Adsorption behavior of uranyl ions onto amino-type adsorbents prepared by radiation-inducedgraft copolymerization?
CHI Hong-Ying(遲洪影),LIU Xi-Yan(劉西艷),MA Hong-Juan(馬紅娟),YANG Xiao-Juan(楊曉娟),YU Ming(虞鳴),ZHANG Jian-Yong(張建勇),WANG Min(王敏),,LI Jing-Ye(李景燁),,Hiroyuki Hoshina,and Noriaki Seko
1TMSR Research Center and CAS Key Lab of Nuclear Radiation and Nuclear Energy Technology, Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,China
2University of Chinese Academy of Sciences,Beijing 100049,China3Quantum Beam Science Directorate,Japan Atomic Energy Agency,1233 Watanuki,Takasaki,Gunma,370-1292,Japan
Adsorption behavior of uranyl ions onto amino-type adsorbents prepared by radiation-induced
graft copolymerization?
CHI Hong-Ying(遲洪影),1,2LIU Xi-Yan(劉西艷),1MA Hong-Juan(馬紅娟),1YANG Xiao-Juan(楊曉娟),1YU Ming(虞鳴),1ZHANG Jian-Yong(張建勇),1WANG Min(王敏),1,?LI Jing-Ye(李景燁),1,?Hiroyuki Hoshina,3and Noriaki Seko3
1TMSR Research Center and CAS Key Lab of Nuclear Radiation and Nuclear Energy Technology, Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,China
2University of Chinese Academy of Sciences,Beijing 100049,China3Quantum Beam Science Directorate,Japan Atomic Energy Agency,1233 Watanuki,Takasaki,Gunma,370-1292,Japan
Amino-type adsorbents(ATAs)were prepared by radiation-induced graft copolymerization of 4-hydroxybutyl acrylateglycidylether(HB)ontoapolyethylene-coatedpolypropylene(PE/PP)duplexf i berofanon-wovenfabric,and modif i ed with different amines of ethylenediamine(EDA),diethylenetriamine(DETA),triethylenetetramine(TETA)and diethylamine(DEA).The adsorption behavior of uranyl ions onto the ATAs was studied in batch experiments.The effects of the contact time,initial concentration of the ions,temperature,and pH value. The salinity were investigated along with the adsorption kinetics and the adsorption isotherms.The kinetic experimental data followed the pseudo second-order kinetic model,and the adsorption isotherms correlated well with the Langmuir model.The ATAs showed good eff i ciency in adsorbing uranyl ions,with the best saturation adsorption capacity being 64.26mgg?1for ATA-DETA within 120min.The temperature dependence of ATADETA was quite abnormal and the quickest behavior was obtained at 25?C.ATAs showed good adsorption capacity over a wide pH range of 4.0–8.5,and HCl could be used in the elution process.Salinity of the solution had great effect on the adsorption capacity,3.5%salinity resulted in a 55%loss of capacity from ATA-DETA.
The selectivity of ATA-DETA showed an order of:UO22+≈Fe3+>Zn2+>VO3–>Co2+>Ni2+.
Amino-type adsorbents,Radiation induced graft polymerization,Uranyl ions,Adsorption,Selectivity
I.INTRODUCTION
Uranium,an important resource of nuclear fuel,mainly resides as sedimentate in terrestrial ores or as dissolved uranyl ions in seawater.The total amount of uranium in seawater is estimated at 4.5 billion tons,about 1000 times the total amount in terrestrial ores,and should be considered as an important fuel sourcefornuclear powerindustry[1–3].However,the low concentration of uranyl ions in seawater,about 3.3μgL?1,is a signif i cant challenge in the developing an economic recovery technique.
In the past decades,various methods,such as solvent extraction,f l otation,ion-exchange and adsorption etc,have been applied for the recovery of uranium from seawater[4–6].As a high eff i ciency and low cost method,the adsorption of seawater uranyl ions with various types of adsorbents has been widely studied and reported.
Of all the adsorbents for uranyl ions coordination reported so far,adsorbents functionalized with amidoxime [AO;?C(NOH)?NH2]groups are considered as the most promising materials due to their high capacity and selectiv-ity[7,8].Radiation-induced graft copolymerization(RIGC) of acrylonitrile onto polymeric fabric or f i bers,and then amidoximed,is now a sophisticated technique in Japan[9].A marine experiment using a braid adsorbent reported a loading capacity of 2mgg?1(uranium per adsorbent)[10].Efforts have been made at SINAP in preparing amidoxime-type adsorbent and studying adsorption behavior of the uranyl ions onto the adsorbent[11,12].
Amines are widely used to form coordination compounds with metal ions.Therefore,an amino-type adsorbent(ATA) is a worthy candidate for studies on uranium extraction from seawater.Several ATAs utilizing RIGC were reported.The precursor monomer containing an epoxy group such as glycidylmethacrylate(GMA)was grafted onto the polymeric matrix and then amino groups were introduced through a ringopening reaction[13].Recently,4-hydroxybutyl acrylate glycidylether(HB)was applied to synthesize metal ion adsorbents,as it contains an epoxy group too,and due to the longer side chain in HB,the adsorbents performed nicely,with higher adsorption amount and faster adsorption rate than those using GMA as the precursor[13].
In this work,ATAs were prepared by grafting HB onto a non-woven fabric made of polyethylene-coated polypropylene(PE/PP)duplex f i ber,and modif i ed with amines of ethylenediamine(EDA),diethylenetriamine(DETA),triethylenetetramine(TETA)and diethylamine(DEA).The adsorption behavior of the uranyl ions onto the ATAs was investigated.
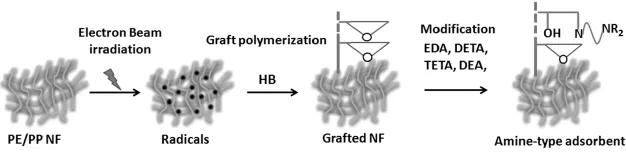
Fig.1.Preparation of the amine-type adsorbents.
II.EXPERIMENTAL SECTION
Materials:PE/PP duplex f i ber non-woven fabric was obtained from Kurashiki MFG Company,Japan.HB was purchased from Tokyo Kasei Kogyo Co.Ltd,Japan.EDA, DETA,TETA,DEA,surfactant sorbitan monolaurate(Span-20),xylene and isopropyl alcohol,were purchased from Kanto Chemical Co.,Ltd.Standard solutions of uranyl,iron,vanadium,nickel,cobalt and zinc were bought from Analytical Laboratory,Beijing Research Institute of Uranium Geology. HNO3and Na2CO3were purchased from Sinopharm Chemical Reagent Co.,Ltd.,All the materials and reagents were used without further purif i cation.
Preparation of the ATA:ATAs were prepared using preirradiation-induced graft copolymerization and an amination procedure(Fig.1).Thenon-wovenfabricsampleswerecooled in dry ice and irradiated 30kGy by 1.5MeV electron beam. Graft copolymerization was carried out in an emulsion system containing 5%HB and 0.5%surfactant Span-20 at 40?C for 2 hours.The degree of grafting(DG)of the obtained fabrics,named as PE/PP-g-PHB,was about 150%.Then,the fabrics were aminated by immerging them in an isopropyl alcohol solution added with EDA,DETA,or TETA(to 70%concentration for each agent),at 60?C for 4h.The amination using DEA was done in a 50%DEA water solution at 30?C for 5h.The resulting ATAs were named ATA-EDA,ATA-DETA, ATA-TETA and ATA-DEA according to the amine used.The amino group density(AGD)of PE/PP?g?PHB fabrics grafted with the EDA,DETA,TETA and DEA were 3.0mmolg?1, 1.7mmolg?1,1.6mmolg?1and 2.2mmolg?1-adsorbent,respectively.
The AGD was estimated by:

where,Z0and Zfare the weights of grafted fabric before and after amination and M the molecular weight of the amine compound.
Batch adsorption experiments:To study the adsorption kinetics,ATAs were put into a uranyl ion solution adjusted at pH 8 and kept at 25?C.The samples were taken out for evaluation at 30min,60min,120min,240min and 480min.Once the best ATA was determined,the ATA-DETA adsorbent was used to study the inf l uences of the adsorption.Equilibrium isotherms were determined by varying initial uranium concentration from 1mgL?1to 7mgL?1and using an equilibrium time of 8h.The effect of temperature on the equilibrium uptake of uranyl ions was investigated under similar experimental conditions,except for changes in temperature from 5?C to 35?C.The inf l uence of the initial solution pH on uranyl ions adsorption was studied with an initial concentration of 1mgL?1and pH value was adjusted between 2.0 and 10.0 usingHNO3andNa2CO3solutionsat25?C.Selectivemetalions adsorption of uranyl,vanadium,iron,nickel,zinc and cobalt ions onto DETA-type adsorbent tests were performed by using a mixture of an aqueous solution of 1mgL?1for each of the six metal ions.The solutions for the batch adsorption experiments were stirred at the same rate at different temperatures forvariouscontacttimesina waterbath.Theconcentrationsof uranium were determined by a trace uranium analyzer(WJGIII).Selective metal ion concentrations after adsorption were determined by using an ICP(Inductively Coupled Plasma)analyzer(NexION 300 D).The adsorption amount of the uranium ion was calculated using Eq.(2):
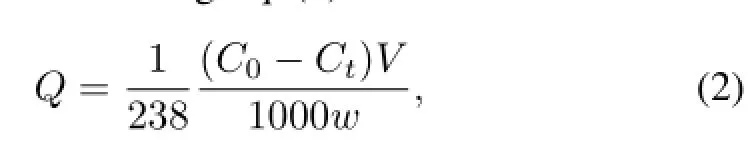
whereQ(molg?1)is the adsorption amount of uranium onto amine-type materials,C0(mgL?1)andCt(mgL?1)are the initial concentrations of the uranium in the solution before and after adsorption for a certain timet,respectively.V(L)is the volume of the solution,andw(g)is the weight of the dry adsorbent.
Characterizations:Infrared spectra were taken on a Tensor 27 FT-IR spectrometer(Germany).The pristine and grafted PE/PP duplex f i ber non-woven fabrics are scanned in the wave number range of 4000–800 cm?1.Scanning electron microscopy(SEM)images of the pristine PE/PP and ATADETA were taken on a JSM-6700F scanning electron microscope(JEOL,Japan).Prior to the SEM observation,the carbon tape for sample attachment were sputtered with gold to enhance the electronic conductivity in vacuum.
III.RESULTS AND DISCUSSION
A.Adsorbent characterization
The HB-graft non-woven fabric and the ATAs were characterized by FT-IR.Fig.2 shows typical FI-IR spectra of the ATA-DETA,the trunk polymer and HB-grafted nonwoven fabric.The spectrum of HB-grafted sample exhibitsstrong absorption at about 1730cm?1(C?O stretching)and 1251cm?1(?C?O?C stretching),and the peak at 848cm?1represented the characteristic vibrations of epoxy groups.All these indicate that the HB-graft chains are introduced onto the PE/PP fabric.The ATA-DETA spectrum shows the new characteristic peaks at 1650cm?1and 1555cm?1(N?H bending vibration),indicating the successful modi fi cation of the DETA group.
SEM images of the virgin PE/PP fabric and ATA-DETA are shown in Fig.3.The surface image of the ATA-DETA revealed the fi ber morphology,crisscrossed by a network of fi bers of about 20μm in diameter(Fig.3(b)).The rough fi ber surface(Fig.3(c))means that the ATAs possess an appropriately high speci fi c surface area,which facilitates the adsorption process of uranyl ions.
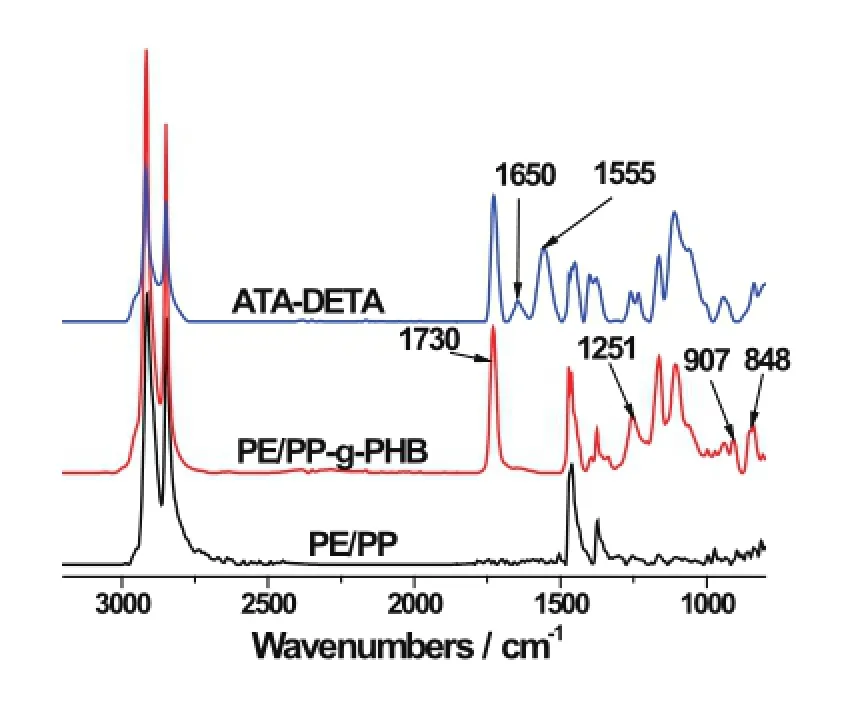
Fig.2.(Coloronline)FT-IRspectraoftrunkPE/PPnon-wovenfabric, HB-grafted non-woven fabric and ATA-DETA.

Fig.3.SEM images of PE/PP(a)and ATA-DETA at low(b)and high (c)magnif i cation.
B.Effect of contact time
The effect of contact time on uranyl ions absorption onto the ATAs was investigated over the time intervals(at 30min, 60min,120min,240min and 480min).As shown in Fig.4(a), the amount of adsorption increased with the contact time and fi nally reached 12.45mgg?1,12.50mgg?1,12.48mgg?1and 12.50mgg?1for ATA-EDA,ATA-DETA,ATA-TETA and ATA-DEA,respectively.After absorption equilibrium,the four ATAs exhibited similar adsorption amount but the time dependence curves show that the adsorption rate is in the order of ATA-DETA>ATA-DEA>ATA-TETA>ATA-EDA.This means that ATA-DETA exhibited the most eff i cient adsorption rate for uranyl ions,especially within the f i rst 30min.As mentioned previously the functional group density of ATAs were 3.0mmolg?1,1.7mmolg?1,1.6mmolg?1and 2.2mmolg?1for ATA-EDA,ATA-DETA,ATA-TETA and ATA-DEA,respectively.Functional groups of the f i rst three type adsorbents were in the form of secondary and tertiary amine when used in aqueous solution and ATA-DEA was of quaternary amine.The most eff i cient adsorbent in this study was ATA-DETA with a functional group density of 1.7mmolg?1.Therefore,the adsorption rate of the materials was affected not only by the density of the amino groups,but also the structure of the amino group.
A good correlation with adsorption kinetics data can fully explain the adsorption process.The pseudo second-order model in Eq.(3),which is based on adsorption ability on the solid phase,was used to f i t the results obtained from the different amine-type materials.
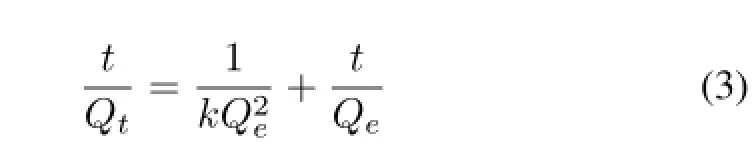
wherekis the rate constants of the pseudo second-order adsorption,QtandQeare the amount of adsorbed uranium at timetand equilibrium state.The f i tting results applying the pseudo second-order model to the kinetic data of different ATAs is given in Fig.4(b),and one sees a good linearity with the correlation coeff i cient(R2>0.99)under the experiment conditions.
As ATA-DETA has the most eff i cient adsorption rate for uranyl ions,we focused on ATA-DETA in the following experiments.
C.Effect of the initial concentrations
The effect of initial uranyl ion concentration on the ATADETA adsorption was obtained from 1 to 7mgL?1at a pH 8.0 at 25?C.Fig.5 shows the amount of uranyl ions adsorbed on the ATA-DETA om 8 hours.The amount of adsorbed uranyl ion increases with the initial concentrations and reached a plateau value of 64.26mgg?1,i.e.the maximum equilibrium adsorption amount.
The Freundlich and Langmuir adsorption models are two typical adsorption equilibrium isotherms widely used to describe adsorption equilibrium.The Freundlich model supposes that a nonideal adsorption surface is heterogeneous with multilayer sorption[14].The linear form of the Freundlich model is given by

whereKFandnare the Freundlich constants to indicate the adsorption capacity and the extent of the adsorption,respectively[15].Qe(mgg?1)is the amount of solute adsorbed atequilibrium,andCe(mmolL?1)is related to the concentration equilibrium.
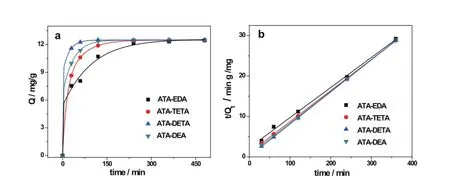
Fig.4.(Color online)(a)Adsorption kinetics of different ATAs(C0=1mgL?1;T=25?C;pH=8)(b)Linearized pseudo-second-order kinetic model for uranium on different ATAs.
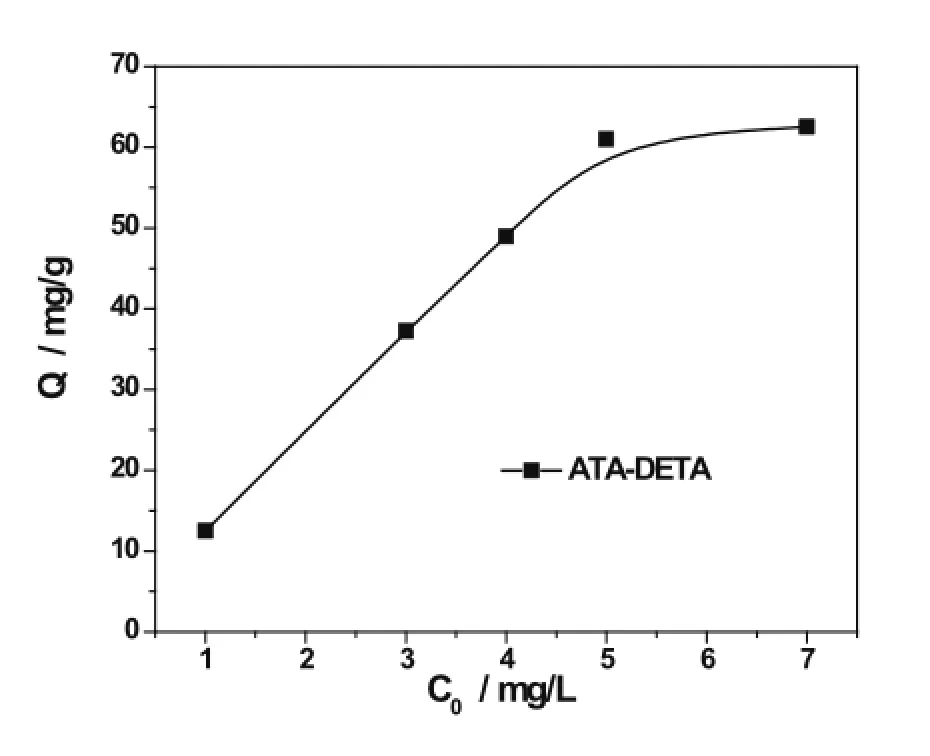
Fig.5.Effecting of the initial concentration on adsorption capacity.
The Langmuir model assumes that the adsorption of metal ions occurs on a homogeneous surface by monolayer adsorption without any interaction between adsorbed ions[16].The linear form of the Langmuir model is given by:
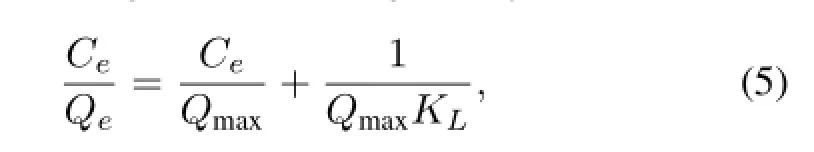
where,Qmaxis the Langmuir monolayer adsorption capacity(mgg?1)andKLis the Langmuir equilibrium constant:the ratio of adsorption and desorption rate coeff i cients(Lg?1).
The plots based on the two linear relationships are shown in Fig.6.The data were linearly correlated,with the correlation coeff i cients(R2)of 0.877 and 0.999 for the Freundlich and Langmuir models,respectively.This indicates that isotherm is a better f i tting model than Freundlich and the adsorption of ATAs for uranyl ions occurred on a homogeneous surface by monolayer adsorption.
D.Effect of the temperature
Temperature is an important parameter for ion adsorption. In the early marine experiments in Japan,scientists found materials set in Okinawa showed higher adsorption capacity than that in the north Japan,indicating that high temperature took advantage for uranyl ion adsorption.It was also reported that higher temperature resulted in higher uranyl ion adsorbance for the amidoxime-type adsorbent in laboratory study[11]. These suggest that the adsorption procedure maybe endothermic.However,in this study,25?C was found to be the most optimal temperature for the adsorption of uranyl ions of ATADETA.The effect of the temperature on uranyl ions adsorption onto ATA-DETA is given in Fig.7(a).The pseudo secondorder curves were used to f i t the data obtained at different temperatures for the ATA-DETA adsorption,with good correlation coeff i cients(R2>0.98)(Fig.7(b)).The adsorption rateswere in the order of 25?C>35?C>15?C>5?C.The Arrhenius equation was used to study the temperature dependence of the reaction rate constant,but the activation energy(Ea)could not be calculated from this empirical relationship.This indicates the adsorption process involves both endothermic and exothermic in the complex step.As the dominant uranyl species in seawater are the carbonato complexes[17,18],the amidoxime must compete with and replace the carbonate groups in the sorption process.Literature reports suggest that the dissociation of the tricarbonato uranyl complex(UO2(CO3)34–)may be the rate-determining step[19].However,each step of the dissociation of the tricarbonato was not clear at present,nor the thermodynamics involved in dissociation of each carbonate.To learn more about the adsorption mechanism,a theoretical simulation will be included in our future study.
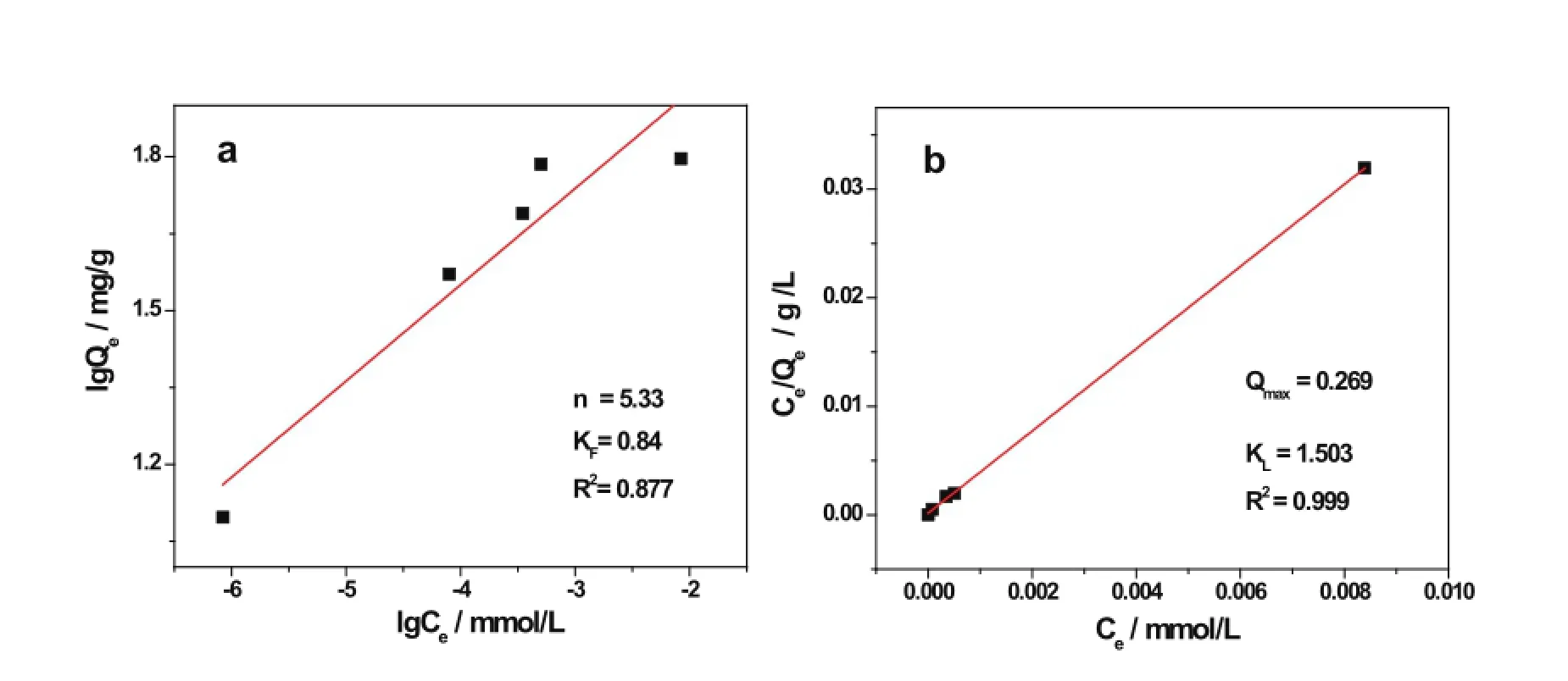
Fig.6.(Color online)Fitting of uranium adsorption on ATA-DETA(T=25?C,pH=8,t=8h)(a)Freundlich adsorption isotherm and(b) Langmuir isotherm.
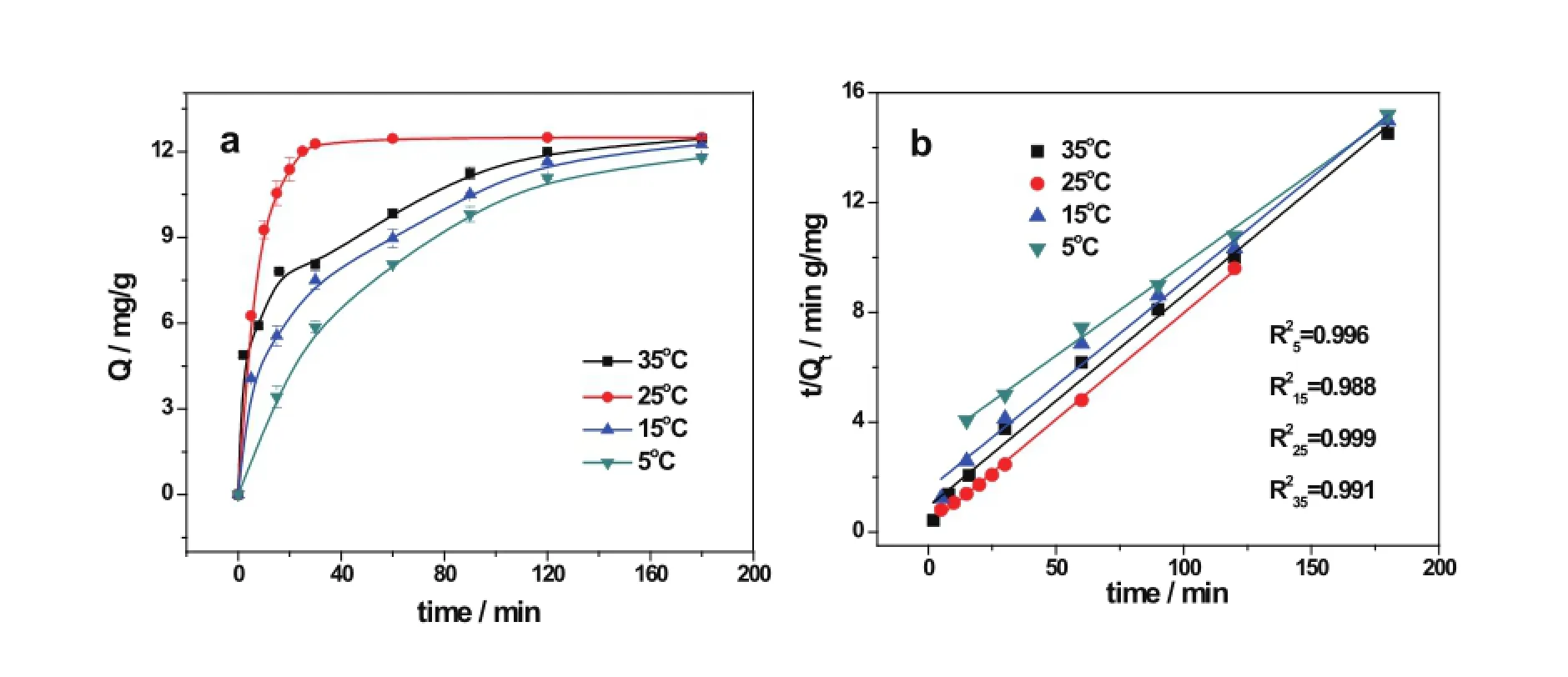
Fig.7.(Color online)(a)Adsorption kinetics of different temperatures with ATA-DETA(C0=1mgL?1;T=25?C;pH=8)(b)Linearized pseudo-second-order kinetic curves for uranyl ion adsortpion at different temperatures.
E.Effect of the pH value
The pH value plays a role in metal ions absorption because of the hydration and complex formation of metal ions.Although the pH value of the seawater is constant at about 7.5–8.5,looking at the effect of the pH value shall be of help to determine the desorption conditions.The results of pH inf l uence(pH 2.0–9.5)is given in Fig.8.
In the range of pH 4.0–8.5,the adsorption ratios and amount of the uranyl ions were almost the same,while a pH value over 8.5 resulted in a slight decrease.The adsorption ratio and adsorbance of uranyl ions dramatically decreased in an acid condition with a pH value below 4.At pH 2,the ATA-DETA is incapable of adsorbing the uranyl ions.
The pH effect can be explained in two ways.First,the existing form of(UO2)2+in aqueous solutions is extremely complex.U(VI)mostly exists as((UO2)2+)in its hydrolysis complexes,carbonate complexes and multinuclear hydroxide as a function of pH and concentration under experimental conditions[18,20–22].(UO2)2+were the main species in acidic solution at pH 2–4[23].At pH 4–8.5,(UO2)2+,[UO2OH]+, [(UO2)3O(OH)3]+,[UO2(CO3)],and[(UO2)2(OH)2]2+coexist[19–22].On the other hand,the protonation of amino groups depends greatly on the pH value of the solution,where the lone pair electrons on N are occupied by hydrogen,which makes coordination of amine absent in strong acid condition. This illustrates that desorption procedure can be carried out in acidic solution.
After adsorption equilibrium in 1mgL?1uranyl ions solution,desorption was carried out with HCl.The desorption ratio of uranyl ions from ATA-DETA under different concentrations of HCl is shown in Table 1.The results show that 0.1M HCl can remove 75.11%uranyl ions from ATA-DETA.Higher concentrationofHClisofhigherdesorptionratioand5MHClcan remove 93.67%uranyl ions from ATA-DETA.
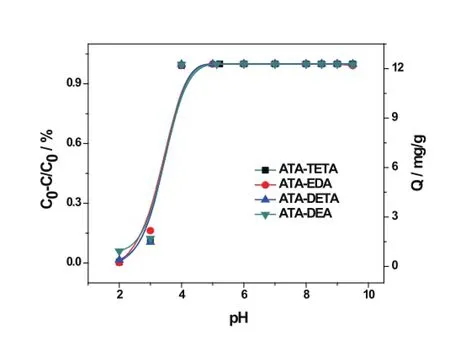
Fig.8.(Color online)Effect of solution pH on the equilibrium adsorption eff i ciency onto ATAs(C0=1mgL?1;T=25?C;t=24h).

TABLE 1.Desorption ratio of uranyl ions from ATA-DETA with different concentration of HCl
F.Effect of salinity
Salinity is important forthe recoveryor separation ofseawater uranium due to the high average salinity(3.5%)of seawater.Fig.9 shows the salinity effect on equilibrium adsorption amount.
The salinity was adjusted with NaCl.The adsorption of uranyl ion was affected a little at salinities below 1%,but it decreased quickly with increasing salinity from 1%to 4%,where it began to decrease slowly until 5%salinity,and reached an equilibrium at 6%salinity and 4.76mgg?1of the adsorption. It has been reported that the salinity had great effect on the adsorption of uranyl ions[15–17].However,with a 55%loss of capacityfromtheATA-DETAatsalinityof3.5%,thesaltresistance property of the adsorbents needs to be improved,which is a challenge of adsorbent design.
G.Effect of interfering ionsy
In a practical application,co-existing ions which would strongly interact with the amino groups will interfere with the adsorption of uranyl ions.Co2+,Fe3+,Zn2+,Ni2+and VO3–were investigated in this study as the concentration of Fe3+, Zn2+,Ni2+and VO3–and uranyl ions in seawater are at the same level of ppb(μgL?1).Moreover,the distribution coeff icient of Co2+,Fe3+and Ni2+from the amidoxime-type adsorbents are higher than that of uranyl ions[24].Vanadium also attracted wide attention recently as it can be collected on the adsorbent,butthevanadium elutionisdiff i cult.Theadsorption amount of uranyl ions in mix solutions at pH 8 decreased from 12.50mgg?1to 11.92mgg?1when the initial concentrations of interference were all set at 1mgL?1.Fig.10 indicates that the sequence of adsorption amount of metal ions is:UO22+≈Fe3+>Zn2+>VO3–>Co2+>Ni2+.The adsorption capacity of uranium was higher than vanadium,which is an advantage over amidoxime-type adsorbents[25].The interference ofFe3+,however,still presents challenges in the preparation of adsorbents with high selectivity.

Fig.9.Salinity effects on the equilibrium adsorption amount.(C0= 1mgL?1;T=25?C;t=24h)
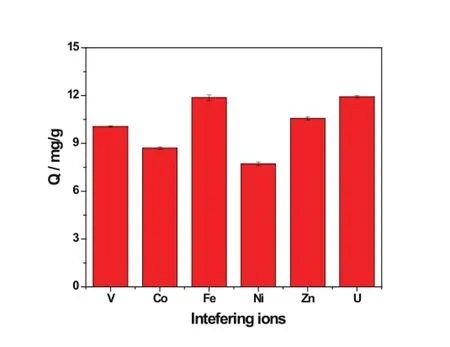
Fig.10.(Color online)Adsorption amount of different metal ions by ATA-DETA(C0=1mgL?1;T=25?C;t=24h,pH=8).
IV.CONCLUSION
ATAs exhibit excellent adsorption behavior of uranium with saturation sorption capacities as high as 64.26mgg?1from the ATA-DETA type adsorbent.The sorption kinetics followed a pseudo second-order kinetic model,while the sorption isotherm followed the Langmuir adsorption isotherm model.ATAs showed good adsorption capacity over a wide pH range of 4.0–8.5.Using 0.1M HCl,75.11%uranyl ion could be removed from the ATA-DETA,and the desorption ratio increased to 93.67%with 5M HCl.The most suitable temperature for the adsorption of uranyl ions was 25?C.A salinity of 3.5%resulted in a 55%loss of capacity from ATA-DETA in this study.The selectivity from ATA-DETA was in the order of UO22+≈Fe3+>Zn2+>VO3–>Co2+>Ni2+.The adsorption capacity of uranium was higher than that of vanadium,which is an advantage over other ATAs.The effect of Fe3+,however, remains a challenge.
[1]Schenk H J,Astheimer L,Witte E G,et al.Sep Sci Technol, 1982,17:1293–1308.
[2]Scanlan J P.J Inorg Nucl Chem,1977,39:635–639.
[3]Koske P H,Ohlrogge K,Peinemann KV.Sep Sci Technol,1988,23:1929–1940.
[4]Elnaggar I M,Elabsy M A,Abdelhamid M M,et al.Solvent Extr Ion Exch,1993,11:521–540.
[5]Williams W J and Gillam A H.Analyst,1978,103:1239–1243.
[6]Tabushi I,Kobuke Y,Nishiya T.Nature,1979,280:665–666.
[7]Egawa H,Kabay N,Jyo A,et al.Ind Eng Chem Res,1994,33: 657–661.
[8]Das S,Pandey A K,Athawale A,et al.Desalination,2008,232: 243–253.
[9]TamadaM,SekoN,YoshiiF.RadiatPhysChem,2004,71:223–227.
[10]Schierz A and Zanker H.Environ Pollut,2009,157:1088–1094.
[11]Liu X Y,Liu H Z,Ma H J,et al.Ind Eng Chem Res,2012,51: 15089–15095.
[12]Liu H Z,Yu M,Deng B,et al.Radiat Phys Chem,2012,81: 93–96.
[13]Ma H J,Yao S D,Li J Y,et al.Radiat Phys Chem,2012,81: 1393–1397.
[14]Parab H,Joshi S,Shenoy N,et al.Bioresour Technol,2005,96:1241–1248.
[15]McGinley P M,Katz L E,Weber W J.Water Resour Res,1996,32:3571–3577.
[16]Langmuir I.J Am Chem Soc,1918,40:1361–1403.
[17]Kyoichi S and Terukatsu M.J Nucl Sci Tech,1982,19:145–150.
[18]Bayramoglu G,Celik G,Arica M Y.J Hazard Mater,2006,137: 1689–1697.
[19]Takao A,Akira G,Tokihiro K.et al.Separation Sci Tech,1992,27:1655–1667.
[20]Kang M J,Han B E,Hahn P S.Environ Eng Res.2002,7:149–157.
[21]Meinrath G,Kato Y,Kimura T,et al.Radiochim Acta,1996,75: 159–167.
[22]Kilincarslan A,Akyil S.JRNC.,2005264:541–548.
[23]Ma H,Hoshina H,Seko N.J Appl Polym Sci,2013,128:4253–4260.
[24]Tamada M.Japan Atomic Energy Agency,2009.
[25]Huheey J M.Inorganic Chemistry,3rd,New York:Harper& Row,1983.
10.13538/j.1001-8042/nst.25.010302
(Received October 15,2013;accepted in revised form December 2,2013;published online February 20,2014)
?Supported by National Natural Science Foundation of China(Nos. 11175234 and 11105210),the“Strategic Priority Research Program”of the Chinese Academy of Sciences(No.XDA02030200),the“Knowledge Innovation Program”of the Chinese Academy of Sciences(No.KJCX2-YW-N49),and Shanghai Municipal Commission for Science and Technology(Nos.11ZR1445400 and 12ZR1453300)
?Corresponding author,wangmin@sinap.ac.cn
?Corresponding author,jingyeli@sinap.ac.cn
 Nuclear Science and Techniques2014年1期
Nuclear Science and Techniques2014年1期
- Nuclear Science and Techniques的其它文章
- Using activation method to measure neutron spectrum in an irradiation chamber of a research reactor?
- A neural network to predict reactor core behaviors?
- Research on GPU-accelerated algorithm in 3D f i nite difference neutron diffusion calculation method?
- Development of a three dimension multi-physics code for molten salt fast reactor?
- Performance simulation and structure design of Binode CdZnTe gamma-ray detector
- Effectiveness and failure modes of error correcting code in industrial 65 nm CMOS SRAMs exposed to heavy ions?
