Structure,electronic,and nonlinear optical properties of superalkaline M3O(M =Li,Na)doped cyclo[18]carbon
Xiao-Dong Liu(劉曉東), Qi-Liang Lu(盧其亮),?, and Qi-Quan Luo(羅其全)
1School of Physics and Material Science,Anhui University,Hefei 230601,China
2Institute of Physical Science and Information Technology,Anhui University,Hefei 230601,China
3Department of Chemical Physics,University of Science and Technology of China,Hefei 230026,China
Keywords: superalkaline doped cyclo[18]carbon,structure and electronic properties,nonlinear optical properties,density functional theory(DFT)
1.Introduction
Carbon has long been a popular research topic.In 1985,Krotoet al.obtained sp-hybridized fullerene C60clusters[1]with a coordination number of three by laser vaporization.This result opened a new door for the study of lowdimensional materials.Since then, carbon nanotubes[2]and graphene[3]have been synthesized successively,and the family of molecular carbon allotropes has continued to expand.In 1966,Hoffman proposed the ring-like C18cluster.[4]Since the first detection of the C18ring in 1989,[5]small carbon clusters have attracted extensive attention from experimental and theoretical studies.[5–8]One of the most impressive advances in this field in recent years is the successful synthesis and characterization of a ring consisting of 18 carbon atoms, named cyclo[18]carbon,on the surface of Cu(111)by Kaiseret al.[9]Recently, Andersonet al.[10]proposed an efficient synthesis of C18from bromocyclic carbon (C18Br6).This discovery has attracted the extensive attention of scholars who wish to study cyclic[n]carbon, and its analogs and derivatives due to its potential use in practical applications.[11–36]Many studies have shown that the C–C bond of the most stable structure of C18cannot be simply described as alternating single and triple bonds but can only be described as alternating long and short bonds.[9,14,16,19]
The diameter of cyclo[18]carbon is about 7.40 ?A,[17]which is equivalent to the size of C60(7.1 ?A).Cyclo[18]carbon provides sufficient space to encapsulate a range of other atoms or small molecules, thus forming a similar endohedral fullerene species.[37–40]This endohedral complex is an interesting structure from the perspective of research and application.Compared with many studies on the structure and properties of cyclo[18]carbon, work on the structure and properties of doped C18[41–49]and other carbon rings[50–53]is limited.Alkali metal atoms have the lowest ionization energy(5.39 eV–3.89 eV) in the periodic table.[54]However, some polyatomic molecules or clusters, which are called “superalkali” by Gutsev and Boldyrev,[55]have lower ionization energies than cesium atoms (3.89 eV).[54]Studies have also shown the formation of an electron donor–acceptor endohedral complex with superalkali inside C60[56]and Si12C12[57]nanocage.Their nonlinear optical properties are significantly improved.Liuet al.[29]systematically studied the electronic structure, electronic spectra, and optical nonlinearity of cyclo[18]carbon.They also investigated the properties of the Li@C18complex and its potential application in an optical molecular switch.[43]It has also been shown that C18molecules have strong electron acceptor properties.[33]Therefore, stable complexes can form between C18and electron donor species, which implies a strong capability of charge transfer within complexes and excellent optical properties.Therefore,investigating the structure and properties of superalkali-doped C18is worthwhile.In this work, we report systematic theoretical studies on the structure and properties of superalkalineM3O(M=Li,Na)-doped cyclo[18]carbons.
2.Computational methods
An extensive structural search for C18M3O (M= Li,Na) was conducted based on the two low-energy isomers of cyclo[18]carbon; namely, the alternating bond length structure and the transition state structure with equal bond length.[9,14,16,19]Superalkaline moleculesM3O (M=Li, Na)were placed on the inner/outer edges of the ring and in different regions above the ring.Geometry optimization was performed at the M06-2X/def2-TZVP level for these different initial structures by using the Gaussian 09 software package.[58]Single-point energy calculations were performed at the CCSD(T)/def2-TZVP level to confirm the lowest energy structure.Vibrational frequency analyses were conducted on optimized structures to determine whether they are at the minimum or saddle point on the potential energy surface.Zero-point energy and the counterpoise procedure for basis set superposition error (BSSE) were utilized to calculate energies.On the basis of the results of Gaussian 09,Mayer bond order[59–61]and localized orbital locator (LOL)analysis[62–64]were performed using Multiwfn 3.8.[65]Electrostatic potential (ESP) analysis was performed using Visual Molecular Dynamics (VMD) software[66]in combination with Multiwfn.Meanwhile,ωB97XD is reliable and robust for studying photophysical properties and optical nonlinearity of C18molecule and its analogs.[29,34,43,46]Therefore,ωB97XD is considered to be capable of describing the optical properties of the C18M3O system.Electron excitations were studied by using the time-dependent density functional theory (TD-DFT) method at theωB97XD/def2-TZVP level.Charge-transfer spectra(CTS)were plotted based on the results of TD-DFT.[58]The (hyper)polarizability of C18M3O complex was calculated by coupled-perturbed Kohn–Sham(CPKS) method at theωB97XD/aug-cc-pVTZ level.[46,67–70]Polarizability,the first hyperpolarizability,and the second hyperpolarizability were analyzed by Multiwfn and VMD.For the parameters of describing the nonlinear optical properties,we follow the formulas for calculating the response properties to electric field provided in Refs.[34,43].
3.Results and discussion
The most stable structures of C18M3O (M=Li, Na) are shown in Fig.1.Compared with the isolated cyclo[18]carbon,the C18ring in C18Na3O has higher structural deformation than that in C18Li3O.TheM3O moiety is not entrapped in the cyclo[18]carbon and is located some distance above the carbon ring.For C18Li3O, the distance between O atom and the center of C18is about 0.99 ?A.The projection of the oxygen atom on the C18plane is almost located in the geometric center of the optimized C18ring.However,for C18Na3O,the projection of the oxygen atom deviates far from the point.The four atoms of the Li3O moiety are no longer coplanar and three Li atoms are close to the C18ring.This finding may be attributed to the attraction between Li atoms and C18ring resulting from charge transfer,as discussed later on.The Li3plane is almost parallel to the C18plane.For C18Na3O,the Na3plane is at an angle to the C18plane.The three shortest distances of Li–C are in the range from 2.24 ?A to 2.27 ?A.The values of Na–C are in the range from 2.62 ?A to 2.68 ?A.
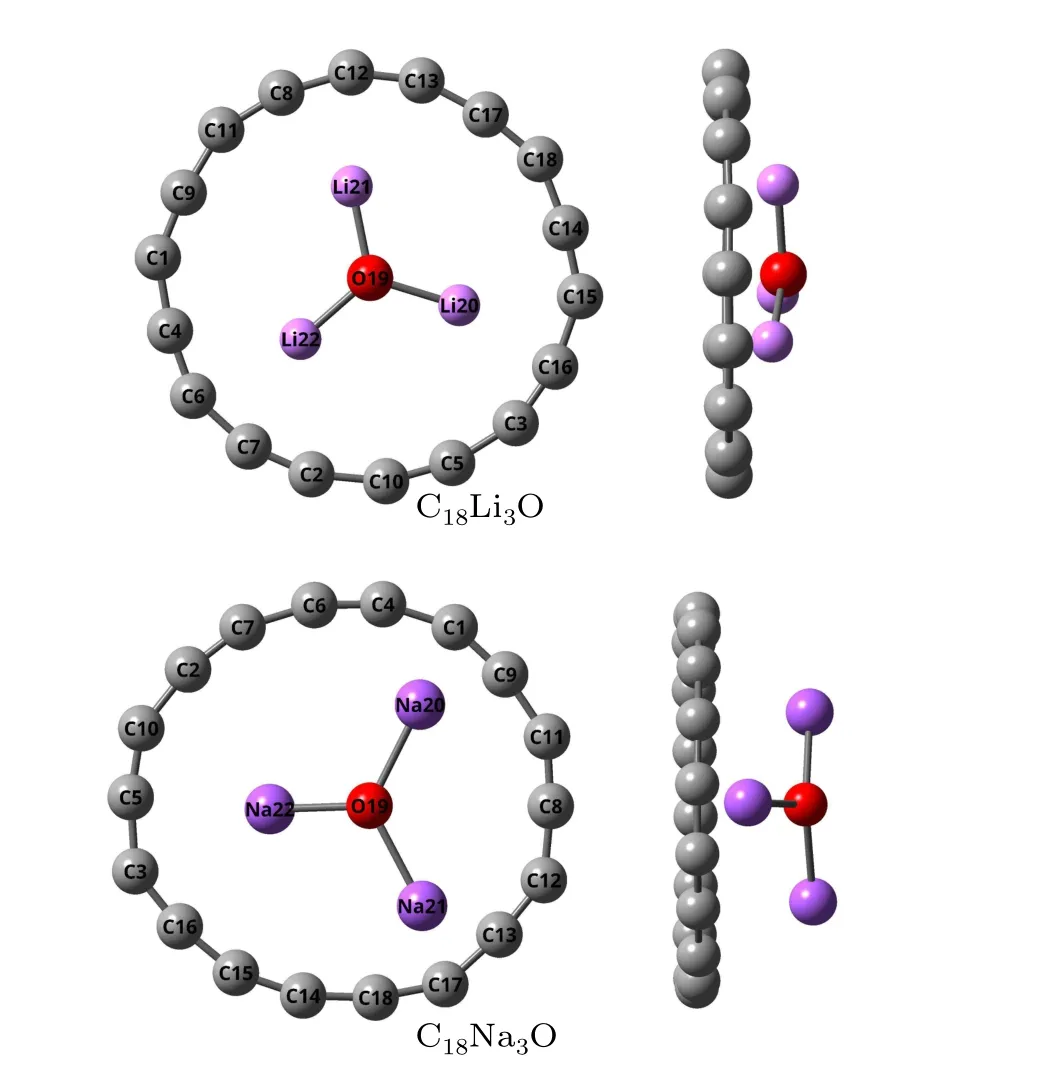
Fig.1.Top and side views of the structure of C18M3O(M=Li,Na).
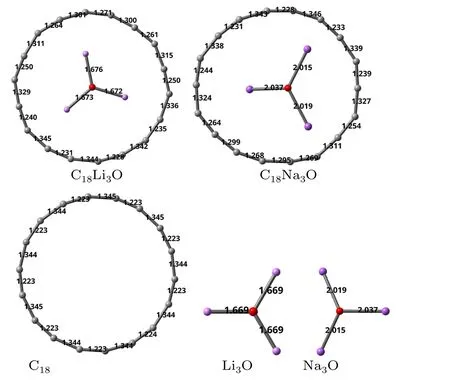
Fig.2.Bond lengths(?A)of C18Li3O and C18Na3O.The lengths of pristine C18,Li3O and Na3O are given for comparison.
Alternating C–C bond lengths (Fig.2) are also found in C18Li3O and C18Na3O.The C–C bond lengths of the isolated cyclo[18]carbon are 1.223 ?A and 1.344 ?A at the present level of theory.Compared with the values of the pristine C18ring,C18M3O has short C–C bonds that become shorter and long C–C bonds that become longer.Little difference can be found between theM–O bond length of C18M3O and the free-stateM3O.
The binding energy (Eb) ofM3O to C18ring can be defined as follows:
whereE(C18M3O) represents the energy of the most stable structure of C18M3O.E(C18) andE(M3O) represent the single-point energies of C18andM3O moieties, respectively,based on the ground-state structure of C18M3O.The obtainedEbvalues of Li3O and Na3O are 3.675 eV and 3.315 eV, respectively.Large binding energies suggest strong interactions betweenM3O and the C18ring.
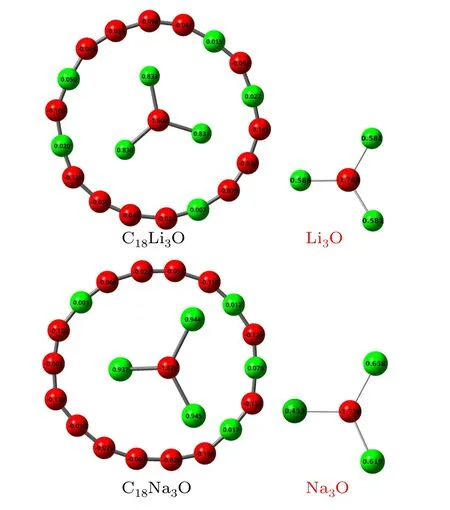
Fig.3.Natural charge populations of C18Li3O and C18Na3O.The values of isolated Li3O and Na3O are given for comparison.
The calculated natural charge populations of C18Li3O and C18Na3O are shown in Fig.3.Their charge transfers are similar.The oxygen atom and C18ring are negatively charged,whereas alkali atoms lose electrons.The oxygen atom obtains about two-thirds of the transferred electrons.The remaining one-third of the transferred electrons are transferred to cyclo[18]carbon,resulting in a negatively charged ring.The C18ring obtains about 0.9eand 1.0echarges from Li3O and Na3O, respectively.The charge transfer betweenM3O and C18forms stable [M3O]+[C18]-ionic complexes.This result supports the previous statement that the C18molecule is a good electron acceptor.[33]Na atoms lose more electrons than Li atoms do.The negative charges are not evenly distributed on carbon atoms of the C18ring.Few carbon atoms located far away from Li and Na carry a positive charge of no more than 0.08e.Other atoms near alkali atoms are negatively charged.The threeMatoms possess almost identical charges in C18M3O.However,for isolatedM3O,significant differences can be found between Li3O and Na3O.The charges are evenly distributed on Li atoms.However, the number of charges of the two Na atoms is remarkably larger than that of the other Na atom.This finding indicates that the charge redistribution of Na3O is greater than that of the Li3O after doping into cyclo[18]carbon.
Fig.4.Deformation electron densities of C18Li3O and C18Na3O(isovalue=0.15|e|).
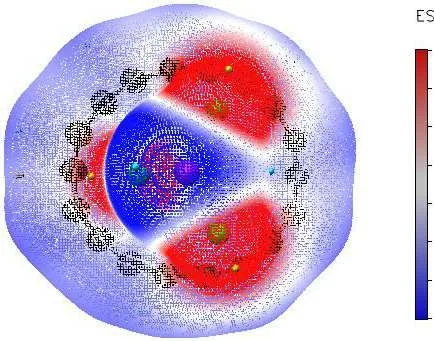
Fig.5.Isosurface maps of the ESP for C18Li3O and C18Na3O.Red and blue colors correspond to positive and negative parts of ESP,respectively.
The deformation electron density of C18M3O is shown in Fig.4.A large number of electrons are predominantly distributed in two carbon atoms of the C18ring, indicating the covalent characteristics of the C–C bond.The alternating electron density also reveals the alternating bond strength between two carbon atoms, which is consistent with the analysis of bond length.Many electrons are distributed around the oxygen atom, indicating the existence of an ionic chemical bonding between alkali and oxygen atoms.This observation is confirmed by the result of natural charge population analysis.The charge transfer fromM3O to C18results in an electric field between the two moieties.ESP analysis[71]is an effective tool to understand the interaction betweenM3O(M=Li,Na)and C18.On the basis of the ESP map in Fig.5,the regions around alkali atoms are positive ESP,whereas the circular regions surrounding the C18ring and oxygen atom show negative values.The maximum ESP is near the alkali atoms,whereas the minimum ESP appears near the oxygen atom.Several local potential minimum points are distributed on the periphery of the C18ring.The distribution pattern of ESP sufficiently reflects intermolecular electrostatic interaction betweenM3O and C18.
Figure 6 shows the Mayer bond order of C18Li3O and C18Na3O to further reveal the nature of theM3O–C18interaction and the bonding situation in the system.The bond orders between O and alkali atoms are no more than 0.41, indicating that the O–M covalent bond does not exist.This finding also confirms the results of population analysis and deformation electron density sections.The bond orders of C–M are less than 0.08,indicating that the chemical bond does not exist.This result probably occurred because the transferred electrons fromM3O are decentralized on other carbon atoms(Fig.3).The C18ring still exhibits alternating C–C bond orders.Most short bonds roughly satisfy double bonds, except for some bonds that deviate from 2.0.The orders of all long C–C bonds are larger than those of pristine cyclo[18]carbon.[16]

Fig.6.Mayer bond orders of C18Li3O and C18Na3O.
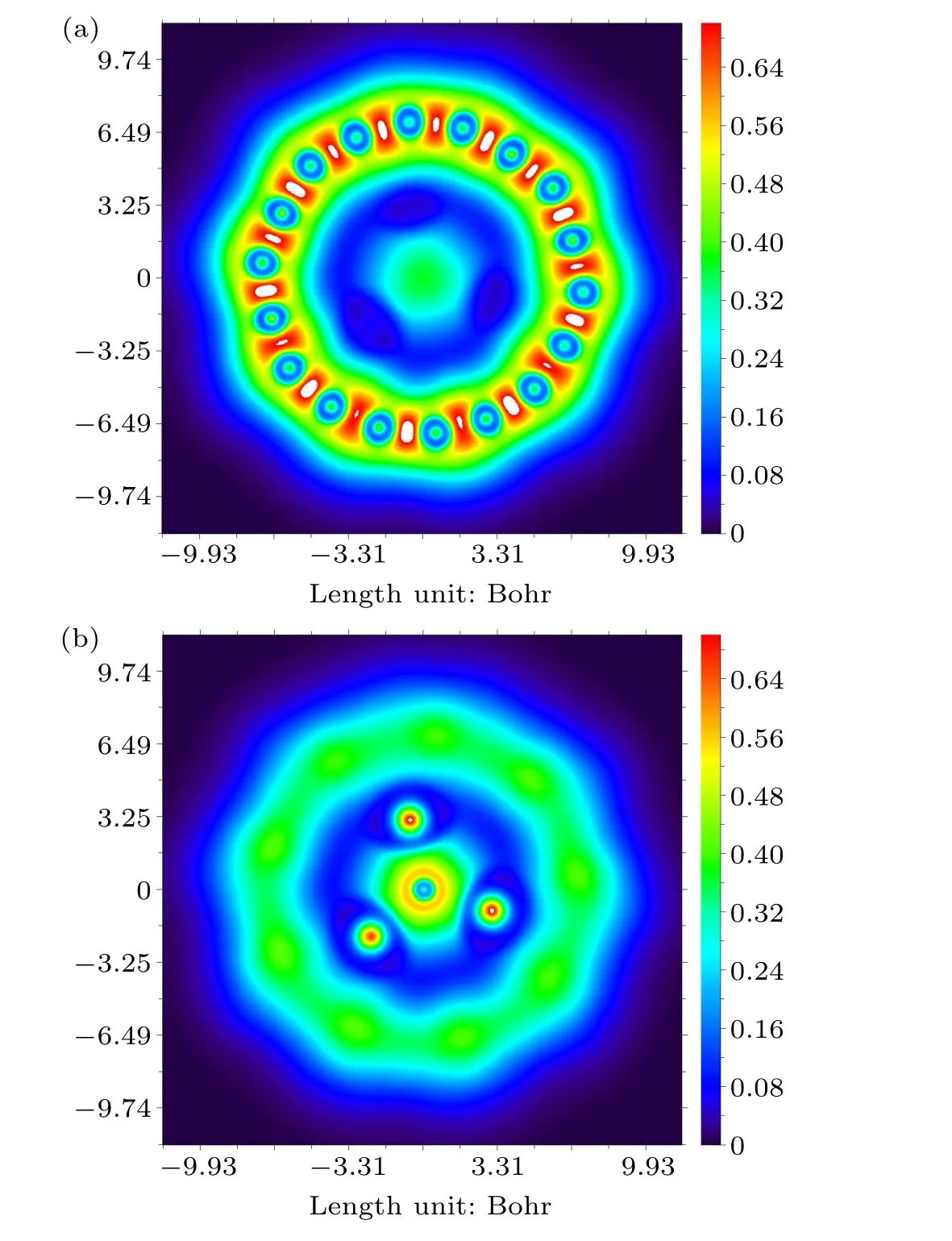
Fig.7.The localized orbital locator of C18Li3O.
The LOL has useful real space functions in the range of 0–1 to unravel the delocalization ofπelectrons of the system.Given that the two planes of C18ring and Li3moiety are almost parallel to each other,only the color-fill mapped LOL of C18Li3O is shown in Fig.7.Vast regions between Li3O and C18ring have LOL values much smaller than 0.5, indicating the absence of covalent bonds.We discussed earlier that they bind together by electrostatic attraction.A large LOL value(>0.5)in the middle of the C–C bond indicates a strong covalent bond and exhibits alternating characteristics.The regions of the short C–C bonds are evidently broader than those of the long C–C bond.This result indicates that theπ-electron delocalization of long C–C bonds is more difficult than that of short ones.Figure 7(b) shows the LOL–πmap of the Li3O at 1.4 Bohr above the C18ring plane.Regions with a value larger than 0.5 are spherically distributed around the O atom.The values in the middle of the O–Li are small.These results further reveal that the O–M is not a covalent bond but an ionic bond.LOL based onπmolecular orbitals can reflect the delocalization ofπelectrons in C18M3O (Fig.S1).The inside isosurface of the C18ring is broader than the outside ones for in-plane occupiedπmolecular orbitals,indicating that the delocalization over theπregion inside the ring occurred somewhat easily.The out-of-plane orbitals centering onM3O are not perpendicular to C18ring; instead, they tilt outward the C18ring slightly.
The electronic absorption spectrum can reflect electronic transition behavior in the C18M3O system.As observed from Fig.8,three absorption peaks exist for C18Li3O,in which two peaks are located at about 430 nm and 720 nm in the visible light region.The other one is a weak absorption at about 970 nm in the far infrared region.The spectrum of C18Na3O is different from that of C18Li3O,showing two absorption peaks at about 424 nm and 725 nm.The absorption range of the C18Li3O is wider than that of the C18Na3O,but its absorption intensity is much weaker than that of the latter.
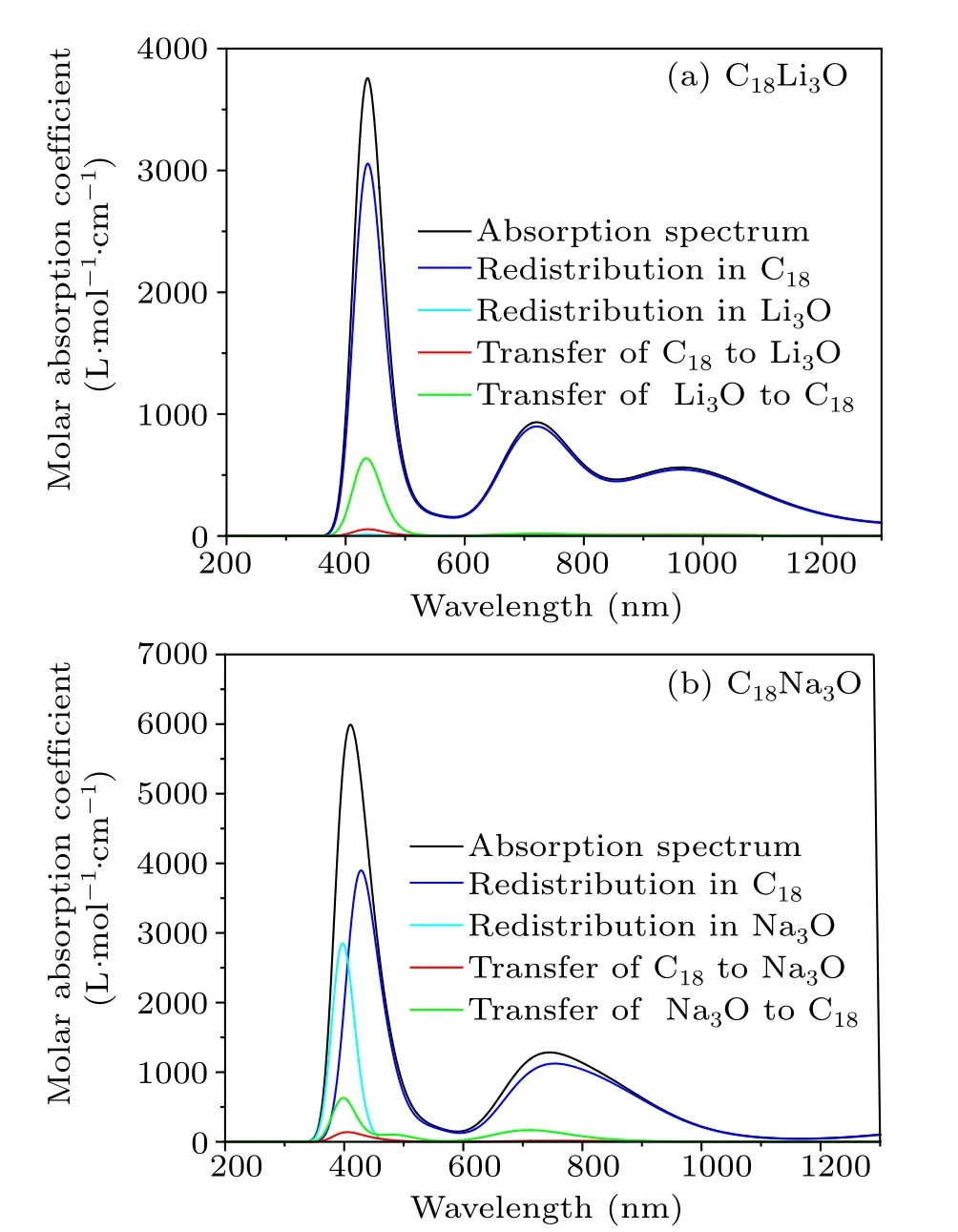
Fig.8.Simulated absorption spectra and charge-transfer spectra of C18Li3O and C18Na3O.Gaussian broaden for the full width at halfmaximum(FWHM)of 2800 cm-1 was employed.
Luet al.proposed a concept called CTS[43,46]to further reveal the nature of electron excitation from the charge redistribution within the fragment and charge transfer between two fragments of C18moiety andM3O.It can graphically present the contribution of charge redistribution.The colored CTSs are shown in Fig.8.For C18Li3O, the contribution for the electron excitation overwhelmingly originates from the electron redistribution inside the C18moiety, while the contribution of Li3O group is almost negligible.The electron transfers from Li3O to C18have a significant proportion.In contrast,the nature of electron excitation of C18Na3O is different from that of the C18Li3O because many excitations of the former in the range of 340 nm–470 nm originate from the electron redistribution inside the Na3O.The contribution of electron transfers from Na3O to C18to the absorption spectrum is also non-negligible.
Hole–electron analysis can intuitively reveal the excitation characteristics of electrons in a system.[29]The isosurface maps of hole and electron distributions related to their maximum absorptions of C18Li3O and C18Na3O are collectively given in the supplementary material (Fig.S2).As discussed earlier, the charges of three Na atoms are not equal before Na3O doping into cyclo[18]carbon.The charge transfer within the Na3O (charge redistribution) results in hole and electron distributions.Figure S2 shows that the absorption peak(397 nm) that corresponds to electron excitation (S0→S47)originates from electron redistribution inside the Na3O.This situation is not observed for C18Li3O.Thus, C18Na3O and C18Li3O have different electron excitations.
Polarizability and hyperpolarizability are the response characteristics of a molecule to an external electric field.Polarizability reflects the change in the dipole moment caused by applying one unit of electric field and hyperpolarizability represents the nonlinear polarization effect.Table 1 shows the calculated isotropic average polarizability (αiso), the projection of the first hyperpolarizability on the molecular dipole(βvec), and the average of the second hyperpolarizability (γ||)of C18M3O(M=Li,Na)under electric fields at different frequencies (λ= ∞; 1907 nm and 1460 nm).The calculated static and dynamic(hyper)polarizability of free C18ring at the present theory level are given in the table.The corresponding results of Ref.[29] are also listed for comparison.Our results are very close to those of Ref.[29].Theαiso(∞)values of C18M3O (M=Li, Na) are 1.20 and 1.25 times that of the free C18ring(293.9 a.u.).[29]Theβvecof the cyclo[18]carbon is zero due to its the centrosymmetric structure.However,the introduction ofM3O causes a considerable magnitude of staticβvec.C18Li3O and C18Na3O present a larger staticγ||(λ=∞)than the pristine C18ring(140909 a.u.).[29]
No significant difference is observed between static and dynamic polarizability (αiso).αisochanges slightly with frequency.Table 1 shows that the hyperpolarizability values(βvecandγ||)for C18Li3O decrease rapidly with the frequency of the external electric field.Atλ=1907 nm and 1460 nm,C18Li3O hasγ||values of 115 and 5.8 times, respectively, which are higher than those of pristine C18ring.[29]Theβvecvalues are 32.4 and 2.8 times,respectively,and theγ||values are 14.6 and 4.7 times,respectively,which are as much as the static values at the two frequencies.C18Na3O also exhibits a strong polarization resonance effect under the dynamic external field at a low frequency becauseβvecis 46 and 47 times higher,andγ||is 723 and 21 times higher than the static values atλ=1907 nm and 1460 nm,respectively.
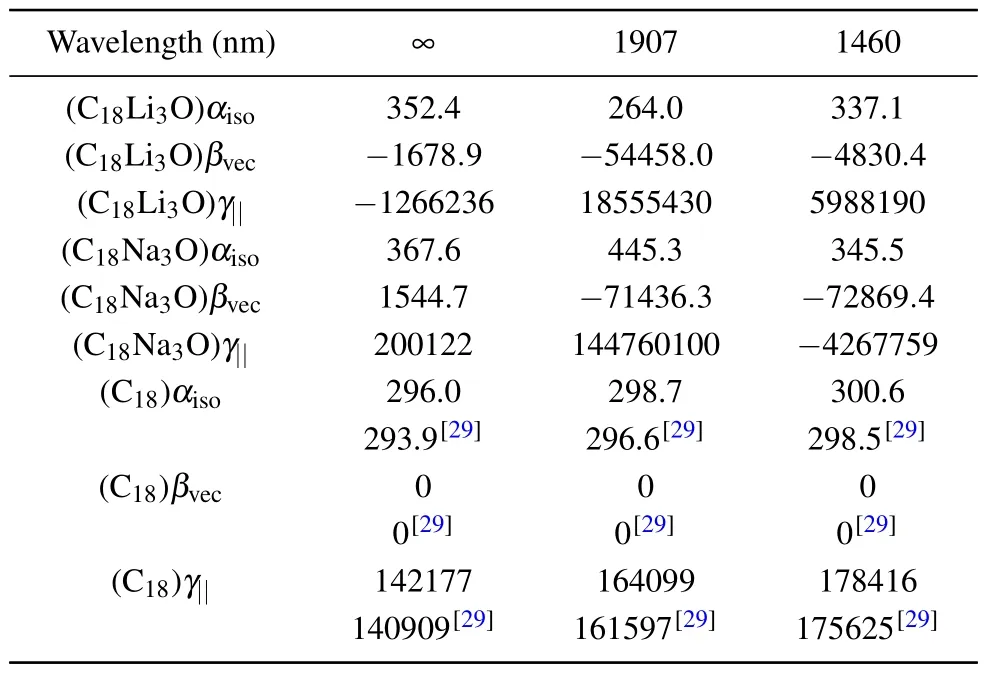
Table 1.Polarizabilities,the first-and the second-order hyperpolarizabilities(in a.u.) of C18Li3O,C18Na3O,and free C18 ring in zero-frequency limit case (λ = ∞) and frequency-dependent case (λ = 1907 nm and 1460 nm).The corresponding results of C18 of Ref.[29]are also listed.
Some values ofβvecandγ||are negative.Luet al.explained the meaning and the reason for their different signs.[43]The molecular volume is defined as the area surrounded by the electron density isosurface and has a close positive correlation with polarizability.This conclusion is also basically applicable to different types of molecules.[72]Therefore,the positive and negative signs ofβxxxandγxxxxcan be attributed to whether the molecular volume increases or decreases when the electric field is applied,which turns the problem into how the electric field affects the electronic structure of the system.The external electric field can affect the electron distribution of molecules.As a result, the effective volume of electron density changes,and polarizability also changes.[43]
The unit sphere representation of (hyper)polarizability can be used to visualize and comprehensively characterize the (hyper)polarizability tensor,[73]and can intuitively reflect the molecular global and local features of response properties.Figure 9 shows the unit sphere representation of (hyper)polarizability of C18M3O (M= Li, Na) under the electrostatic field.The molecular plane of C18ring is set as the xy-plane.The(hyper)polarizability of C18M3O(M=Li,Na)in the electrostatic field exhibits evident anisotropy.Thexandy- components of polarizability on the molecular plane are evidently larger than the component in the vertical direction(z-component).However, thez-component cannot be ignored because of dipole moments that result from the charge transfer between the C18ring andM3O.The tensor of all(hyper)polarizability suggests that some vector distributions(blue arrow) are perpendicular to the C18plane, indicating that the response of (hyper)polarizability in this direction is not negligible.C18Li3O and C18Na3O exhibit similar characteristics of first-order hyperpolarizability tensors.Theirx- andz-components are zero and have vanished in Figs.9(b) and 9(e).However, a significant difference is observed in terms of the second-order hyperpolarizability.Thex-component of C18Li3O is remarkably larger than that of C18Na3O.In contrast, they- andz-components of C18Na3O are much larger than those of C18Li3O.The two components of C18Li3O are small and negligible.
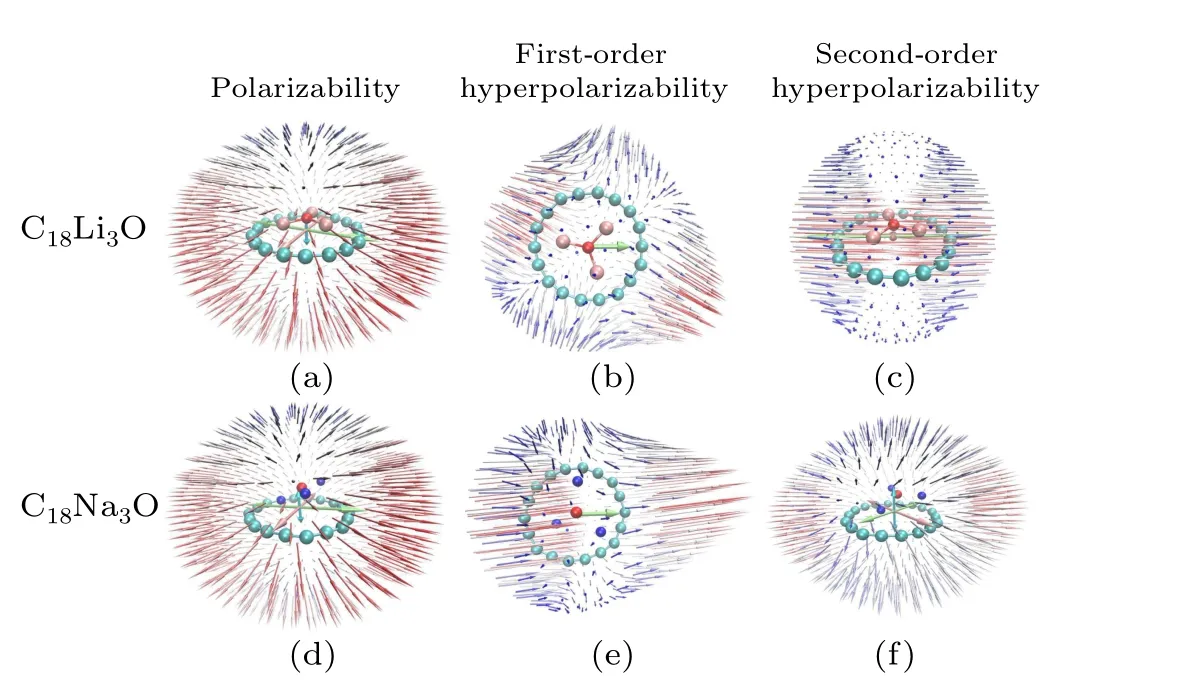
Fig.9.Unit sphere representation of (hyper)polarizability for C18Li3O and C18Na3O in static electric field.The longer and redder arrow indicates a larger tensor value in the corresponding direction.
4.Conclusion
In this paper, we theoretically investigate the structural,electronic,electronic absorption spectrum,and nonlinear optical properties ofM3O (M=Li, Na)-doped cyclo[18]carbon.M3O and the C18ring are not coplanar.Alternating C–C bond lengths are found in the two complexes.The charge,electrostatic potential, bond order, and delocalization ofπelectrons are analyzed.Specifically, C18Li3O and C18Na3O show striking optical nonlinearity,i.e.,their first-and secondorder hyperpolarizability (βvecandγ||) increase significantly atλ=1907 nm and 1460 nm.The relevant results are expected to provide theoretical guidance for the development of advanced cyclo[18]carbon-based functional molecules.
Acknowledgments
Project supported by the Natural Science Foundation of Anhui Province(Grant No.1908085MA12)and the National Natural Science Foundation of China(Grant No.21703222).
——山東省某區(qū)開發(fā)區(qū)管委會原副主任劉曉東(正處級)受賄案紀(jì)實
- Chinese Physics B的其它文章
- Quantum synchronization with correlated baths
- Preparing highly entangled states of nanodiamond rotation and NV center spin
- Epidemic threshold influenced by non-pharmaceutical interventions in residential university environments
- Dynamical behavior of memristor-coupled heterogeneous discrete neural networks with synaptic crosstalk
- Dynamics and synchronization in a memristor-coupled discrete heterogeneous neuron network considering noise
- Spatial search weighting information contained in cell velocity distribution

