Kinetic and Thermodynamic Studies of Acid Scarlet 3R Adsorption onto Low-cost Adsorbent Developed from Sludge and Straw*
(任曉莉)**(楊玲)(劉敏)
Department of Environmental and Safety Engineering, Taiyuan Institute of Technology, Taiyuan 030008, China
Kinetic and Thermodynamic Studies of Acid Scarlet 3R Adsorption onto Low-cost Adsorbent Developed from Sludge and Straw*
REN Xiaoli(任曉莉)**, YANG Ling(楊玲)and LIU Min(劉敏)
Department of Environmental and Safety Engineering, Taiyuan Institute of Technology, Taiyuan 030008, China
A low-cost adsorbent was prepared from sludge and straw by pyrolysis in a dried state with the surface area of the adsorbent of 829.49 m2·g?1, micropore volume of 0.176 cm2·g?1and average pore radius of 5.0 nm. The kinetic, equilibrium isotherm and thermodynamic characteristics of trisodium 1-(1-naphthylazo)-2-hydroxynaphthalene-4′,6,8-trisulphonate (acid scarlet 3R) onto the adsorbent from sludge and straw were investigated. The results indicated that the pseudo second order adsorption was the predominant adsorption mechanism of acid scarlet 3R. Thus, the adsorption phenomenon was suggested as a chemical process. The adsorption data were fitted better with Langmuir model than Freundlich model, indicating that the adsorption of acid scarlet 3R belonged to the monolayer adsorption and mainly occurred in micropores.
kinetic, thermodynamic, acid scarlet 3R, adsorbent, sludge, straw
1 INTRODUCTION
Sewage sludge is the inevitable by-products of wastewater purification. The world is currently undergoing a rapid increase in sludge production that is expected to continue for many years [1]. Sewage sludge is composed largely of the substances responsible for the offensive, pathogenic and toxic characteristics of untreated wastewater. Therefore, if proper treatment disposal is not implemented, this sludge will lead to serious environmental pollution. Various methods have been used to dispose or utilize municipal sewage sludge including incineration, land filling, land application, road surfacing, conversion to fertilizer, compression into building blocks, etc [2]. However, the environmental and legislative constraints, increasing sludge production, more limited disposal options and reducing availability of land call for more efficient and environmentally friendly approaches to its utilization [3-6]. It is well known that sewage sludge is carbonaceous in nature. Hence, it might be promising to prepare adsorbent from sewage sludge [7-9].
Dye wastewater discharged from dyeing industries is highly colored and is toxic to aquatic life in the receiving water bodies. However, wastewater containing dyes is difficult to treat, since the dyes are recalcitrant organic molecules, resistant to aerobic digestion and are stable to light, heat and oxidizing agents. Adsorption technology is considered to be most effective and proven technology for applications in dye wastewater treatment. An alternative treatment for the discoloration of wastewaters from the textile industry is the usage of some non-conventional adsorbents (natural materials, bio-adsorbents and waste materials from industry and agriculture) with lower cost and high efficiency [10-16].
In this work, excess sludge and straw were prepared into adsorbents by chemical activation. In order to improve the adsorption performance of the adsorbents and reduce heat energy and activating agent consumption, the immersion method was substituted with a mixing method that consisted of mixing dried sludge with solid ZnCl2. Sludge-derived absorbents were investigated for kinetic, equilibrium isotherm and thermodynamic studies of acid red 3R adsorption.
2 MATERIALS AND METHODS
2.1 Materials
Dewatered surplus sludge (SS) was collected from the Northern Suburb Municipal Wastewater Treatment Plant of Taiyuan, China. Proximate analysis of the raw materials showed water content of 76%, an ash content of 47.1%, a volatile mass of 47.55%, and fixed carbon of 5.35% (dry basis).
The molecular formula of trisodium 1-(1-naphthylazo)-2-hydroxynaphthalene-4′,6,8-trisulphonate (acid scarlet 3R) is C20H11N2Na3O10S3, molecular mass is 604.48, and the UV-VIS spectrophotometer UV-2100PC scanning spectrum indicated that the maximum absorption wavelength was 506 nm.
2.2 Methods
The study of adsorption kinetics and thermodynamics is essential in supplying the fundamental information required for the design and operation of adsorption equipment for wastewater treatment.
2.2.1 Adsorption kinetics experimentation
The dye solutions were taken in a 250 ml Erlenmeyer flask for conducting the tests. The varying initial concentration was 50 mg·L?1, 100 mg·L?1and 150 mg·L?1with the adsorbent dosage of 0.2 g. The mixturewas agitated in an orbital shaker at a constant speed of 120 r·min?1for 24 h at a temperature of 20 °C. At predefined time intervals the solutions of the specified flask were separated from the adsorbent materials. The residual concentration of acid scarlet 3R was determined by using a UV-VIS spectrophotometer at a wavelength of 506 nm. The adsorption capacity (qt, mg·g?1) at predefined moments was calculated by using Eq. (1):
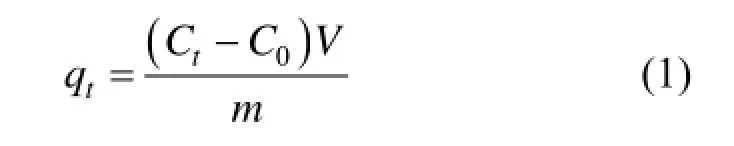
where qtis monolayer adsorption capacity of the adsorbent at time (mg·g?1), C0is initial dye concentration (mg·L?1), Ctis dye concentration at time (mg·L?1), m is adsorbent mass (g) and V is volume of the adsorbate solution (L).
2.2.2 Adsorption thermodynamic experimentation
The dye solutions were put in a 250 ml Erlenmeyer flask for conducting the tests. The initial concentration varied from 50 mg·L?1to 400 mg·L?1with an adsorbent dosage of 0.2 g. The experiments were carried out at 20 °C, 30 °C and 40 °C, respectively. The dye concentration retained in the adsorbent phase (qe, mg·g?1) was calculated by using Eq. (2):
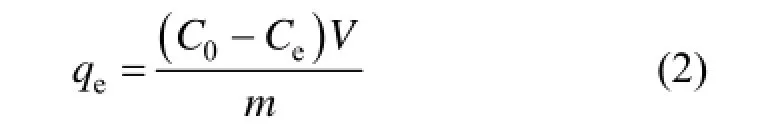
where C0is the initial dye concentration (mg·L?1), Ceis the equilibrium concentration of the dye (mg·L?1), m is the adsorbent mass (g), V is volume of the adsorbate solution (L) and qeis the adsorption capacity of the adsorbent at equilibrium (mg·g?1).
3 PREPARATION OF ADSORBENT
Dried sludge samples and straw were crushed to an average particle size of 75 μm and mixed with solid ZnCl2to a final concentration of 30% (g·g?1). The mixture was pyrolyzed in a stainless steel container (60 mm inside diametor) in a hypoxic environment at 400 °C for 120 min. The resulting sample was washed several times with 3 mol·L?1HCl and rinsed with deionized water until the pH of the washing effluent reached between 6 and 7. The product was then dried to constant mass in a drum drier at 105 °C.
The physical characteristics of the adsorbents, including the specific surface area, pore volume distribution and pore diameter, were measured with a surface area analyzer (ASAP 2020 M, Micro-meritics Instrument Co., USA) by using the adsorption isotherms for gas adsorption (N2, 77 K) [17]. The specific surface area of the adsorbent was 829.49 m2which was calculated by using the Langmuir equation. Micropore volume (Vmi) was 0.176 cm3·g?1obtained by the t-plot method. Desorption average pore radius (2 V·A?1) was 5.0 nm calculated by the Barrette Joyner Halenda (BJH) method.
4 RESULTS AND DISCUSSION
4.1 Adsorption kinetics studies
The influence of adsorption time on adsorption capacities of acid scarlet 3R were investigated with three dye concentration of 50 mg·L?1, 100 mg·L?1, and 150 mg·L?1at 25 °C and 4 g·L?1adsorbent dosage. The results are shown in Fig. 1.
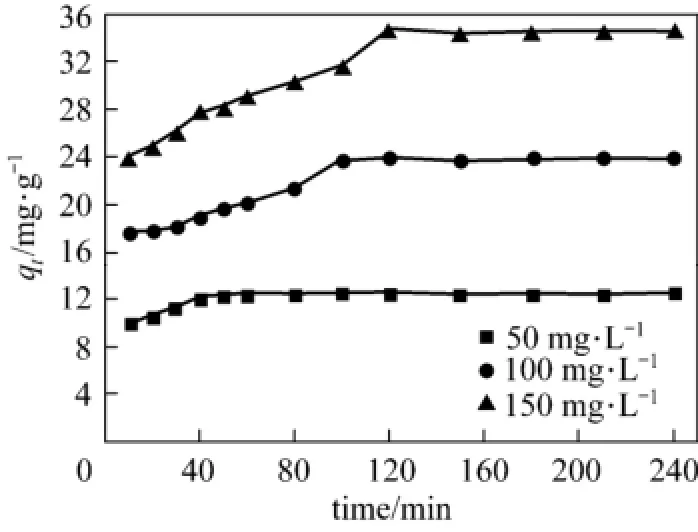
Figure 1 qt-t curve of acid scarlet 3R on low-cost adsorbent
Figure 1 reveals that with the increase in concentration, the equilibrium adsorption capacity of acid scarlet 3R onto the adsorbent also increases. Furthermore, the lower the concentration, the shorter time it needs to reach the equilibrium.
In order to comprehensively study adsorption kinetics characteristics of acid scarlet 3R onto the adsorbent and find the most suitable the kinetics model to describe the adsorption process, this paper chooses the pseudo first order kinetics equation, pseudo second kinetics equation, and particle diffusion equation to fit adsorption kinetics data, respectively. The suitability of three kinds of kinetics models was determined according to the linear correlation coefficient R of the fitting equation.
The pseudo first order kinetics equation has been widely used to describe adsorption dynamics. The model assumes that the adsorption is a pseudo chemical reaction process and the limiting factor for adsorption is the resistance of mass transfer inside granules. Its expression is as follows:
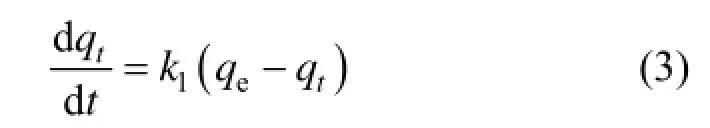
The boundary condition: t=0 and qt=0.
Integrating Eq. (3) and applying the initial conditions, we have:
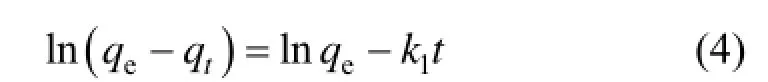
where k1is the adsorption rate constant of pseudo first order (min?1).
The pseudo second order kinetics equation has also been widely used to describe the adsorption dynamics. The model also assumes that adsorption is a pseudo chemical reaction process. However, unlike the pseudo first order kinetics equation, this modeldescribes the whole process of adsorption and assumes that the limiting factor for adsorption is the adsorption mechanism rather than the resistance of mass transfer inside granules. The model of pseudo second order kinetics is expressed as Eq. (5):
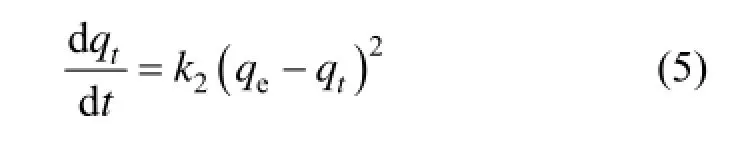
The boundary condition is t=0 and qt=0.
After definite integration by applying the initial conditions, Eq. (5) becomes:
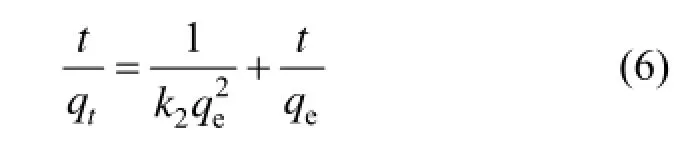
where k2is the adsorption rate constant of pseudo second order (g·mg?1·min?1).
Intra-particle diffusion process is often the rate-controlling step in many adsorption processes. The possibility of intra-particle diffusion was explored by using Eq. (7):

where kpis the adsorption rate constant of the intra-particle diffusion (mg·g?1·min?0.5). Fitting the adsorption kinetics data of acid scarlet 3R with the above motioned three kinetics models, the linear fitting curve and fitting equation can be obtained. The equation parameters can also be calculated by Eqs. (3)-(7) and the results are shown in Figs. 2 to 4 as well as Table 1.
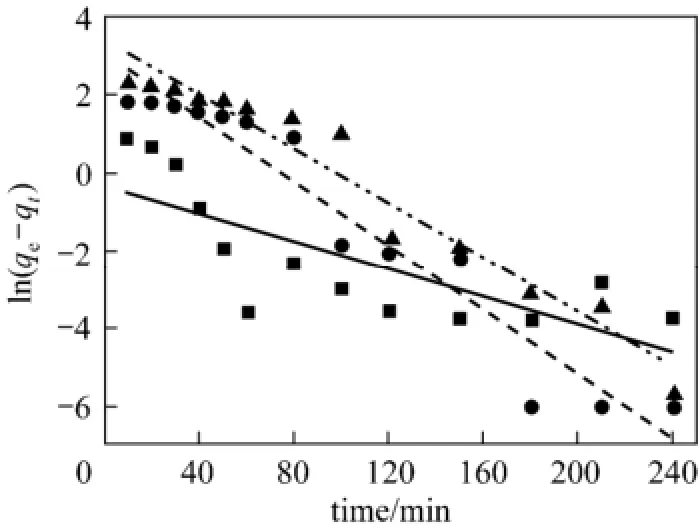
Figure 2 Pseudo first order kinetics fitting curve of acid scarlet 3R on low-cost adsorbent■ 50 mg·L?1, Exp.; ● 100 mg·L?1, Exp.; ▲ 150 mg·L?1, Exp.;50 mg·L?1, model;100 mg·L?1, model;150 mg·L?1, model
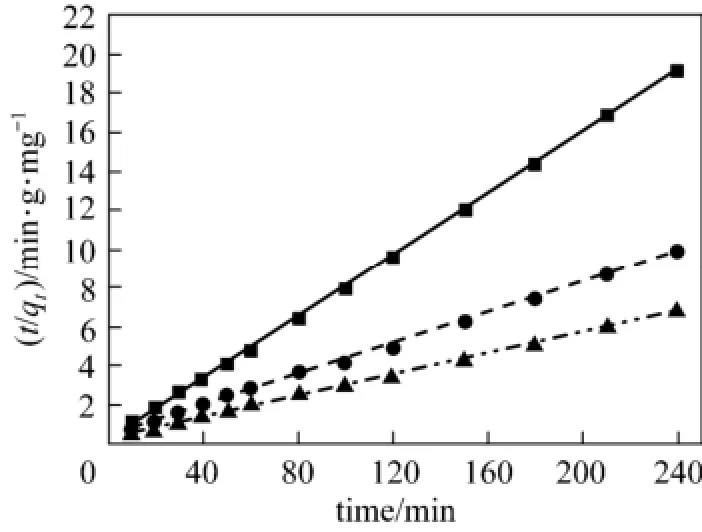
Figure 3 Pseudo second order kinetics fitting curve of acid scarlet 3R on low-cost adsorbent■ 50 mg·L?1, Exp.; ● 100 mg·L?1, Exp.; ▲ 150 mg·L?1, Exp.;50 mg·L?1, model;100 mg·L?1, model;150 mg·L?1, model
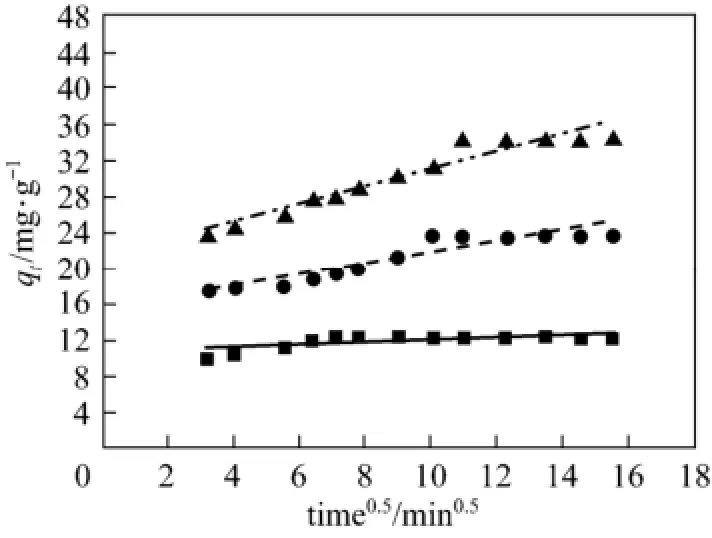
Figure 4 Diffusion equation fitting curve of acid scarlet 3R on low-cost adsorbent■ 50 mg·L?1, Exp.; ● 100 mg·L?1, Exp.; ▲ 150 mg·L?1, Exp.;50 mg·L?1, model;100 mg·L?1, model;150 mg·L?1, model
From Figs. 2 to 4 and Table 1, the best model to generate a good fit to the experimental data is the pseudo-second-order model (R2>0.99). It shows that acid scarlet 3R adsorption mechanism on the sludgestraw adsorbent includes all the adsorption process, such as external liquid film diffusion, surface adsorption, and intra-particle diffusion. In addition, the maximum adsorption capacity obtained by experiment is close to that calculated by model, which further verified the validity of the above mentioned conclusion.
According to the linear regression correlation coefficient R2values (R2: 0.91-0.93), the intra-particle diffusion model shows limited applicability for theadsorption process. The fitting lines of qtvs. t0.5do not pass through the origin (C≠0), indicating that the presence of intra-particle diffusion process is one of the rate-controlling steps, besides many other processes controlling the rate of adsorption, such as surface adsorption and liquid film diffusion control, all of which are more likely to be operating simultaneously. These findings are in agreement with those obtained by other workers [16, 18].
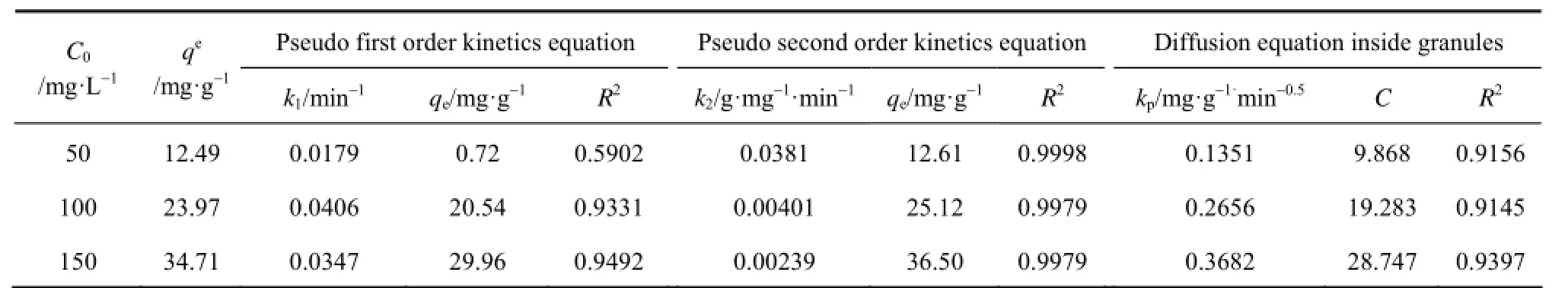
Table 1 Fitting kinetics parameters of acid scarlet 3R
4.2 Adsorption thermodynamics studies
The adsorption of acid scarlet 3R onto the adsorbent was investigated at 20 °C, 30 °C and 40 °C, respectively. The concentration of acid scarlet 3R varied from 50 mg·L?1to 400 mg·L?1. Primary discussion for the adsorption mechanism of acid scarlet 3R was progressed.
The commonly used adsorption isotherm equations are the Langmuir isothermal adsorption equation and Freundlich isothermal adsorption equation.
The Langmuir isothermal adsorption equation assumes that the adsorption is a chemical process, one adsorption site only adsorbs one molecule adsorbate, that is to say, the adsorption is assumed to occur at a specific uniformity site on the surface of the adsorbent. Langmuir isothermal adsorption equation is mainly used to describe the single molecular layer adsorption. Its linear expression is as follows:
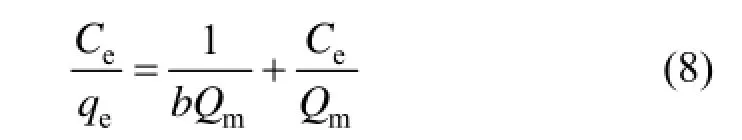
where Ceis the equilibrium concentration (mg·L?1), b is the Langmuir constant (L·mg?1) and Qmis the saturated absorption capacity (mg·g?1).
Freundlich isothermal adsorption equation is an empirical formula, which describes equilibrium on heterogeneous surfaces and hence does not assume monolayer capacity. The linear expression is as follows:
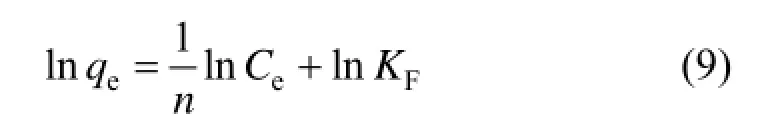
where KFis the Freundlich constants, 1/n is the index of concentration, KFand 1/n are indicators of the adsorption capacity and adsorption intensity. 1/n values between 0.1 and 0.5 represent good adsorption potential of the adsorbent.
The adsorption experiments of acid scarlet 3R were conducted at 20 °C, 30 °C and 40 °C, respectively. The experimental data were fitted using Eqs. (8) and (9) respectively. The fitting isotherm equations are shown in Figs. 5 and 6.
From Figs. 5 and 6, the experimental data are better fitted to the Langmuir isotherm than Freundlich isotherm. It indicates that the adsorption of acid scarlet 3R belongs to the monolayer adsorption and mainly occurs in micropores.
The parameters of the fitting isotherm equation are shown in Tables 2 and 3.
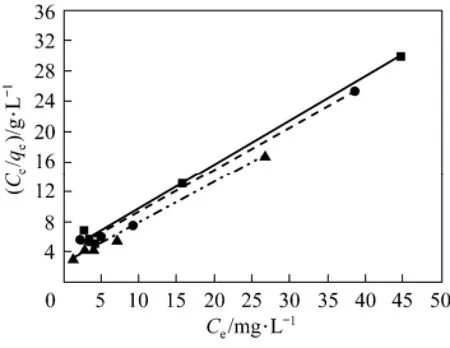
Figure 5 Fitting curve of Freundlich isotherm equation of acid scarlet 3R on low-cost adsorbent■ 20 °C, Exp.; ● 30 °C, Exp.; ▲ 40 °C, Exp.;?20 °C, model;?30 °C, model; ? 40 °C, model
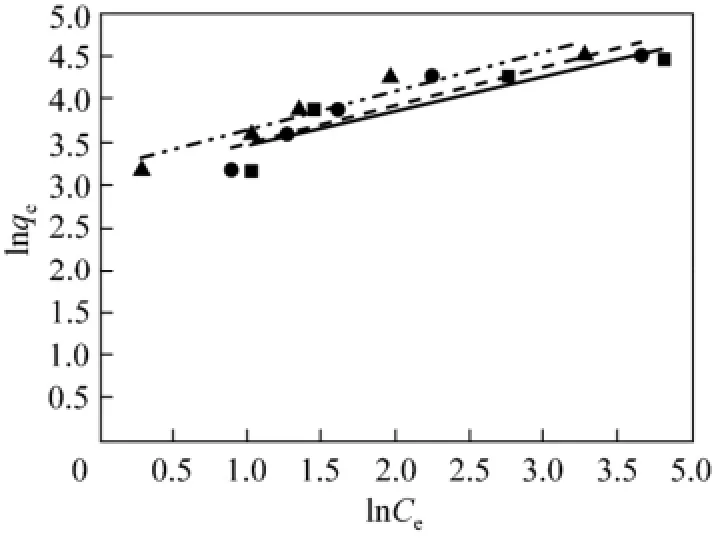
Figure 6 Fitting curve of Freundlich isotherm equation of acid scarlet 3R on low-cost adsorbent■ 20 °C, Exp.; ● 30 °C, Exp.; ▲ 40 °C, Exp.;20 °C, model;30 °C, model;40 °C, model
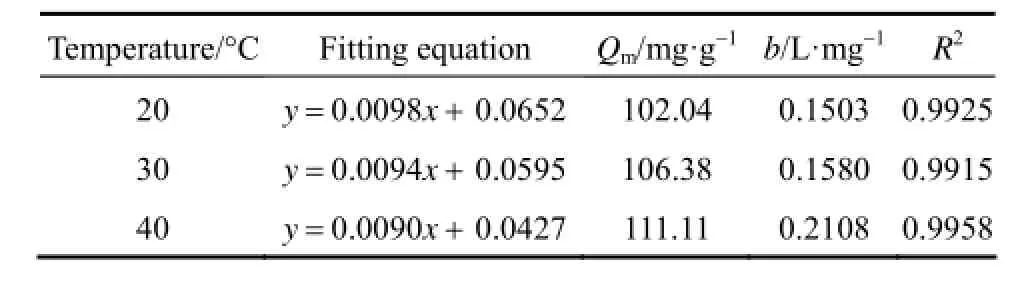
Table 2 Fitting parameters of Langmuir isotherm equation
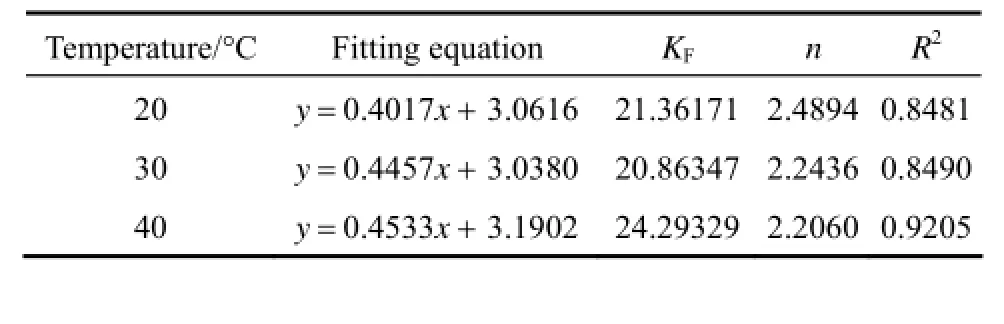
Table 3 Fitting parameters of Freundlich isotherm equation
From Tables 2 and 3, it can be seen that Langmuir equation parameters Qmincrease in accordance with the increase of temperature, which implies that the adsorption of acid scarlet 3R onto the adsorbent is an endothermic process where a higher temperature is more conducive to adsorption. The concentration index of the Freundlich model 1/n is between 0.1 and 0.5. Thus, it is easier to adsorb acid scarlet 3R from wastewater.
Wang et al. [18] have studied adsorption kinetics of methylene blue, crystal violet and basic fuchsine onto dried waste sludge. The experimental data were analyzed by using several kinetic equations. The results showed that the adsorption of respective dye could be best described by pseudo second order equation. The diffusion process inside granules played an important role in adsorption and Langmuir isotherm was found to be more suitable than Freundlich isotherm for correlation of equilibrium data. Similar results were shown by Phanikumar [13], Yong [14], Yue [16], Zhang [19] and Gupta [20] in the removal of dyes. The above mentioned results were basically consistent with the conclusion of this study.
4.3 Adsorption effects of sludge-straw adsorbent
The adsorption effects of acid scarlet 3R onto the sludge-straw adsorbent were investigated. A comparative study with a commercial coal-based activated carbon (the iodine value of 950 mg·g?1) was conducted. The experimental conditions were as follows: pH of 3, adsorption time of 2 h, adsorption temperature of 26 °C and initial concentration of 250 mg·L?1. Fig. 7 shows the decolorization rate of acid scarlet 3R onto sludge-straw adsorbent and commercial activated carbon at different dosages.
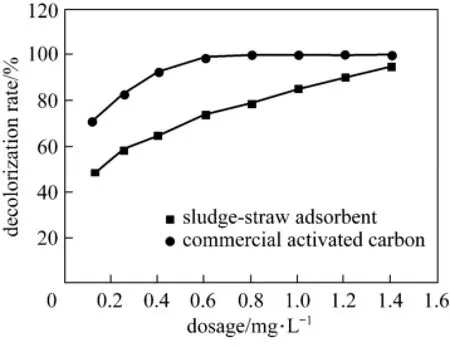
Figure 7 Decolorization rate of acid scarlet 3R at different dosage
Both sludge-straw adsorbent and commercial activated carbon have good performance for acid scarlet 3R adsorption. Although the adsorption capacity of acid scarlet 3R onto sludge-straw adsorbent is inferior to that of commercial activated carbon at the same dosage, the decolorization rate of acid scarlet 3R onto sludge-straw adsorbent increases to 94.77% close to that of commercial activated carbon when the dosage is up to 1.4 g·L?1. Commercial activated carbon was prepared from high quality coal and expensive for dye removal in wastewater, while sludge-straw adsorbent was prepared from solid waste and relatively cheap compared with commercial activated carbon. The potential lower cost of the sludge-straw adsorbent might make it more attractive for practical applications.
5 CONCLUSIONS
The studies indicated that the adsorbent developed from sludge and straw was potential to act as an active carbon for the removal of the acid scarlet 3R from colored wastewater. The kinetics and thermodynamic experimentation were investigated, finding that the adsorption was best suited to the pseudo second order kinetics equation with higher correlation coefficients R and the thermodynamic data were also correlated better with the Langmuir model than the Freundlich isotherm model.
REFERENCES
1 Méndez, A., Gascó, G., Freitas, M.M.A., Siebielec, G., Stuczynski, T., Figueiredoa, J.L., “Preparation of carbon-based adsorbents from pyrolysis and air activation of sewage sludges”, Chem. Eng. J., 108, 169-177 (2005).
2 Hwang, H.R., Choi, W.J., Kim, T.J., Kim, J.S., Oh, K.J., “The preparation of an adsorbent from mixtures of sewage sludge and coal-tar pitch using an alkaline hydroxide activation agent”, J. Anal. Appl. Pyrol., 83, 220-226 (2008).
3 Martin, M.J., Serra, E., Ros, A., Balaguer, M.D., Rigola, M., “Carbonaceous adsorbents from sewage sludge and their application in a combined activated sludge-powdered activated carbon (AS-PAC) treatment”, Carbon, 42, 1389-1394 (2004).
4 Fang, P., Cen, C.P., Chen, D.S., Tang, Z.X., “Carbonaceous adsorbents prepared from sewage sludge and its application for Hg0adsorption in simulated flue gas”, Chin. J. Chem. Eng., 18 (2), 231-238 (2010).
5 Otero, M., Rozada, F., Calvo, L.F., García, A.I., Morán, A., “Elimination of organic water pollutants using adsorbents obtained from sewage sludge”, Dyes and Pigm., 57, 55-65 (2003).
6 Xu, G.R., Zhang, W.T., Li, G.B., “Adsorbent obtained from CEPT sludge in wastewater chemically enhanced treatment”, Water Res., 39, 5175-5185 (2005).
7 Pan, Z.H., Tian, J.Y., Xu, G.R., Li, J.J., Li, G.B., “Characteristics of adsorbents made from biological, chemical and hybrid sludges and their effect on organics removal in wastewater treatment”, Water Res., 45, 819-827 (2011).
8 Ren, X.L., Liang, B.H., Liu, M., Xu, X.Y., Cui, M.H., “Effects of pyrolysis temper ature, time and leaf litter and powder coal ash addition on sludge-derived adsorbents for nitrogen oxide”, Bioresour. Technol., 125, 300-304 (2012).
9 Anfruns, A., María, M.J., Montes-Morán, M.A., “Removal of odourous VOCs using sludge-based adsorbents”, Chem. Eng. J., 166, 1022-1031 (2011).
10 Shukla, A., Zhang, Y.H., Dubey, P., Shyam, S.S., “The role of sawdust in the removal of unwanted materials from water”, J. Hazard. Mater., 95 (1/2), 137-152 (2002).
11 Forgacs, E., Cserhati, T., Oros, G., “Removal of synthetic dyes from wastewaters: A review”, Environ. Int., 30 (7), 953-971 (2004).
12 Crini, G., “Non-conventional low-cost adsorbents for dye removal: A review”, Bioresour. Technol., 97 (9), 1061-1085 (2006).
13 Geethakarthi, A., Phanikumar, B.R., “Adsorption of reactive dyes from aqueous solutions by tannery sludge developed activated carbon: Kinetic and equilibrium studies”, J. Environ. Sci. Tech., 8 (3), 561-570 (2011).
14 Fan, H., Yang, J.S., Gao, T.G., Yong, H.L., “Removal of low-moleculor basic dye (azure blue) from aqueous solutions by anative biomass of a newly isolated adsorption sp.: Kinetics equilibrium and biosorption simulation”, J. Taiwan Ins. Chem. Eng., 43, 382-392 (2012).
15 Nandi, B.K., Goswami, A., Purkait, M.K., “Adsorption characteristics of brilliant green dye on kaolin”, J. Hazard. Mater., 161 (1), 387-395 (2009).
16 Xie, J.K., Yue, Q.Y., Gao, B.Y., Li, Q., “Adsorption kinetics and thermodynamics of anionic dyes onto sewage sludge derived activated carbon”, Int. J. Environment and Pollution, 45 (1-3), 123-144 (2011).
17 Chiang, H.M., Chen, T.C., Pan, S.D., Chiang, H.L., “Adsorption characteristics of orange II and chrysophenine on sludge adsorbent and activated carbon fibers”, J. Hazard. Mater., 161 (2/3), 1384-1390 (2009).
18 Wang, X.S., Lin, H.Q., “Adsorption of basic dyes by dried waste sludge: Kinetic, equilibrium and desorption studies”, Desalin. and Water Treat., 29, 10-19 (2011).
19 Zhang, L.N., Chen, Q.Y., Ke, Y.J., “Investigation on preparation and decolorization for dye waste water of activated carbon from sludge”, Saf. Environ. Eng., 16, 44-47 (2009).
20 Gupta, V.K., Jain, R., Siddiqui, M.N., Saleh, T.A., Agarwal, S., Malati, S., Pathak, D., “Equilibrium and thermodynamic studies on the adsorption of the dye Rhodamine-B onto mustard cake and activated carbon”, J. Chem. Eng. Data, 55, 5225-5229 (2010).
Received 2013-06-24, accepted 2013-10-09.
* Supported by the Shanxi Science and Technology Agency Research Project (20100321085) and the Scientific Research Foundation of the Shanxi Education Department (20111029).
** To whom correspondence should be addressed. E-mail: xlren66@126.com
 Chinese Journal of Chemical Engineering2014年2期
Chinese Journal of Chemical Engineering2014年2期
- Chinese Journal of Chemical Engineering的其它文章
- Kinetics of Glucose Ethanolysis Catalyzed by Extremely Low Sulfuric Acid in Ethanol Medium*
- Synthesis of Sub-micrometer Lithium Iron Phosphate Particles for Lithium Ion Battery by Using Supercritical Hydrothermal Method
- Hydrogenation of Silicon Tetrachloride in Microwave Plasma
- Effects of Solvent and Impurities on Crystal Morphology of Zinc Lactate Trihydrate*
- Large-eddy Simulation of Ethanol Spray-Air Combustion and Its Experimental Validation*
- Performance of EDAB-HCl Acid Blended System as Fracturing Fluids in Oil Fields*
