maintaining high proton conductivity, for example, by synthesizing SPEEK with various hydrophobic block: hydrophilic block ratios [10], by introducing cross-links between some of the sulfonic groups in the SPEEK membrane [11], and by blending the SPEEK polymer with non-conductive engineering thermoplastics (.., SPEEK/PEI, SPEEK/PES, SPEEK/PBI) [12-14].
Addition of inorganic particles (.., ZrO2) into the SPEEK matrix is also an important approach in PEM research [15]. This approach has two objectives: one is to improve the mechanical properties of the composite membranes and the other is to physically counteract methanol crossover [16, 17]. It has also been suggested that the size of the particles (nano or micro), surface properties (acid or basic), and the functionalization determine whether the filler, besides acting as a reinforcing components as above mentioned, can impart a significant improvement in proton conductivity [16-22].
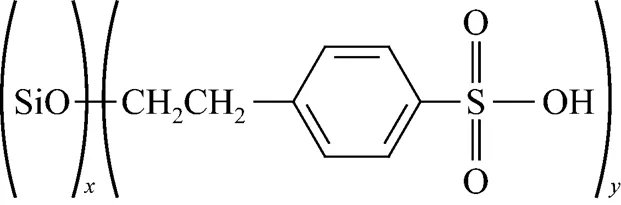
Figure 1 Molecular structure of the functionalized silica with sulfonic acid groups (SiO-S)
2 EXPERIMENTAL
2.1 Materials

2.2 Preparation of SiOx-S powder
The SiO-S gel can be prepared from SiO-Cl hydrolyzed for 6 h at 80°C as described in a previous study [17]. The gel was washed repeatedly with deionized water until pH of the rinse water was 7 and then dried at 70°C for 24 h to remove water. The resultant SiO-S was ground to fine powder by a QM-ISP04 ball mill and finally stored in an airtight bottle before being used.
The back-titration of sulfonic groups within the SiO-S powder was used to find whether the SiO-Cl hydrolyzed into the SiO-S completely.
2.3 Synthesis of SPEEK
PEEK (10g) was added gradually to 100 ml of concentrated sulfuric acid in a three-necked flask with vigorous stirring at 60°C. At a prescribed time point, the acid polymer solution was added to a large excessive ice-cold water with continuous agitation. The SPEEK precipitate was rinsed repeatedly with deionized water until the water reached pH 7. Then the SPEEK was dried at room temperature for 2 days followed by drying at 60°C for 24 h under vacuum. The IEC and DS of the sulfonated polymers were determined by a classical back-titration method as described in a previous study[23].
2.4 Membrane preparation
The membranes were prepared by solution casting. When the mass content of SiO-S powder is higher than 20%, the composite membrane becomes brittle in dry state at room temperature. Therefore, SPEEK/SiO-S (3%–20%) composite membranes (the degree of sulfonation for the SPEEK polymer is 55.1%, the mass content of SiO-S powder in the composite membranes is from 3% to 20%) were studied in this article.
SPEEK and SPES-C in prescribed amount were dissolved separately in DMF (10%, by mass) and then the two solutions were mixed and stirred for 6 h. The mixed solution was cast onto a glass plate and dried overnight at 60°C in a vacuum oven, followed by annealing at 100°C for 4 h. After cooling to room temperature, the membrane was peeled from the glass plate with deionized water.
SiO-S powder in prescribed amount was uniformly dispersed in DMF solvent with mechanical stirring. The desired amount of SPEEK was then added to the solvent to make a 10% (by mass) solution. After stirring for 6 h and degassing, the solution was cast onto a glass plate and dried overnight at 60°C in a vacuum oven, followed by annealing at 100°C for 4 h. After cooling to room temperature, the membrane was peeled from the glass plate with deionized water. Finally, the membrane was treated with 1 mol·L-1sulfuric acid at room temperature for 24 h and subsequently rinsed with deionized water several times to remove acid completely. Membranes were kept in deionized water before testing. The thickness of the dried membranes was 80-95 μm.
2.5 Membrane characterization
Fourier transform infrared spectroscopy (FT-IR) were measured in absorbance mode by using an FT-IR spectrometer (Bio-RAD FTS 6000) in the range of wave numbers 600-4000 cm-1to compare position of IR bands and to check the presence of functional groups and their interaction in composite membranes. Prior to FT-IR measurement, the samples were dried at 80°C for 24 h.
Thermogravimetric analysis (TGA) was used to estimate the thermal stability of the composite membranes. We used a TGA thermogravimetric analyzer (TA-50 Instrument Shimadzu TGA) at a heating rate of 10 K·min-1in nitrogen gas in the temperature range 30-800°C. All specimens were dried overnight at 90°C under vacuum before measurements.
The morphology of the cross-section of samples was examined with an environment-scanning electron microscope (PHILIPS XL30 ESEM). The samples werecryo-fractured in liquid nitrogen to obtain fresh cross-sections, which were coated with gold before measurements.
The swelling degree (SD) of the specimens was obtained by measuring the area difference between the dry and the wet states as described in Ref. [23]. The membranes were cut into 3 cm×4 cm rectangles and dried overnight at 90°C under vacuum before measuring the area (d). The dried membranes were immersed for 48 h in 1 mol·L-1methanol to reach equilibrium at the desired temperature. The wet membranes were wiped dry with tissue paper and the area was measured again (w). SD was calculated (in area percent) as follows:
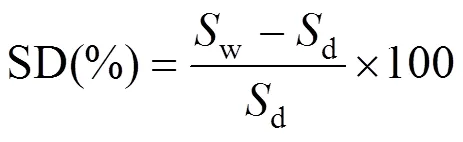
wheredandware the areas of dry and corresponding wet membrane sheets, respectively. Three sheets of each membrane composition were measured by the above method, and the average was calculated.
The proton conductivity of samples in the lateral direction was measured with a measurement cell and a frequency response analyzer (FRA) (Autolab PG-STAT20). Two stainless steel electrodes connected to the FRA were pressed against the membrane to be tested. The measurement temperature was controlled from room temperature to 160°C. The conductivity,, was calculated from the impedance data, using the relation/(×), whereandare the distance between the electrodes and the cross-section area of the membrane, respectively, andwas derived from the low intersection of the high-frequency semi circle on a complex impedance plane with the() axis. For membranes that dissolved below 160°C, their dissolution was tested by measuring the weight before and after the proton conductivity measurement. Besides, the proton conductivity of SiO-S powder was obtained as described in a previous study [23]. The powder needs to be pressed into a slice before testing.
3 RESULTS AND DISCUSSION
3.1 Preparation of the SiOx-S
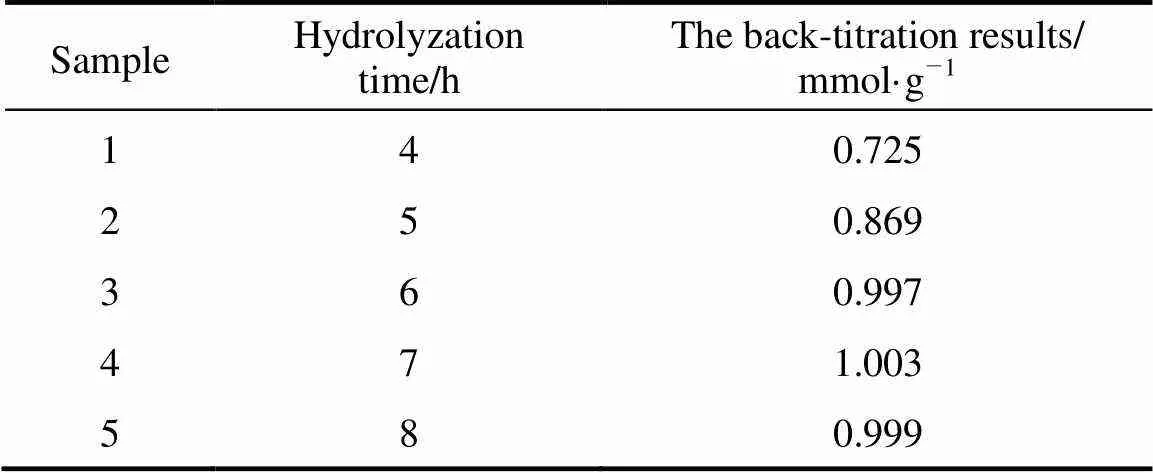
Table 1 The back-titration results of sulfonic groups within the SiOx-S hydrolyzed at different time
3.2 Sulfonation
3.3 FT-IR spectra
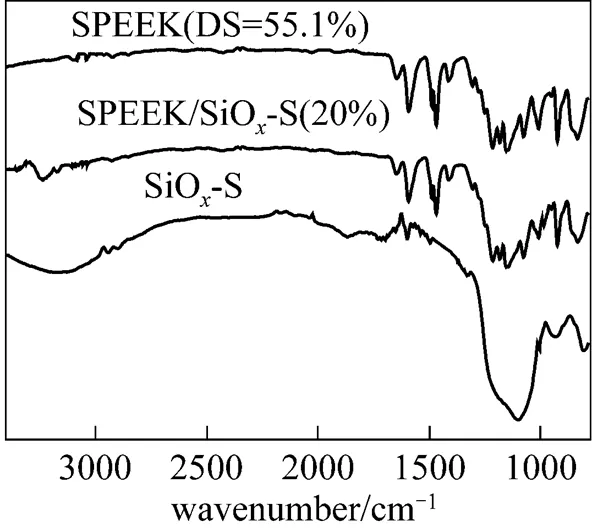
3.4 Thermal stability
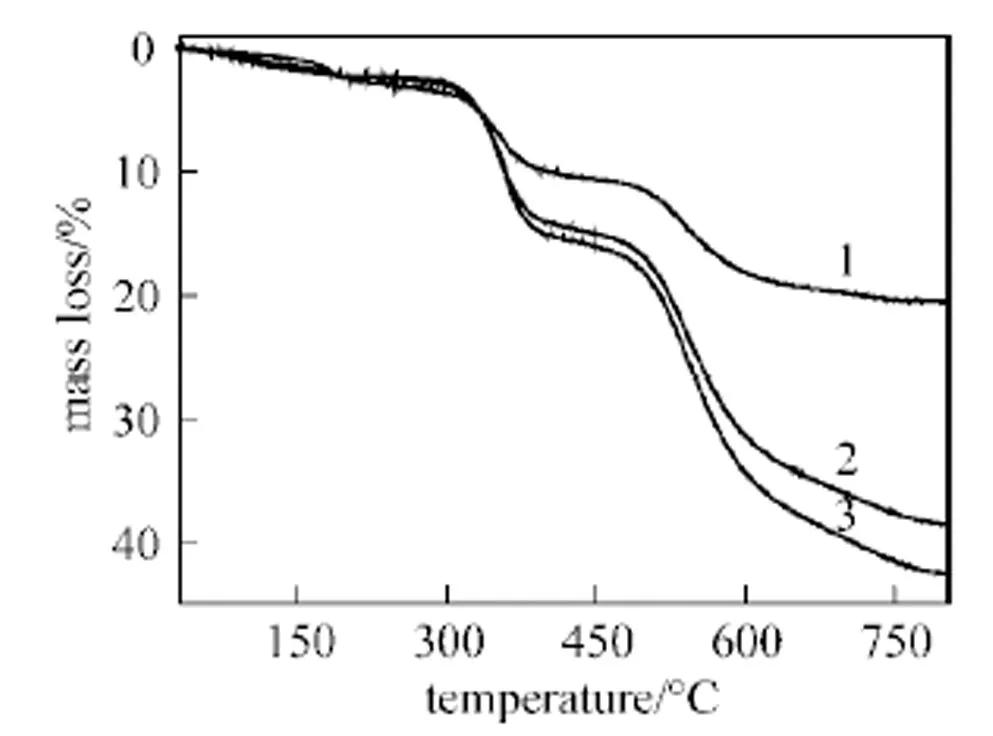
Figure 3 TGA curves of SiOx-S powder, SPEEK/SiOx-S (18%) composite membrane and pure SPEEK membrane
1—SiOx-S; 2—SPEEK/SiOx-S (18%); 3—SPEEK (DS = 55.1%)
3.5 Morphology
The basic homogeneous distribution of SiO-S powder within the SPEEK matrix and no sign of evident aggregation can be observed from the SEM images of the SPEEK/SiO-S composite membranes at magnification of up to 10000× (Fig. 4) although the SiO-S content reaches 20%. The nominal powder size is less than 500 nm as indicated by the SEM images.

Figure 4 Cross-section images of SPEEK/SiO-S (5%) composite membrane and SPEEK/SiO-S (20%) composite membrane
3.6 Swelling behavior
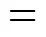
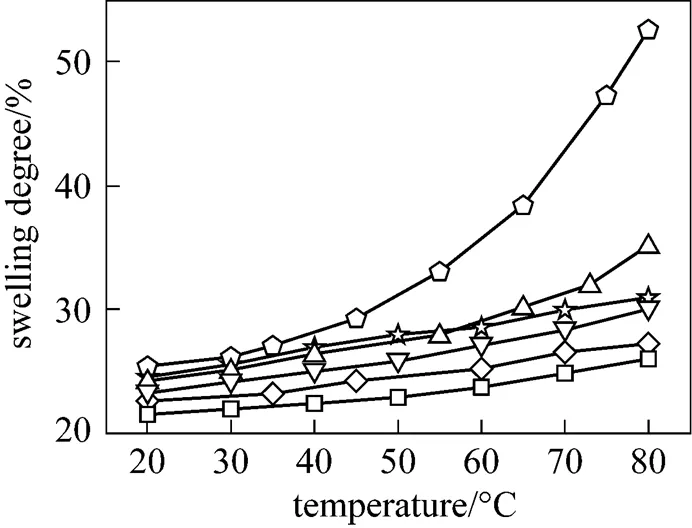
Figure 5 Swelling degree of Nafionò115, SPEEK membrane and SPEEK/SiO-S composite membranes in 1 mol·L-1methanol solution at different temperatures

Figure 6 Arrehenius plots of methanol permeability for Nafionò115, pure SPEEK membrane, and SPEEK/SiO-S composite membranes
Figure 7 Arrehenius plots of proton conductivity at 100%RH for Nafionò115, SiO-S powder, pure SPEEK membrane and SPEEK/SiO-S (15%, 20%) composite membranes under 100% RH
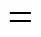
3.7 Methanol permeability
Methanol fuel is fed in the form of liquid below 80°C, thus the methanol crossover is more serious than in the form of gas at higher temperatures [2]. We analyze the methanol permeability of the membranes below 80°C here. Fig. 6 shows the methanol permeability as a function of temperature for Nafionò115, pure SPEEK and SPEEK/SiO-S composite membranes. The methanol permeability of Nafionò115 at room temperature is 1.02×10-6cm2·s-1, which is very close to the value reported by Tricoli. [24, 26, 27], while that of SPEEK and SPEEK/ SiO-S membranes are approximately an order of magnitude lower than Nafionò115.
The difference in methanol permeability between Nafionò115 and the SPEEK membrane can be explained qualitatively by the differences in their microstructures and the acidity of their sulfonic acid functional groups [4]. Nafionò115 macromolecules consist of very hydrophobic perfluorinated backbones and very hydrophilic side chains with sulfonic acid functional groups. The very different components lead to relatively large microphase separation in Nafionò115, which results in the low resistance to methanol permeation. The situation for SPEEK polymer is rather different. The carbon-hydrogen main chains with ether links, phenyl rings, and carbonyl groups in SPEEK make it less hydrophobic and more rigid compared with Nafionò115. Furthermore, the acidity of sulfonic acid functional groups of the SPEEK polymer is weaker than that of Nafionò115. Therefore, the microphase separation in the SPEEK membrane is not obvious and the hydrophilic ion channels are narrower, which results in low methanol permeability. This can also be proved by the results of schematic representation of the microstructure of Nafionò115 and SPEEK reported by Kreuer[4].
As can be seen in Fig. 6, the methanol permeability of the SPEEK/SiO-S composite membranes is even lower and decreases with the increased SiO-S content in the membrane. The addition of SiO-S inhibits effectively the swelling of SPEEK matrix (Fig. 5) and thus imposes higher resistance to methanol crossover. The higher resistance to methanol crossover of the SPEEK/SiO-S composite membranes is beneficial to improve open circuit voltage (OCV) of DMFCs [2].
3.8 Proton conductivity
The relation between the conductivity and the reciprocal of the temperature for all the SPEEK and SPEEK/SiO-S membranes, before they dissolve, can be described by the Arrhenius equation and exhibits straight lines in a semilogarithmic graph (Fig. 7). The apparent activation energy of proton transfer, which is equal to the slope of the corresponding lines of different membranes, is obtained from the Arrhenius plot. It is noteworthy that the apparent activation energy of the SPEEK membrane has approximately 38 kJ×mol-1, in contrast to 9 kJ×mol-1for Nafionò115. The very high apparent activation energy of the SPEEK membrane is believed to be a consequence of its low swelling degree and slow variation with temperature (as shown in Fig. 7). Narrow ion channels and rich branches with dead-end “pockets” in the membrane will contribute to the high barrier to proton transfer [4].
SPEEK and SiO-S materials are weakly acidic compared with Nafionò115. The dissociation of sulfonic acid functional groups in these materials increases at high temperatures [5, 23], whereas that of Nafionò115 approaches 100% at room temperature. Therefore, the elevation of temperature increases both the proton mobility and the proton content in the SPEEK and SPEEK/SiO-S membranes, which results in a much faster increase of proton conductivity with temperature.
The proton conductivity of SiO-S powder is 0.018 S×cm-1at room temperature and 100% RH, and reaches 0.086 S·cm-1at 120°C. The proton conductivity of composite membranes increases slightly as mass content of SiO-S powder increases, although SiO-S powder has lower proton conductivity than the pure SPEEK membrane. The increase in conductivity upon addition of SiO-S can be rationalized based on previous studies [16-22, 28]. This phenomenon has been observed by Kim. [29] in composite PEMs based on heteropolyacid in sulfonated polysulfones. The presence of the additive was found to enhance the proton conductivity, while at the same time decrease the water uptake. In this case, it is not completely understood whether the filler participates actively in the proton conduction by enhancing proton dissociation or by providing favorable pathways for the proton along polymer-particle interfaces [16-18, 22]. The conductivity results obtained in this study are very similar to that of previous studies above, and it is likely that a similar mechanism is in place. The lower apparent activation energy of proton transfer through the composite membranes than through the pure SPEEK membrane also shows that the similar mechanism exists. The apparent activation energy of the SPEEK/SiO-S (20%) composite membrane is 33.09 kJ×mol-1. The conductivity of SPEEK/SiO-S (20%) exceeds slightly that of Nafionò115 at 145°C and 100% RH and approaches 0.17S×cm-1.
3.9 Working temperature
Figure 8 shows*as a function of the mass content of SiO-S in the composite membranes. SPEEK membranes of 55.1% DS is useful only below 90°C. The*of the composite membranes is, however, markedly increased as the mass content of SiO-S powder increases. As discussed in Section 3.6, the addition of SiO-S to SPEEK matrix inhibits effectively the swelling and dissolution of the membranes below 80°C. The membranes’ swelling at temperature above 100°C was quickly measured by measuring the area changes after their conductivity was tested at the temperature. It can be found that the content of SiO-S increased from 10% to 20%, the swelling degree of composite membranes decreased from 67.8% to 30.4% at 110°C and the content of SiO-S increased from 15% to 20%, the swelling degree of composite membranes decreased from 72.3% to 35.8% at 130°C. The increase of*with the increased content of SiO-S in the composite membrane should be attributed to the depressed swelling and dissolution of the composite membranes at high temperatures.
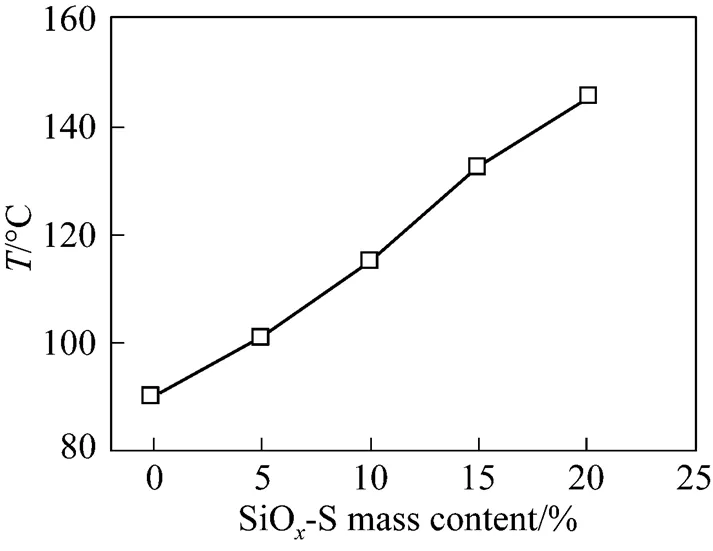
Figure 8*of the SPEEK/SiO-S composite membranes with different SiO-S mass contents
The extension of the working temperature of the membrane is beneficial for DMFC in many aspects. Working at higher temperature, the membrane exhibits higher proton conductivity, which leads to lower ohmic losses in DMFC. The electrocatalysts are also more active and more tolerant to carbon monoxide poisoning [30] at high temperatures. When methanol fuel is fed in the form of gas at high temperature, the methanol crossover and its negative effects can be reduced markedly. Operating at high temperature also simplifies the management of water and heat in the DMFC systems [30].
4 CONCLUSIONS
The results of this study indicate a strong potential of these composite membranes for use in DMFCs. SPEEK/SiO-S composite membranes with high levels of dimensional stability were prepared. The methanol permeability of the membranes was shown to be about one order of magnitude lower than that of Nafionò115, and to decrease with the increase of SiO-S content. The addition of the SiO-S into the membranes not only increases slightly the proton conductivity of composite membranes but also effectively inhibits their swelling, which enables them to be used at higher temperature, thus presenting higher proton conductivity. The results of this study indicate a strong potential of these composite membranes for use in DMFCs.
1 Kuver, A., Kamloth, K.P., “Comparative study of methanol crossover acrosselectropolymerized and commercial proton exchange membrane electrolytes for the acid direct methanol fuel cell”,., 43 (16), 2527-2535 (1998).
2 Ren, X., Zawadzinski, T.A., Uribe, F., Dai, H., “Methanol cross-over in direct methanol fuel cells”,..., 95 (23), 284-289 (1995).
3 Heinzel, A., Barragan, V.M., “A review of the state-of-the-art of the methanol crossover in direct methanol fuel cells”,., 84, 70-74 (1999).
4 Kreuer, K.D., “On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells”,..., 185, 29-39 (2001).
5 Yang, B., Manthiram, A., “Sulfonated poly(ether ether ketone) membranes for direct methanol fuel cells”,.., 6, A229-A231 (2003).
6 Wycisk, R., Lee, J.K., Pintauro, P.N., “Sulfonated polyphosphazene- polybenzimidazole membranes for DMFCs”,..., 152, A892-A898 (2005).
7 Dai, H., Guan, R., Li, C.H., Liu, J.H., “Development and characterization of sulfonated poly(ether sulfone) for proton exchange membrane materials”,., 178, 339-345 (2007).
8 Shahi, V.K., “Highly charged proton-exchange membrane: Sulfonated poly(ether sulfone)-silica polyelectrolyte composite membranes for fuel cells”,., 177, 3395-3404 (2007).
9 Park, K.T., Jung, U.H., Choi, D.W., Chun, K., Lee, H.M., Kim, S.H., “ZrO2-SiO2/Nafion (R) composite membrane for polymer electrolyte membrane fuel cells operation at high temperature and low humidity”,., 177, 247-253 (2008).
10 Zhao, C., Li, X., Na, H., “Synthesis of sulfonated poly(ether ether ketone) (S-PEEKs) material for proton exchange membrane”,..., 280, 643-650 (2006).
11 Mikhailenko, S.U.D., Wang, K.P., Kaliaguine, S., Xing, P.X., Robertson, G.P., Guiver, M.D., “Proton conducting membranes based on cross-linked sulfonated poly(ether ether ketone) (SPEEK)”,..., 233, 93-99 (2004).
12 Mikhailenko, S.D., Zaidi, S.M.J., Kaliaguine, S., “Electrical properties of sulfonated polyether ether ketone/polyetherimide blend membranes doped with inorganic acids”,...,:.., 33, 1386-1395 (2000).
13 Manea, C., Mulder, M., “Characterization of polymer blends of polyether sulfone/sulfonated polysulfone and polyether sulfone/sulfonated polyetherether ketone for direct methanol fuel cell applications”,..., 206, 443-453 (2002).
14 Zhang, H.Q., Li, X.F., Zhao, C.J., Fu, T.Z., Shi, Y.H., Na, H., “Composite membranes based on highly sulfonated PEEK and PBI: Morphology characteristics and performance”,..., 308, 67-74 (2008).
15 Silva, V.S., Ruffmann, B., Silva, H., Gallego, Y.A., Mends, A., Madeira, L.M., Nunes, S.P., “Proton electrolyte membrane properties and direct methanol fuel cell performance/I. Characterization of hybrid sulfonated poly (ether ether ketone)/zirconium oxide membranes”,., 140, 34-40 (2005).
16 Uchida, H., Ueno,Y., Hagihara, H., Watanabe, M., “Self-humidifying electrolyte membranes for fuel cells—Preparation of highly dispersed TiO2particles in Nafion 112”,.., 150, A57-A62 (2003).
17 Martinelli, A., Matic, A., Jacobsson, P., borjesson, L., Navarra, M.A., Fernicola, A., Panero, S., Scrosati, B., “Structural analysis of PVA-based proton conducting membranes”,., 177, 2431-2435 (2006).
18 Kim, D.S., Park, H.B., Rhim, J.W., Lee, Y.M., “Preparation and characterization of crosslinked PVA/SiO2hybrid membranes containing sulfonic acid groups for direct methanol fuel cell applications ”,..., 240, 37-48 (2004).
19 Croce, F., Persi, L., Scrosati, B., Serraino-Fiory, F., Plichta, E., Hendrickson, M.A., “Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes”,., 46, 2457-2461 (2001).
20 Shao, Z.G.., Joghee, P., Hsing, I.M., “Preparation and characterization of hybrid Nafion-silica membrane doped with phosphotungstic acid for high temperature operation of proton exchange membrane fuel cells”,.., 229, 43-51 (2004).
21 Xu, W.L., Liu, C.P., Xue, X.Z., Su, Y., Lv, Y.Z., Xing, W., Lu, T.H., “New proton exchange membranes based on poly (vinyl alcohol) for DMFCs ”,., 171, 121-127 (2004).
22 Sambandam, S., Ramani, V., “SPEEK/functionalized silica composite membranes for polymer electrolyte fuel cells”,., 170, 259-267 (2007).
23 Li, L., Wang, Y.X., “Sulfonated polyethersulfone Cardo membranes for direct methanol fuel cell”,..., 246, 167-172 (2005).
24 Li, L., Wang, Y.X., “A hybrid membrane of poly(vinyl alcohol) and phosphotungstic acid for fuel cells”,...., 10 (5), 614-617 (2002).
25 Zaidi, S.M.J., Mikhailenko, S.D., Robertson, G.P., Guiver, M.D., Kaliaguine, S., “Proton conducting composite membranes from polyetherether ketone and heteropolyacids for fuel cell applications”,..., 173, 17-34 (2000).
26 Tricoli, V., Carretta, N., Bartolozzi, M., “A comparative investgation of proton and methanol transport in fluorinated ionomeric membranes”,..., 147, 1286-1290 (2000).
27 Huang, M.Y., Wang, Y.X., Cai, Y.Q., Xu, L., “Sulfonated poly(ether ether ketone)/zirconium tricarboxybutylphosphonate composite proton-exchange membranes for direct methanol fuel cells”,, 4, 337-342 (2007).
28 Gasa, J.V., Boob, S., Weiss, R.A., Shaw, M.T., “Proton-exchange membranes composed of slightly sulfonated poly(ether ketone ketone) and highly sulfonated crosslinked polystyrene particles”,..., 269, 177-186 (2006).
29 Kim, Y.S., Wang, F., Hickner, M., Zawodzinski, T.A., McGrath, J.E., “Fabrication and characterization of heteropolyacid (H3PW12O40)/ directly polymerized sulfonated poly(arylene ether sulfone) copolymer composite membranes for higher temperature fuel cell applications”,..., 212, 263-282 (2003).
30 Li, Q.F., Huang, R.H., “Approaches and recent development of polymer electrolyte membranes for fuel cells operating above 100°C”,.., 15, 4896-4915 (2003).
2008-07-24,
2008-11-20.
the State Key Development Program for Basic Research of China (2008CB617502), the National Natural Science Foundation of China (20606025), and Program for Changjiang Scholars and Innovative Research Team in University of China (IRT0641).
** To whom correspondence should be addressed. E-mail: xuli620@eyou.com
 Chinese Journal of Chemical Engineering2009年2期
Chinese Journal of Chemical Engineering2009年2期
- Chinese Journal of Chemical Engineering的其它文章
- Kinetics of Reactive Extraction of Nd from Nd2O3 with TBP-HNO3Complex in Supercritical Carbon Dioxide*
- Activity Coefficient Models to Describe Vapor-Liquid Equilibrium in Ternary Hydro-Alcoholic Solutions*
- Position Group Contribution Method for Predicting the Normal Boiling Point of Organic Compounds
- Experimental Study on the Initial Position Distribution of Taylor Bubbles in Cryogenic Upward Inclined Tubes*
- Enhancement of Proton Exchange Membrane Fuel Cell Performance Using a Novel Tapered Gas Channel*
- Tert-butylation of Toluene with Tert-butyl Alcohol over Realuminated H-mordenite Zeolite*
