Synthesis of ternary compound in H–S–Se system at high pressures?
Xiao Zhang(張曉)
1Key Laboratory of Materials Physics,Institute of Solid State Physics,HFIPS,Chinese Academy of Sciences(CAS),Hefei 230031,China
2University of Science and Technology of China,Hefei 230026,China
Keywords: hydride,high pressure,Raman spectroscopy
1. Introduction
Metallic hydrogen[1]attracts a lot interest due to its charming properties, especially room-temperature superconductivity.[2]However, the enormous pressure required makes such a state of hydrogen remain a challenge.Chemical precompression in hydrides has been proposed to facilitate metallization of hydrogen.[3]Based on this, many hydrides are predicted to be candidates for high temperature superconductors.[4–6]Moreover, high temperature superconductivity of hydrides is proved in H3S which shows a remarkably high superconducting transition temperatureTCof 203 K at 150 GPa.[7]Since then, a lot of new hydrides are synthesized[8–10]and record of the highestTCis broken by hydrides in rapid succession.[11–13]
As H3S is the first material to demonstrate such a high temperature superconductivity, the investigation into mechanism of the highTCof H3S has been a hot topic and fruitful.[14–17]It was suggested that superconductivity of H3S can be attributed to conventional phonon-mediated mechanism as it exhibits strong covalent bonds giving rise to large electron–phonon couplings.[14,18]Improving the covalent characteristics of the sulfur-hydrogen bond by replacing S atoms with mixtures of chalcogens or other atoms is a good choice to further enhance superconducting properties of H3S.[19]Several ternary hydrides based on the H3S structure were predicted to exhibit high-TCbehavior.[19–22]Recently,a carbonaceous sulfur hydride exhibited room-temperature superconductivity.[13]However, superconductivities in other doping systems are still to be confirmed experimentally. It is of fundamental importance to investigate the synthesis conditions of these hydrides.
Superconducting H3S is of cubicIm-3mstructure.[16,23]It can be considered as being stoichiometrically disproportionate H2S[7,24]or synthesized by the directly laserheated elements.[25]Besides, a molecular host–guest compound (H2S)2H2with the same stoichiometry as H3S was found at low pressures,[26]and it remained stable at up to 160 GPa.[27,28]The room-temperature superconductor, carbonaceous sulfur hydride, was suggested to have a similar host–guest structure(CH4)x(H2S)(2?x)H2based on its Raman spectra,[13,28]but different from H3S.[29]On the other hand,owing to similar kinetic diameters of H2S, CH4, and H2Se,it is possible to expect a similar van der Waals compound in H–S–Se system by substituting H2S for H2Se. Moreover,this ternary compound of H–S–Se promises to be achieved by using a similar route carbonaceous sulfur hydride.[13,29]
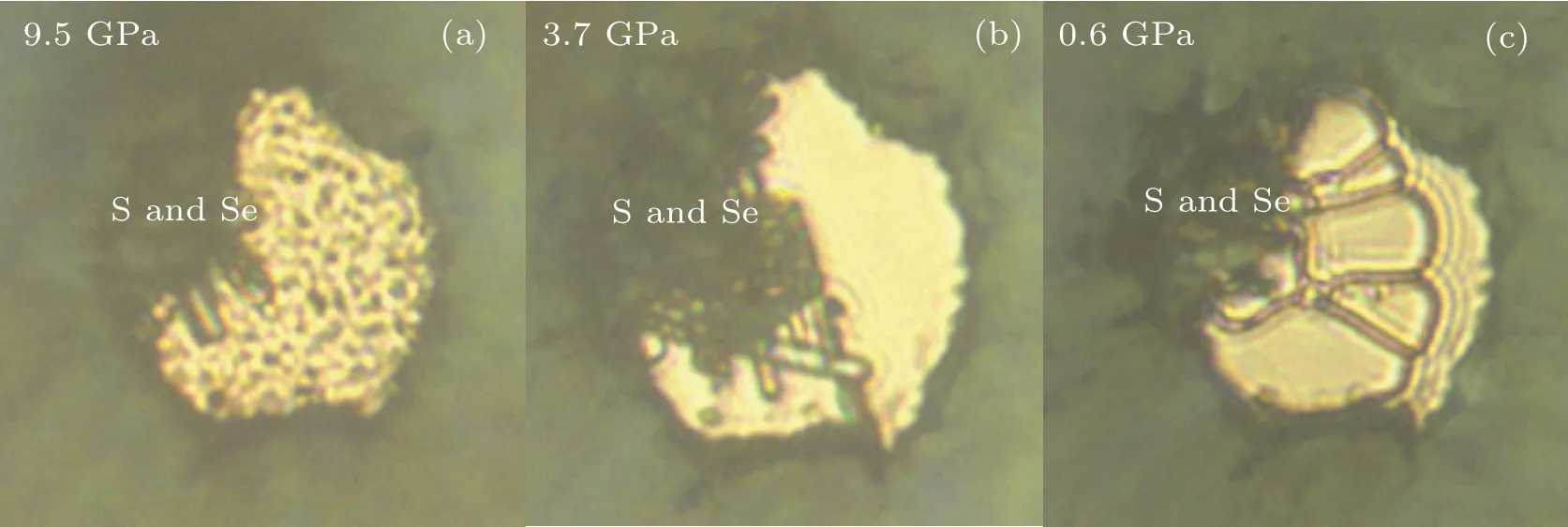
Here in this work, the ternary compound in H–S–Se system at high pressures is synthesized. This compound has a molecular host–guest structure and can be named(H2S)x(H2Se)(2?x)H2(0 Symmetric diamond anvil cells (DACs) equipped with anvils with central culets of 250 μm in diameter were employed in the experiments. Small pieces of S and Se were positioned close to a hole in a rhenium gasket and filled with H2gas at~200 MPa. Two samples (A and B) were used in this experiment. Because H2S solifies at a low pressure of about 1.1 GPa,[26]in order to get fluid H2S and H2Se, laser heating are performed at pressures lower than 1 GPa. Samples A and B were heated at 0.9 GPa and 0.4 GPa,respectively. Laser sharply focused on a spot of 2μm–3μm in diameter irradiated two solids alternatingly for a few seconds and moved back and forth. The 532-nm solid state laser at power about 460 mW was used in laser heating to melt S and Se. Synthesis of H2S and H2Se are judged from Raman spectra measured. Once the mixture of H2S, H2Se, and H2became fluids, the DAC was compressed up to 37 GPa at room temperature. Pressure was determined by using the ruby fluorescence.[31]For the Raman experiments, a backscattering geometry was adopted for confocal measurements with an incident laser wavelength of 532 nm.[32]The Raman notch filter is of a very narrow bandpass(Optigrate),allowing Raman measurements down to 10 cm?1in the Stokes and anti-Stokes.One of these notch filters was used as a beam splitter to inject the laser into the optical path. After being heated by laser, both solid S and Se melt as observed visually. The transparent region looks uniform without boundary of fluids. Meanwhile,Raman spectra measured at different positions in H2region all show two new sharp peaks at about 2350 cm?1and 2620 cm?1(Fig. 3). These two peaks can be assigned to Se–H[33]and S–H[5]stretching modes,respectively. The S–H stretching mode has higher intensity than Se–H stretching mode. Summing up the above,fluids H2S and H2Se are synthesized and mix well with each other and also together with H2. The two samples A and B show similar outcomes in the further experiment. However,some different phenomena are observed. It is worthwhile to describe the changes in two samples respectively. In sample A,at 6.8 GPa,H2region is filled with punctate black solids(Fig.1(a)).However,they are too small to be characterized by Raman spectra. On the other hand,high-intensity S–H and Se–H stretching modes can be detected simultaneously at boundary of S and Se, along with an additional Raman peak at lower frequency in H–H vibron. Although there are some peaks in lattice region at about 200 cm?1, no peak can be attributed to S–Se bond.[34]The Raman spectrum measured shows the coexistence of molecular H2S,H2Se,and prolonged H2, indicating the formation of van der Waals S–Se–H ternary hydride like (H2S)2H2[26,28]and (H2Se)2H2.[8]On the other hand,owing to similar kinetic diameters of H2S and H2Se,[8]the substitution of H2Se for H2S is possible and it is a good choice to describe this new ternary compound as(H2S)x(H2Se)(2?x)H2(0 When pressure decreased to 9.5 GPa, three transparent single crystals emerge (Figs. 1(b) and 2(a)). These crystals have similar spectra to ternary compound at boundary of S and Se (Figs. 1(a) and 1(b)). Some differences including no sharp peak at about 200 cm?1are caused by measurement relatively far from S and Se. These transparent single crystals can be recognized as being different form of(H2S)x(H2Se)(2?x)H2.At 3.7 GPa,(H2S)x(H2Se)(2?x)H2turns into H2S–H2Se van der Waals compound which does not have prolonged H2molecules(Fig.2(b)). Meanwhile,small punctate black solids disappear. Finally, DAC is successfully decompressed to 0.6 GPa. At this pressure,all kinds of crystals decompose. Instead, some scale-like vesicles spatially separated appear in chamber(Fig.2(c)),figuring out immiscibility of fluid H2S,H2Se,and H2. Furthermore,after being exposed to the laser, the transparent part quickly turns into a uniform three-fluid mixture. Fig. 1. Microscopic images and Raman spectra of (H2S)x(H2Se)(2?x)H2 in different forms at about 6 GPa,showing(a)compound at boundary of S and Se, (b) single-crystalline compound obtained on decompression, (c) crystal attached on Se,and(d)mall black solid compound. Spectra are measured at positions marked as red circles. In sample B,van der Waals compound of H2S and H2Se is also observed on compression at 4.1 GPa. This compound grows up gradually as a transparent crystal adjoining Se approaches to fluid H2region. Its Raman spectrum is shown in Fig.3. Fig.2. Microscopic images of sample A at different pressures on decompression,showing(a)growing-out crystalline(H2S)x(H2Se)(2?x)H2,(b)emerging H2S–H2Se van der Waals solid,(c)immiscibility of fluid H2S and H2Se with H2. Fig.3. Raman spectra of H–S–Se compounds in sample B at different pressures. At 6.1 GPa, the van der Waals compound of H2S and H2Se disappears. Instead, the same small black solids which are present in sample A emerge in H2region (Fig. 1(d)).These solids are much bigger on this occasion. Their Raman spectra are in common with spectra of single-crystalline(H2S)x(H2Se)(2?x)H2obtained on decompression (Figs. 1(b)and 1(d)). Besides, spectra with S–H and Se–H stretching modes and extra H–H vibron are detected at boundary of S and Se,as done in sample A.These two spectra figure out that the H–S–Se ternary compound (H2S)x(H2Se)(2?x)H2is also synthesized in sample B. And H–S–Se ternary compound is in the form of small black solid. To obtain bigger crystalline solid for high-quality spectra, sample B is decompressed to 5.3 GPa. At this point, small black solids disappear, and the ternary compound cannot be detected on S or Se either.Meanwhile,fluid H2S comes back and shows a sharp peak at about 2650 cm?1. When compressing sample again to 6.7 GPa,ternary compound regains as a black solid at edge of Se where H2S–H2Se van der Waals solid was located before.It is not big but shows a good Raman spectrum with high-intensity Raman characteristic peaks(Figs.1(c)and 2). At 20.4 GPa, a new component of stretching mode appears at 2600 cm?1(Figs.3 and 4). This splitting of S–H Raman vibrational stretching mode is due to strengthening of H bonding between neighboring H2S molecules. The same clues can also be found in(H2S)2H2[26,28]and pure H2S.[35,36]Besides, the H–H vibron splitting observed in (H2S)2H2is also observed in ternary compound at 2.4 GPa. But the pressure of peak splitting is hysteretic in comparison with (H2S)2H2.The later material finishes this phase transition at 16.7 GPa.Because some peaks at about 200 cm?1arise from S and Se,the lattice modes of compound cannot be distinguished well.But orientational ordering of H2S molecules which are concurrent with splitting of S–H stretching mode and H–H vibron in(H2S)2H2disappear at 20.4 GPa in(H2S)x(H2Se)(2?x)H2. Above 29.9 GPa, Se–H stretching becomes weak but can still be resolved. Its lifespan has been prolonged while(H2Se)2H2[8]and pure H2Se[30]are not stable at room temperature and chemically decomposes above 22 GPa. Even though this binary compound is stable at low temperature,its Raman signal will lose above 29 GPa. Above 31.6 GPa, stretching modes turns into a broad band. Meanwhile,two peaks of H–H vibron belonging to compound are equal in intensity. Without regard to different initiate or terminate pressures,the pressure dependence of S–H and Se–H stretching modes of(H2S)x(H2Se)(2?x)H2is in line with that of(H2S)2H2[28]and(H2Se)2H2,[8]respectively (Fig. 4). These correspondences indicate that H2S and H2Se exist in the same coordinate as their binary guest–host compounds. Because of similar structure of(H2S)2H2and(H2Se)2H2,with the addition of similar kinetic diameters of H2S and H2Se, substitution of H2Se for H2S orvice versais possible to form H–S–Se ternary compound. Fig. 4. The pressure-dependent S–H and Se–H stretching modes of(H2S)x(H2Se)(2?x)H2 in comparison with those of (H2S)2H2[28] (dashed lines)and(H2Se)2H2[8] (dotted line),respectively. From pressure-dependent H–H vibron(Fig.5),the transition of H2subsystem in (H2S)x(H2Se)(2?x)H2only conforms to that of (H2S)2H2,[28]while H2molecule in (H2Se)2H2are abandoned. It is shown that the ternary compound has(H2S)2H2as framework and the substitution of H2Se for H2S has more chances. In addition, no contribution of H2Se in H2subsystem provides a clue to certifying the formation of ternary compound.If ternary compound does not exist,but the mixture of two binary compounds (H2S)2H2and (H2Se)2H2participates in Raman measurement,the H–H vibron mode of(H2Se)2H2should be detected. Although the earlier theoretical studies predict that the S–Se–H ternary hydride should be based on a cubic structure inImˉ3mphase with a strong covalent character through atomic substitution,[20,21]our experiments suggest the synthesized S–Se–H ternary compound is a van der Waals compound with guest–host structure like C–S–H room temperature superconductor.[13,29]The new S–Se–H ternary hydride is also possible to be a superconductor with high transition temperature driven by strong electron–phonon coupling to highfrequency hydrogen phonon modes.[38,39]To further reveal the structural and superconducting properties of the S–Se–H ternary hydride, synchrotron x-ray diffraction and charge transport studies are needed in future. Fig.5. Pressure-dependent H–H vibron of(H2S)x(H2Se)(2?x)H2 in comparison with (H2S)2H2[28] (dashed lines) and H3Se[8] (dotted line). Solid line refers to literature data for H2.[37] Chemical transformations in H–S–Se system are detected with Raman spectroscopy in this experiment. The H2S and H2Se can combine into a van der Waals solid at about 4 GPa.More importantly, a ternary van der Waals compound of H2S–H2Se–H2is synthesized at about 6 GPa. This compound has a guest–host structure and can be identified as(H2S)x(H2Se)(2?x)H2because H2Se replaces partial H2S in(H2S)2H2. This compound is stable at up to 37 GPa at least and down to 3.6 GPa. The changes in properties of this material with pressure are very well consistent with the behavior of (H2S)2H2. Moreover, the stability of H2Se molecule is improved in this ternary system,besides the decomposition in (H2Se)2H2and pure H2Se. These results pave the way to studying the properties of this material at higher pressures and are conducive to the study of other ternary hydrides.2. Experimental methods
3. Results and discussion




4. Conclusions