Kinetics of Reactive Extraction of Nd from Nd2O3 with TBP-HNO3Complex in Supercritical Carbon Dioxide*
ZHU Liyang (朱禮洋), DUAN Wuhua (段五華), XU Jingming (徐景明) andZHU Yongjun(朱永)
?
Kinetics of Reactive Extraction of Nd from Nd2O3with TBP-HNO3Complex in Supercritical Carbon Dioxide*

Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing 102201, China
The process based on supercritical fluid extraction for reprocessing of the spent nuclear fuel has some remarkable advantages over the plutonium-uranium extraction (PUREX) process. Especially, it can minimize the generation of secondary waste. Dynamic reactive extraction of neodymium oxide (Nd2O3) in supercritical carbon dioxide (SC-CO2) containing tri--butyl phosphate-nitric acid (TBP-HNO3) complex was investigated. Temperature showed a positive effect on the extraction efficiency, while pressure showed a negative effect when the unsaturated TBP-HNO3complex was employed for the dynamic reactive extraction of Nd2O3in SC-CO2. Both temperature and pressure effects indicated that the kinetic process of the reactive extraction was controlled by the chemical reaction. A kinetic model was proposed to describe the extraction process.
supercritical carbon dioxide, kinetics, reactive extraction, TBP-HNO3complex, Nd2O3
1 INTRODUCTION
Reprocessing of the spent nuclear fuel is commercially carried out by the plutonium-uranium extraction (PUREX) process. In the PUREX process, the spent nuclear fuel is dissolved in nitric acid followed by co-extraction of uranium and plutonium from acid solution using the tri--butyl phosphate (TBP)/kerosene system. The fission products are left in the raffinate stream [1]. Therefore, considerable volume of the high level liquid waste (HLLW) is generated, leading to further treatment with high cost.
Recently, supercritical fluid extraction (SFE) of lanthanides and actinides has been extensively studied for its potential application in reprocessing of the spent nuclear fuel because it can simplify the process and reduce the secondary liquid waste [2-11]. TBP-HNO3complex has been found to have high solubility in supercritical carbon dioxide (SC-CO2). It is capable of dissolving directly lanthanide and actinide oxides. This process integrates chemical reactions with SFE, which is free of any aqueous solution to dissolve metal oxides prior to extraction. Therefore, this process is named reactive extraction.
There are extensive mathematical models proposed for SFE of natural products from the solid matrix, where the interactions between target compounds and the matrix are carefully considered [12-14]. Cui. [15] studied the kinetics of chelating extraction of heavy metal ions in SC-CO2. However, little is known about modeling supercritical fluid reactive extraction of metal oxides, especially lanthanide and actinide oxides, which is very important to explore the extraction mechanisms. In this study, Nd2O3as a typical lanthanide oxide in the spent nuclear fuel is selected to start our research on application of SFE in reprocessing of the spent nuclear fuel. The reactive extraction of Nd2O3is conducted using TBP-HNO3complex in SC-CO2. Effects of temperature and pressure are investigated. A kinetic model is proposed to describe the extraction process.
2 EXPERIMENTAL
2.1 Materials
Tri--butyl phosphate (AR, >98%) and HNO3(AR, 15.5 mol·L-1) were provided by Beijing Chemical Plant, China. NaOH (AR) was obtained from Tianjin Unionlab Chemical Reagent Ltd., China. Ethylene diamine tetraacetic acid disodium salt (EDTA, 99.96%) was purchased from National Research Center for Certified Reference Materials, China. Carbon dioxide (99.995%) was purchased from Beijing Bei Temperature Gas Factory, China. Nd2O3powder was obtained from Sinopharm Chemical Reagent Co., Ltd, China, and sieved before use with standard sieve of 0.025-0.031 mm.
TBP-HNO3complex was prepared by vigorously mixing TBP with equal volume of 15.5 mol·L-1HNO3, followed by centrifugation for 0.5 h. The concentration of HNO3in TBP-HNO3complex was determined by acid-base titration using 0.1 mol·L-1NaOH. The content of water was measured by a 787 KF Titrino instrument (Metrohm Ltd., Switzerland). The density of the complex was calculated by weighing a known volume of complex with Mettler AE 200 balance (Mettler Toledo, Switzerland). The concentration of TBP in TBP-HNO3complex could be calculated by combining the density of complex with the content of H2O and HNO3in the complex [16, 17]. The complex in this study had a formula of TBP(HNO3)1.63(H2O)0.53. Since TBP in the complex was saturated with HNO3and H2O, excess water droplets would form and separate from the supercritical fluid phase during the extraction process. If the metal ion is distributed into the water droplets, effective extraction is not achieved [18]. Thus, another kind of TBP-HNO3complex was prepared by diluting the TBP(HNO3)1.63(H2O)0.53complex with equal volume of anhydrate TBP and used in the experiments. Accordingly, its formula was determined to be TBP(HNO3)0.79(H2O)0.26, which could be called an unsaturated complex wherein the concentration of H+was 2.52 mol·L-1and the content of water was 1.48% (by mass).
2.2 Procedure
A schematic diagram of the apparatus (SFT-100, Supercritical Fluid Technologies, Inc. USA) for the SFE experiments is shown in Fig. 1. The main part of the apparatus consists of a 50-ml stainless steel reaction vessel, which is settled in a thermostatic air bath that can control the temperature within ±0.1 K. A plunger pump is used to flow CO2. It is coupled with a pressure control plane, which can display and control both the flow rate of CO2and the pressure of the system.
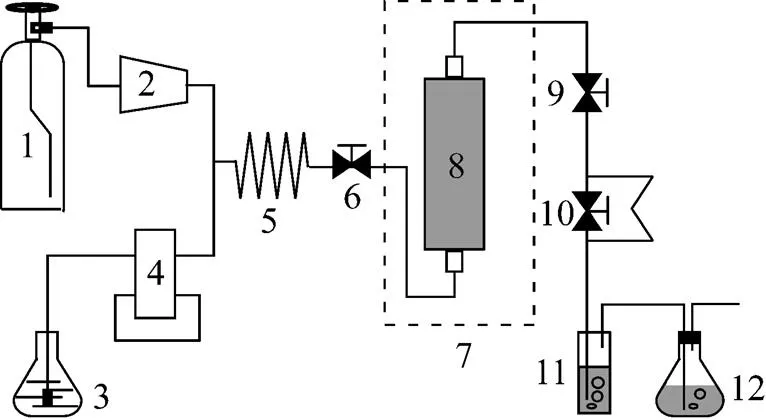
Figure 1 Schematic diagram of the apparatus for SFE
1—CO2cylinder; 2—plunger pump; 3—TBP-HNO3complex container; 4—syringe pump; 5—pre-heating coil; 6—inlet valve; 7—thermostatic air bath; 8—reaction vessel; 9—static/dynamic valve; 10—back pressure restrictor; 11—collection vessel; 12—tail gas filter
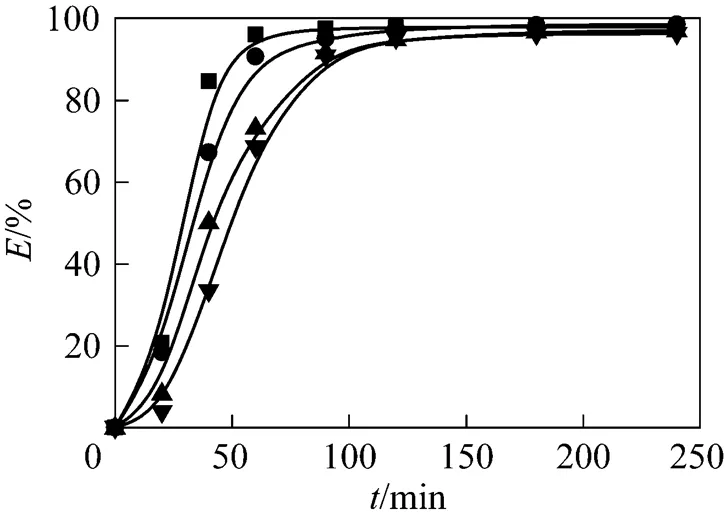
Figure 2 Effect of pressure on the extraction efficiency at 323.15 K
flow rate of CO2/ml·min-1: ■?2.51±0.28;●?2.56±0.23;▲?2.61±0.20;▼?2.58±0.28pressure/MPa: ■?15; ●?21; ▲?25; ▼?30
The thermostatic air bath was first heated to the desired temperature. The reaction vessel was sealed after 0.5 g Nd2O3powder was charged into it. The liquid CO2was subsequently purged into the system. The restrictor valve was closed for pressurizing CO2with the plunge pump. When the pressure of the system reached the desired pressure, the restrictor valve was opened carefully to let CO2flow through the system. The flow rate of the liquid CO2at the outlet of the plunger pump was about 2.50 ml·min-1. It was difficult to keep the pressure and the flow rate of CO2constant in the flow SFE process, so the pressure was kept constant throughout the experiments in the present work, while slight fluctuation of the flow rate of liquid CO2was observed, which was also reported by Tomioka [19]. Then, TBP-HNO3complex was supplied into the system by the syringe pump at a constant rate of 0.5 ml·min-1, and mixed with CO2flow at the mixing joint of the system. In the reaction vessel, TBP-HNO3complex in SC-CO2reacted with Nd2O3. As a result, the soluble Nd-TBP complex was formed in SC-CO2, carried out of the reaction vessel by SC-CO2. At last, the soluble Nd-TBP complex was retained in the collection vessel filled with the hydrogenating kerosene when SC-CO2was gasified under the atmospheric pressure by opening the back pressure restrictor. The collection vessel was replaced at a designed time interval for the study of dynamic extraction.
The quantity of Nd collected was determined by the complexometric titration method with the EDTA solution. The residual Nd2O3in the reaction vessel after being dissolved in 1 mol·L-1nitric acid was determined by ICP-AES (IRIS. Adr, Thermo Jarrell Ash, USA). Consequently the mass balance could be estimated.
The extraction efficiency () is defined as follows:

3 RESULTS AND DISCUSSION
3.1 Effect of pressure on the extraction efficiency
The effect of pressure on the reactive extraction of Nd2O3in SC-CO2was investigated by fixing the temperature at 323.15 K and varying pressure from 15 to 30 MPa. The extraction curve is shown in Fig. 2 by plotting the extraction efficiency () against the extraction time (). The extraction efficiency increased exponentially with the extraction time. The extraction process nearly finished after 180 min of dynamic extraction because Nd2O3in the reaction vessel was completely reacted and extracted by TBP-HNO3complex in SC-CO2under different pressure. The extraction efficiency could reach 96% for all the cases. Pressure usually has positive effect in the SFE processes, namely the extraction efficiency increases with pressure [20], because the higher the pressure is, the higher the solubility of the solute in SC-CO2is. In contrast, pressure showed a negative effect on the reactive extraction of Nd2O3, namely the extraction efficiency declined with pressure. A similar effect of pressure was also observed when U3O8was dissolved and extracted using SC-CO2containing TBP-HNO3complex [19]. It is possible that increase of pressure in those reactive extraction systems reduces the reactivity of TBP-HNO3complex.
3.2 Effect of temperature on the extraction efficiency
The effect of temperature on the extraction efficiency was examined by fixing pressure at 21 MPaand varying temperature in the range of 313.15-333.15 K. The extraction curve is shown in Fig. 3. The temperature shows a different effect on the extraction efficiency from the pressure, namely, the higher the temperature is, the higher the extraction efficiency is. The temperature effect may attribute to the influence of temperature on the chemical reaction rate of TBP-HNO3complex with Nd2O3. The reaction rate generally increases with temperature.
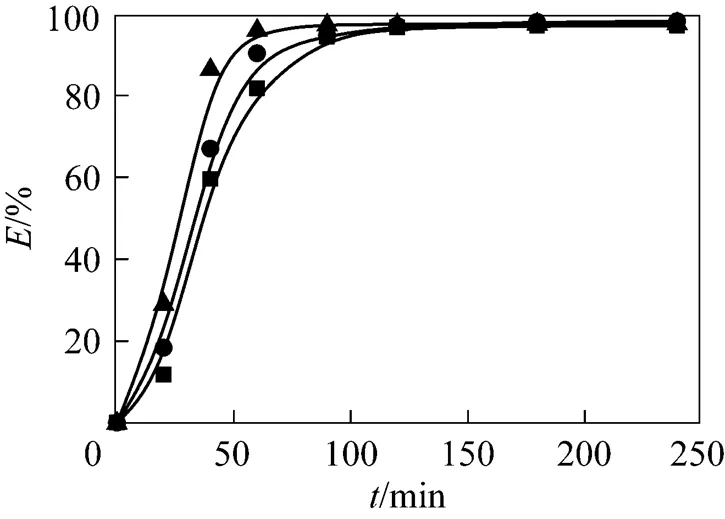
Figure 3 Effect of temperature on the extraction efficiency at 21 MPa
flow rate of CO2/ml·min-1:■?2.77±0.53;●?2.56±0.23; ▲?2.51±0.15temperature/K:■?313.15;●?323.15;▲?333.15
4 PROCESS KINETICS
Both effects of temperature and pressure suggest that the process kinetics is controlled by chemical reaction before 120 min. The kinetics process is composed of the following steps (see Fig. 4).
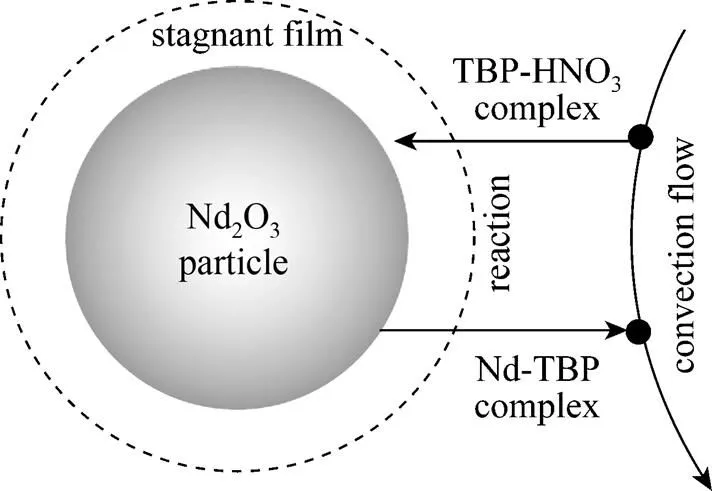
Figure 4 Schematic diagram of the kinetic process
(1) A stagnant film is formed by TBP-HNO3complex near Nd2O3particle surface [21, 22].
(2) TBP-HNO3complex diffuses to the stagnant film from SC-CO2phase in a convective-diffusion way. Subsequently, TBP-HNO3complex penetrates the stagnant film, followed by reaching the particle surface.
(3) TBP-HNO3complex reacts with Nd2O3at the surface of the Nd2O3particle, forming the Nd-TBP complex. It was reported that 1︰4 complex was formed when neodymium nitrate complexes with TBP in SC-CO2[23]. Therefore the reaction can be described as follows:


A united reaction equation is obtained for simplification:
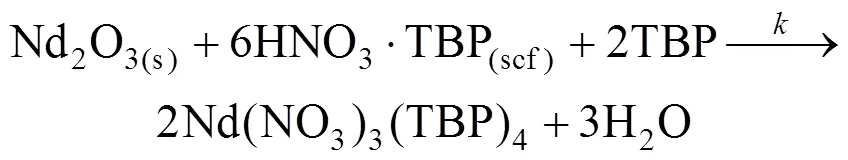
(4) The Nd(NO3)3(TBP)4product diffuses out of the stagnant film to SC-CO2phase, and is transported out of the reaction vessel by convective flow.
With a small quantity of the Nd2O3sample loaded in the reaction vessel, some assumptions can be made: 1) The Nd2O3particle exhibits a spherical shape. 2) SC-CO2is a well-mixed flow and well distributed in every cross-section of the reaction vessel. 3) Temperature and pressure are uniformly distributed in the reaction vessel. Thereby, the reaction and mass transport should be carried out under isothermal conditions. 4) Both the stagnant film resistance and the convective mass transfer resistance may be neglected. Taken these assumptions together with the reaction taking place at particle surface, the conservation equation can be written as follows [15]:
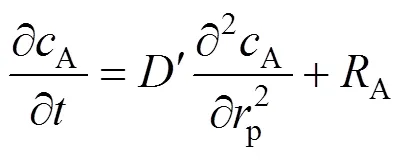
In addition, the diffusivity of SC-CO2is excellent, so that the radial distribution of concentration on particle surface may be neglected, provided that the size of the Nd2O3particle is small. The first term on the right hand side of Eq. (4) is zero. Thus Eq. (4) is further reduced to:
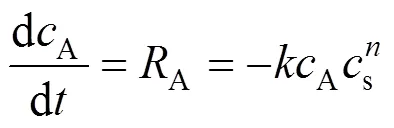

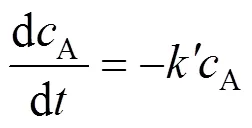
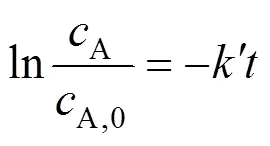
The extraction efficiencycan be expressed as (8):
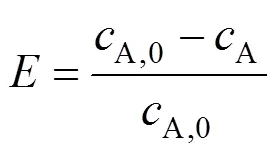
so Eq. (7) is reduced to:
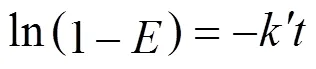
The experimental results under various temperature and pressure are fitted using Eq. (9). The results are listed in Table 1. The linear correlation coefficients (value) are all above 96%. The probabilities of completely nonlinear (value) are less than 0.05 when the confidence level is 95% for all the case.
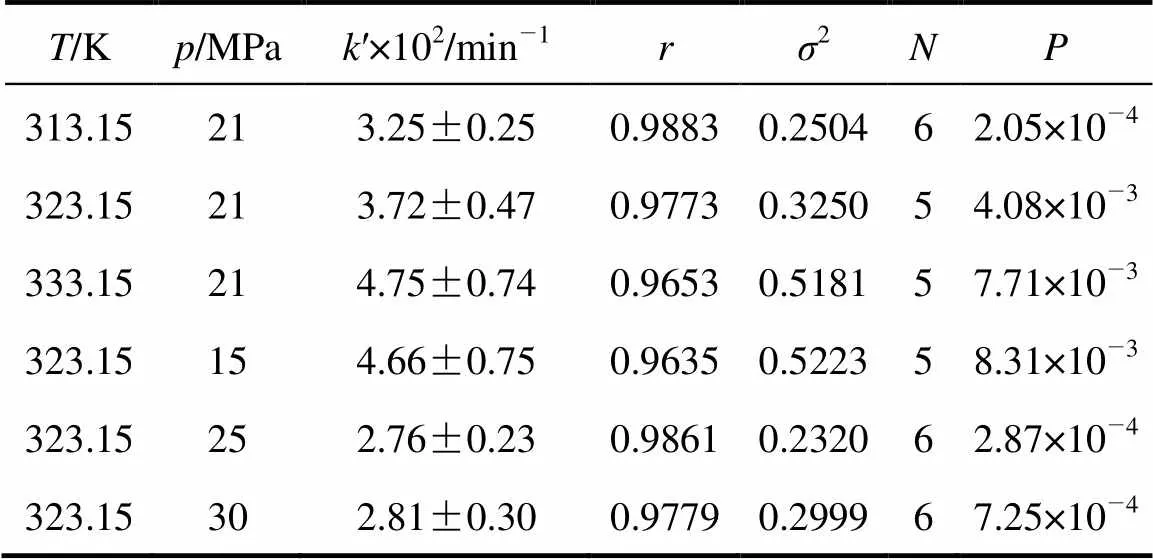
Table 1 Exponential fitting result for the dynamic reactive extraction of Nd2O3 into SC-CO2
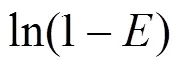
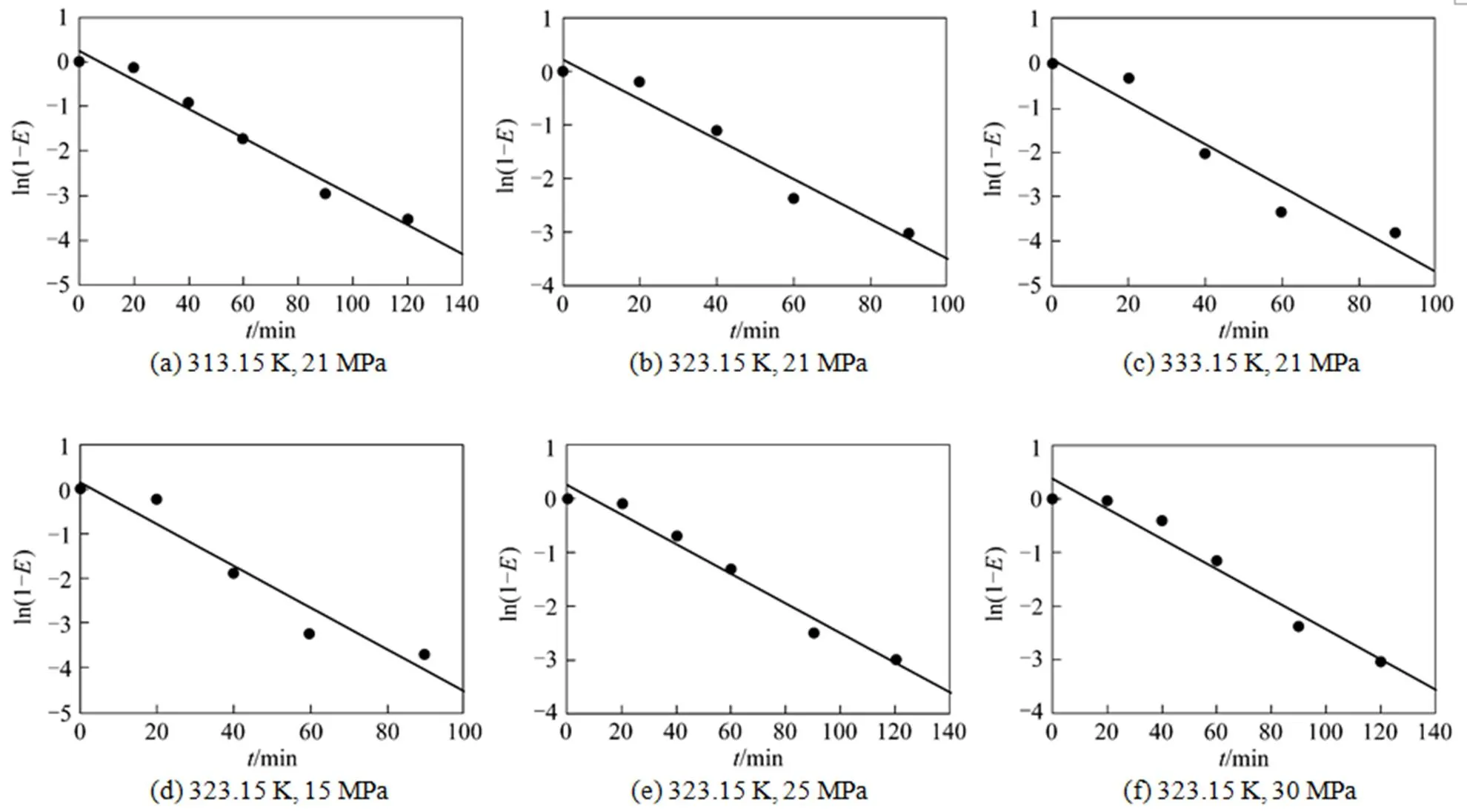
Figure 5 Exponential fitting for the reactive extraction
The effect of temperature on the reaction can be represented by the Arrhenius equation:
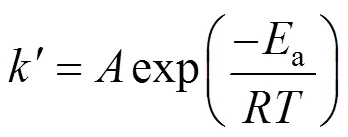
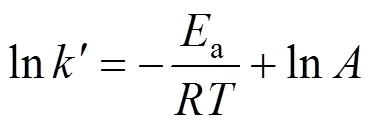
The apparent activation energyais calculated to be (16.47±3.02) kJ·mol-1by plotting ln′ against 1/as shown in Fig. 6.
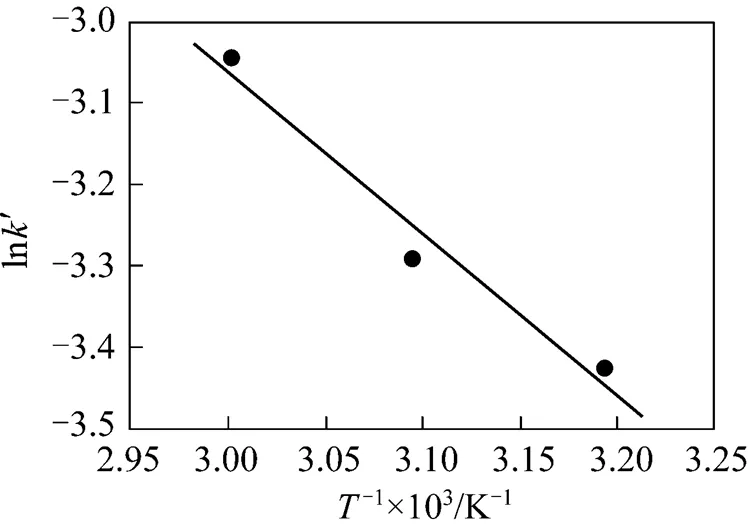
Figure 6 Arrhenius plots for the reactive extraction of Nd2O3at 21 Mpa
5 CONCLUSIONS
Quantitative extraction of Nd2O3by SC-CO2containing TBP-HNO3complex was achieved. The extraction efficiency increased with temperature in the range of 313.15-333.15 K and decreased with pressure in the range of 15-30 MPa. The reactive extraction process was controlled by chemical reaction, and the kinetic process could be well described by a simple mathematic model. The reaction could be represented by a pseudo-first-order reaction, and the apparent reaction rate constants of the reaction of TBP-HNO3complex with Nd2O3under various pressures and temperatures were obtained. The apparent activation energy of the present system was calculated to be (16.47±3.02) kJ·mol-1based on the experimental data in the range of 313.15-333.15 K at 21 MPa.
NOMENCLATURE
pre-exponential factor, min-1
ANd2O3remained in the reaction vessel, mol
A,0Nd2O3loaded in the reaction vessel, mol
collectedNd2O3collected in the collection vessel, mol
sconcentration of TBP-HNO3complex, mol·L-1
′ effective diffusion coefficient, m2·min-1
extraction efficiency, %
aapparent activation energy, kJ·mol-1
′ apparent reaction rate constant, min-1
number of experimental data points
probability (thatis zero)
pressure, MPa
Aformation reaction rate, mol·min-1
linear correlation coefficient
pparticle size, m
temperature, K
time, min
2standard deviation
1 Jiang, S.J., Ren, F.Y., Reprocessing Engineering of Nuclear Fuel, Nuclear Energy Press, Beijing, China, 8-19 (1995). (in Chinese)
2 Laintz, K.E., Wai, C.M., Yonker, C.R., Smith, R.D., “Extraction of metal ions from liquid and solid materials by supercritical carbon dioxide”,.., 64 (22), 2875-2878 (1992).
3 Lin, Y.H., Brauer, R.D., Laintz, K.E., Wai, C.M., “Supercritical fluid extraction of lanthanides and actinides from solid materials with a fluorinated β-diketone”,.., 65 (18), 2549-2551 (1993).
4 Tomioka, O., Enokida, Y., Yamamoto, I., “Solvent extraction of lanthanides from their oxides with TBP in supercritical carbon dioxide”,...., 35 (7), 515-516 (1998).
5 Tomioka, O., Enokida, Y., Yamamoto, I., “Selective recovery of neodymium from oxides by direct extraction method with supercritical CO2containing TBP-HNO3complex”,..., 37 (5), 1153-1162 (2002).
6 Trofimov, T.I., Samsonov, M.D., Kulyako, Y.M., Myasoedov, B.F., “ Dissolution and extraction of actinide oxides in supercritical carbon dioxide containing the complex of tri--butylphosphate with nitric acid”,.., 7 (12), 1209-1213 (2004).
7 Shimada, T., Ogumo, S., Sawada, K., Enokida, Y., Yamamoto, I., “Selective extraction of uranium from a mixture of metal or metal oxides by a tri--butylphosphate complex with HNO3and H2O in supercritical CO2”,.., 22 (11), 1387-1391 (2006).
8 Duan, W.H., Jing, S., Zhu, Y.J., Chen, J., “Research progress on supercritical fluid chelating extraction of lanthanides and actinides”,..., 41 (4), 429-437 (2007). (in Chinese)
9 Wai, C.M., “Supercritical fluid extraction of radionuclides: A green technology for nuclear waste management”, In: A.C.S. symposium series 943, American Chemical Society, Washington, DC, 161-170 (2006).
10 Wai, C.M., “Reprocessing spent nuclear fuel with supercritical carbon dioxide”, In: A.C.S. symposium series 933, American Chemical Society, Washington, DC, 57-70 (2006).
11 Enokida, Y., Sawada, K., Shimada, T., Yamamoto, I., “An option making for nuclear fuel reprocessing by using supercritical carbon dioxide”, In: Proceedings of Global 2007 Conference on Advanced Nuclear Fuel Cycles and Systems, Boise, Idaho, Sept. 9-13, 1029-1032 (2007).
12 Yin, J.Z., Liu, Y., “Kinetics analysis of supercritical fluid extraction for natural products”,...., 22 (2), 216-222 (2008). (in Chinese)
13 Ruetsch, L., Daghero, J., Mattea, M., “Supercritical extraction of solid matrices model formulation and experiments”,...., 33 (2), 103-107 (2003).
14 Vargas, R.M.F., Cassel, E., Gomes, G.M.F., Longhi, L.G.S., Atti-Serafini, L., Atti-Santos, A.C., “Supercritical extraction of carqueja essential oil: Experiments and modeling”,...., 23 (3), 375-382 (2006).
15 Cui, H.Y., Wang, T., Guan, Y.F., Shen, Z.Y., “Kinetics of chelating extraction of heavy metals by supercritical CO2”,...., 52 (9), 829-833 (2001). (in Chinese)
16 Enokida, Y., Tomioka, O., Lee, S.C., Rustenholtz, A., Wai, C.M., “Characterization of a tri--butyl phosphate-nitric acid complex: A CO2-soluble extractant for dissolution of uranium dioxide”,...., 42 (21), 5037-5041 (2003).
17 Duan, W.H., Zhu, L.Y., Jing, S., Zhu, Y.J., Chen, J., “Study on properties of TBP-HNO3complex used for direct dissolution of lanthanide and actinide oxides in supercritical fluid CO2”,..., 25 (3), 319-322 (2007).
18 Shimizu, R., Sawada, K., Enokida, Y., Yamamoto, I., “Supercritical fluid extraction of rare earth elements from luminescent material in waste fluorescent lamps”,.., 33 (3), 235-241 (2005).
19 Tomioka, O., Meguro, Y., Enokida. Y., Yamamoto, I., Yoshida, Z., “Dissolution behavior of uranium oxides with supercritical CO2using HNO3-TBP complex as a reactant”,...., 38 (12), 1097-1102 (2001).
20 Kumoro, A.C., Hasan, M., “Supercritical carbon dioxide extraction of andrographolide from andrographis paniculata: Effect of the solvent flow rate, pressure, and temperature”,...., 15 (6), 877-883 (2007).
21 Liao, C.H., Huang, Z.R., Gu, G.L., “Mass transfer model for supercritical CO2extraction of solid raw material”,., 21 (7), 502-506 (2004). (in Chinese)
22 Goto, M., Roy, B.C., Kodama, A., Hirose, T., “Modeling supercritical fluid extraction process involving solute-solid interaction”,...., 31 (2), 171-177 (1998).
23 Fox, R.V., Ball, R.D., Harrington, P.D.B., Rollins, H.W., Jolley, J.J., Wai, C.M., “Praseodymium nitrate and neodymium nitrate complexation with organophosphorus reagents in supercritical carbon dioxide solvent”,.., 31 (3), 273-286 (2004).
2008-08-26,
2009-01-19.
the National Natural Science Foundation of China (20506014).
** To whom correspondence should be addressed. E-mail: dwh203@mail.tsinghua.edu.cn
——以五華提線木偶傳習(xí)所為例
 Chinese Journal of Chemical Engineering2009年2期
Chinese Journal of Chemical Engineering2009年2期
- Chinese Journal of Chemical Engineering的其它文章
- Activity Coefficient Models to Describe Vapor-Liquid Equilibrium in Ternary Hydro-Alcoholic Solutions*
- Position Group Contribution Method for Predicting the Normal Boiling Point of Organic Compounds
- Experimental Study on the Initial Position Distribution of Taylor Bubbles in Cryogenic Upward Inclined Tubes*
- Enhancement of Proton Exchange Membrane Fuel Cell Performance Using a Novel Tapered Gas Channel*
- Tert-butylation of Toluene with Tert-butyl Alcohol over Realuminated H-mordenite Zeolite*
- A Fully Flexible Potential Model for Carbon Dioxide*
