Production of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by Recombinant Pseudomonas stutzeri 1317 from Unrelated Carbon Sources*
WEI Xiaoxing (魏曉星), LIU Feng (劉峰), JIAN Jia (簡嘉), WANG Ruiyan (王瑞妍)and CHEN Guoqiang (陳國強),4,**
1Department of Basic Medicine, Medical College of Qinghai University, Xining 810016, China
2MOE Key Lab of Bioinformatics and Systems Biology, Department of Biological Science and Biotechnology, School of Life Sciences, Tsinghua-Peking Center for Life Sciences, Tsinghua University, Beijing 100084, China
3Tuspark Kunshan Biotech Science Park Development Center, 4/F, Science & Technology Plaza, Tsinghua Science Park, Kunshan, Jiangsu 215347, China
4Center for Nano and Micro Mechanics, Tsinghua University, Beijing 100084, China
Production of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by Recombinant Pseudomonas stutzeri 1317 from Unrelated Carbon Sources*
WEI Xiaoxing (魏曉星)1,2, LIU Feng (劉峰)2,3, JIAN Jia (簡嘉)2, WANG Ruiyan (王瑞妍)2and CHEN Guoqiang (陳國強)2,4,**
1Department of Basic Medicine, Medical College of Qinghai University, Xining 810016, China
2MOE Key Lab of Bioinformatics and Systems Biology, Department of Biological Science and Biotechnology, School of Life Sciences, Tsinghua-Peking Center for Life Sciences, Tsinghua University, Beijing 100084, China
3Tuspark Kunshan Biotech Science Park Development Center, 4/F, Science & Technology Plaza, Tsinghua Science Park, Kunshan, Jiangsu 215347, China
4Center for Nano and Micro Mechanics, Tsinghua University, Beijing 100084, China
Synthetic biology promises to simplify the construction of metabolic pathways by assembling the detached modules of the whole pathway. This gives new approaches for the microbial production of industrial products such as polyhydroxyalkanoates (PHA). In this study, to produce poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) by Pseudomonas stutzeri 1317 from unrelated carbon sources such as glucose, the phaC1-phaZ-phaC2 operon of P. stutzeri 1317 was knocked out to generate the PHA deficient mutant P. stutzeri 1317LF. Then three modules containing phaCAhAReBRe, phaCAhBReGPpand phaCAhPAhwere introduced into P. stutzeri 1317LF separately. The shake flask results indicated that the precursor supply and PHA synthase activity were the vital factors for the PHBHHx accumulation of P. stutzeri 1317LF. Furthermore, the PHBHHx accumulation of the recombinants from different carbon resources were performed. The highest PHBHHx content was 23.7% (by mass) with 58.6% (by mole) 3HB fraction. These results provide basis for further improving the PHBHHx accumulation of P. stutzeri from unrelated carbon sources.
Pseudomonas stutzeri 1317, PHBHHx, unrelated carbon sources
1INTRODUCTION
Polyhydroxyalkanoates (PHA) are biodegradable polyesters synthesized by many microorganisms as carbon and energy storage substances under certain stress conditions [1, 2]. PHA has attracted increasing interests not only for its similar properties to petrochemical plastics but also its excellent biocompatibility, chirality and high combustion value. It can be widely applied in the fields of new materials, tissue engineering, pharmacy and bio-energy [3-5]. According to the monomer compositions, PHA can be categorized into three classes: short-chain-length (SCL) PHA, mediumchain-length (MCL) PHA and PHA consisting of both SCL and MCL monomers (SCL-MCL PHA) [6]. One of the SCL-MCL PHA copolymers, poly(3-hydroxybutyrateco-3-hydroxyhexanoate) (PHBHHx) consisting of 3-hydroxybutyrate (3HB) and 3-hydroxyhexanoate (3HHx) monomers is considered as the third-generation commercial PHA product. Compared with other PHAs, PHBHHx shows better biocompatibility and material processing properties [5, 7]. However, PHBHHx is mainly produced from expensive substrates such as lauric acid, the high cost of which greatly limits the industrial promotion and application of PHBHHx [8]. Reducing the cost of PHBHHx production by using proper strains and unrelated carbon sources, such as glucose or gluconate, has been the focus of the research.
Aeromonas hydrophila 4AK4 is a commonly used strain for PHBHHx production, because the PHA synthase from A. hydrophila 4AK4 shows the specificity for accumulating PHA with C4-C6 monomers [9]. However, it can only utilize lauric acid as carbon sources but not glucose or any other unrelated carbon sources [10]. Pseudomonas stutzeri 1317 has been studied for accumulating PHA, which contains complicated monomer compositions from the related or unrelated carbon sources [11-13]. Combining the ability of P. stutzeri 1317 to provide 3HA precursors from unrelated carbon sources and the specificity of PHA synthase from A. hydrophila 4AK4 on C4-C6 monomers will be advantageous for the production of PHBHHx from cheap substrates and thus reduce the cost of PHBHHx production.
Synthetic biology offers efficient and versatile DNA assembly systems to facilitate the building of new modules/pathways from basic DNA parts in a standardized way. The modules with specific functions are integrated according to pre-designed order for new biological functions [14]. In this paper, a new PHBHHx biosynthesis pathway is designed in P. stutzeri 1317LF, a phaC deficient mutant of P. stutzeri 1317.
The designed PHBHHx biosynthesis pathway in P. stutzeri 1317LF can be constructed as Fig. 1 (a). The3HB monomers can be generated by glycolysis pathway. Two acetyl-CoA molecules can be condensed into acetoacetyl-CoA, which is then reduced to generate 3-hydroxybutyryl-CoA with the participation of the enzyme β-ketothiolase and acetoacetyl-CoA reductase encoded by phaAReBRefrom Ralstonia eutropha. The 3HHx precursors can be produced from the fatty acid de novo biosynthetic pathway. The (R)-3-hydroxyacyl in this pathway is converted from their acyl carrier protein (ACP) form to the CoA form catalyzed by the enzyme acyl-ACP: CoA transacylase, encoded by phaG from P. putida. The PHA synthase from A. hydrophila 4AK4 finally catalyzes the polymerization. The phasin gene, phaP, from A. hydrophila 4AK4 can be used for regulating the expression of phaC to enhance the PHBHHx accumulation.
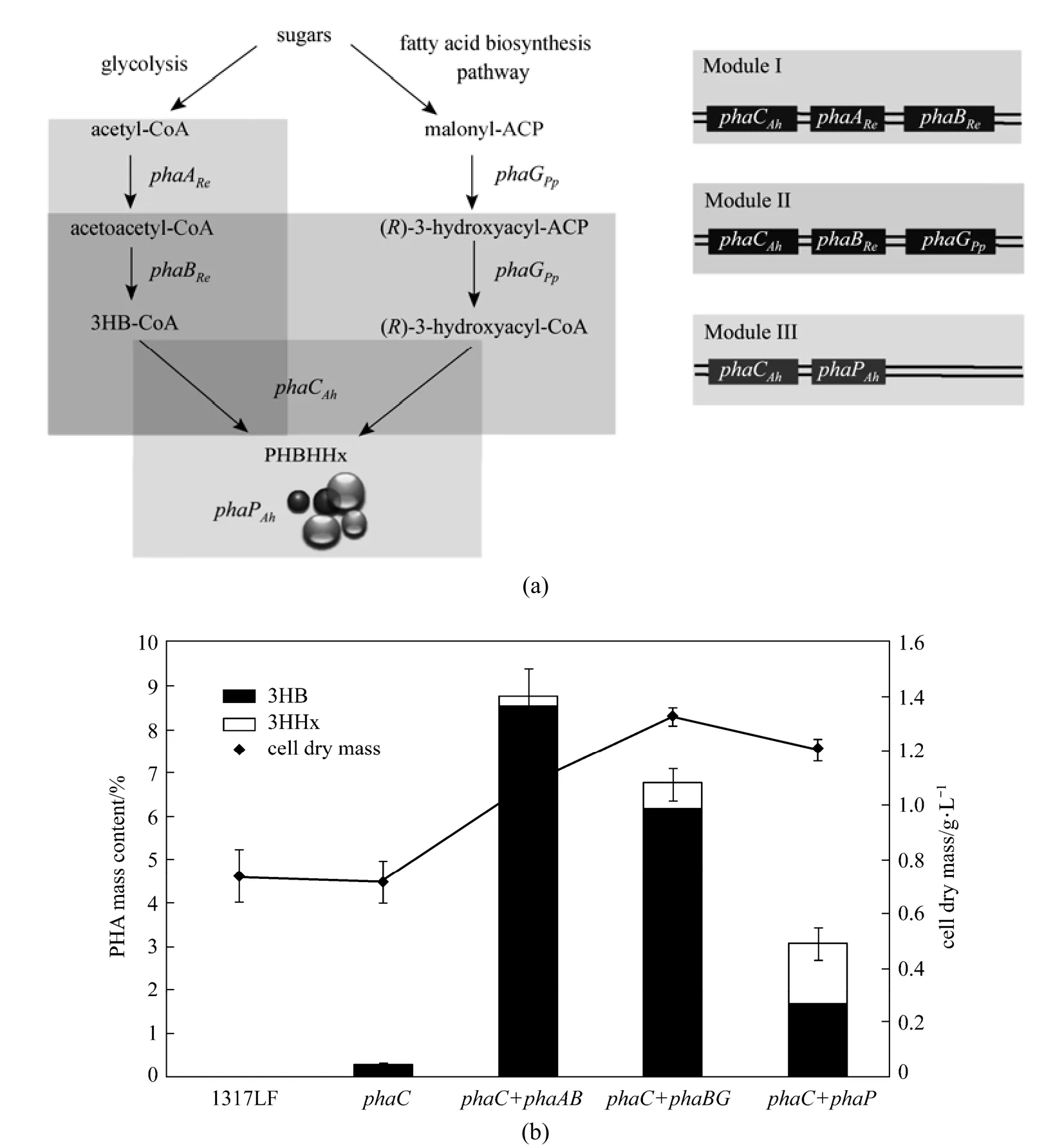
Figure 1Engineered PHBHHx synthesis pathway and PHBHHx production in P. stutzeri 1317LF and its recombinants(a) The PHBHHx synthesis pathway and constructed modules, with genes: phaCAh, PHA synthase from A. hydrophila with C4-C6 substrate specificity; phaARe, β-ketothiolase from R. eutropha; phaBRe, NADPH dependent acetoacetyl-CoA reductase from R. eutropha; phaGPp, acyl-ACP: CoA transacylase from P. putida; phaPAh, phasin gene from A. hydrophila; (b) The PHBHHx production of P. stutzeri 1317LF and its recombinants, with strains cultured in MS medium containing 20 g·L?1glucose and 0.5 g·L?1(NH4)2SO4at 30 °C, 200 r·min?1for 48 h
For the PHBHHx biosynthesis pathway in P. stutzeri 1317 described above, each of the five genes can be regarded as a unit and assembled separately to form three different functional modules including phaCAhAReBRe, phaCAhBReGPpand phaCAhPAh. In this study, the native phaC1-phaZ-phaC2 operon that encodes PHA synthase is deleted from the genome of P. stutzeri 1317 to obtain P. stutzeri 1317LF. Then the three modules are heterologously introduced into the P. stutzeri 1317LF strain to facilitate the production of PHBHHx from glucose and other unrelated carbon sources.
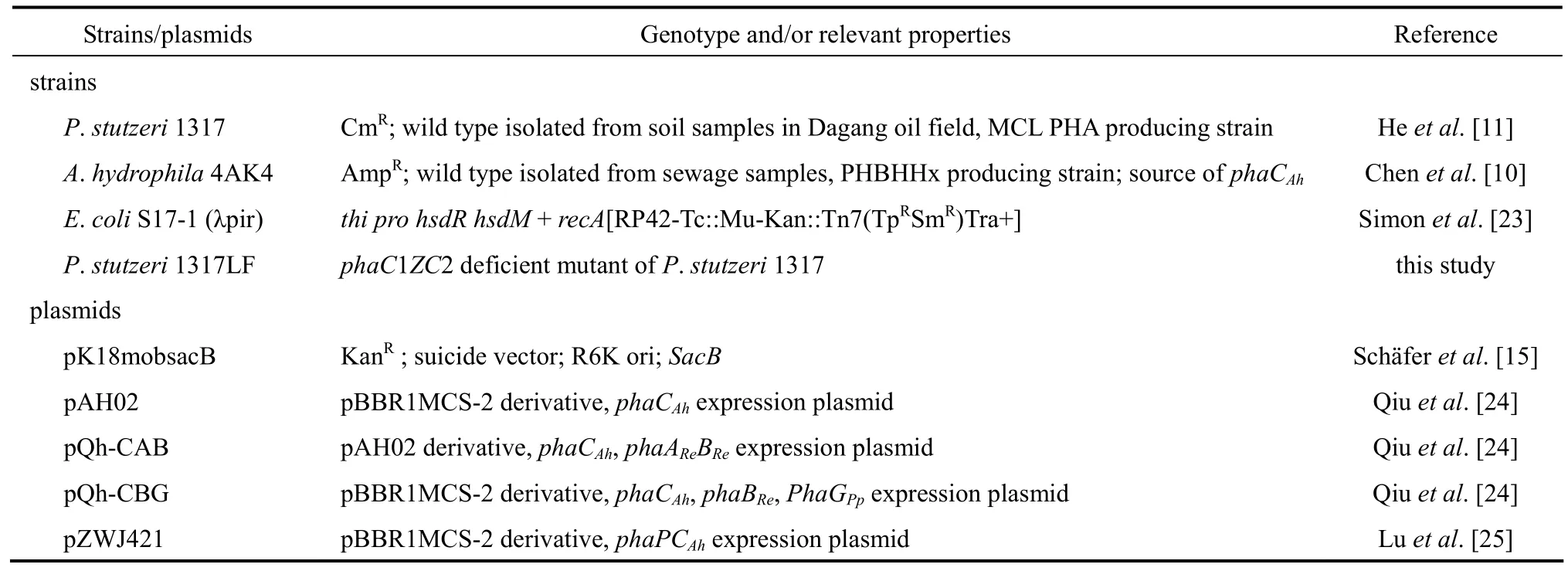
Table 1Strains and plasmids used in this study
2MATERIALS AND METHOD
All bacterial strains and plasmids used in this study were described in previous studies (Table 1) except strain P. stutzeri 1317LF, which was constructed in this study. The PHA synthase gene operon phaC1-phaZ-phaC2 in P. stutzeri was deleted through the homologous double-exchange method described by Sch?fer et al [15-17]. The shake flask and cultural conditions for the recombinant P. stutzeri strains were determined as previously described [18]. PHA accumulation and monomer composition were analyzed by gas chromatography (GC) [19].
3RESULTS AND DISCUSSION
3.1Construction of P. stutzeri mutant strain with phaC1-phaZ-phaC2 deletion
The wild type strain of P. stutzeri was found to be able to synthesize mcl-PHA through de novo fatty acid biosynthetic pathway with glucose as the sole carbon source. In this study, phaC1ZC2-deficient mutant P. stutzeri 1317LF was constructed by gene deletion and this mutant strain failed to accumulate PHA [Fig. 1 (b)].
3.2Heterologous expression of three modules containing phaCAhAReBRe, phaCAhBReGPpand phaCAhPAhin P. stutzeri1317LF
In order to accumulate PHBHHx through fatty acid de novo synthetic pathway, phaCAhgene from A. hydrophila 4AK4 was incorporated to P. stutzeri 1317LF via the plasmid pAH02. Shake flask results are shown in Fig. 1 (b). The introduction of pha CAhgene restored the PHA-producing ability of the mutant P. stutzeri 1317LF from glucose, but it only accumulated a small amount of PHBHHx [0.23% (by mass) of CDW]. Probably, insufficient supply of intracellular 3-hydroxybutyrate (3HB) and 3-hydroxy acid (3HHx) as PHBHHx precursors may result in the low PHBHHx accumulation. To explore the effect of precursor supply, two modules containing phaCAhAReBReand phaCAhBReGPpwere introduced into P. stutzeri 1317LF separately to enhance the PHBHHx production [Fig. 1 (a)]. Plasmid pQh-CAB was constructed harboring phaCAhencoding PHA synthase from A. hydrophila 4AK4 and phaAReBReencoding β-ketothiolase and acetoacetyl-CoA reductase from R. eutropha, to provide 3-hydroxybutyryl-CoA from acetyl-CoA. Meanwhile, pQh-CBG was constructed containing phaCAhphaBReand phaGPp, which encodes acyl-ACP: CoA transacylase from P. putida, in order to increase 3-hydroxybutyryl-CoA by phaBReand the native phaA from P. stutzeri and 3-hydroxyhexanoyl-CoA from fatty acid de novo biosynthesis pathway by phaGPp. Shake flask results revealed that the expression of phaCAhAReBRein
P. stutzeri 1317LF improved the 3HB precursor supply, resulting in 8.75% (by mass) PHBHHx containing 98.45% (by mole) 3HB. When phaCAhBReGPpwas expressed in P. stutzeri 1317LF, both 3HB and 3HHx precursors increased. On this basis, the PHBHHx accumulation achieved 6.77% (by mass) with 6.45% (by mole) 3HHx [Fig. 1 (b)]. Although phaAPsand phaBPsgenes were found in the genome of P. stutzeri [20], only a small amount of 3HB monomers can be produced by P. stutzeri 1317LF. Expression of phaAReBReor phaBRealone improved the 3HB precursor supply, resulting in the production of 8.61% (by mass) and 6.33% (by mass) 3HB, respectively. This result indicates that native PhaA and PhaB exhibit low enzyme activities in P. stutzeri 1317, so that 3HB precursor is provided ineffectively. PhaGPpfrom P. putida KT2440 is a 3-hydroxyacyl- ACP: CoA transferase with a widerange substrate specificity for C6-C14 substances [21]. Heterologous expression of PhaGPpgene significantly increases the 3HHx monomers for PHBHHx accumulation in P. stutzeri 1317LF.
Studies showed that phasin gene, phaP, canregulate the gene expression of phaC and contribute to the accumulation of PHA [22]. To study the effect of phaPAhgene on the PHBHHx accumulation of P. stutzeri 1317LF with phaCAh, plasmid pZWJ421 harboring phaCAhand phaPAhthat encodes phasin gene from A. hydrophila was constructed and studied in P. stutzeri 1317LF [Fig. 1 (a)]. The results showed 3.05% (by mass) of PHBHHx accumulation in glucose medium [Fig. 1 (b)]. Compared with the trace amount of PHA synthesized in P. stutzeri 1317LF (phaCAh), incorporation with phaP gene enhanced the efficiency of phaCAhand the PHBHHx accumulation from glucose. In view of the above, low activity of PHA polymerase was another possible reason for trace amount of PHA accumulation in P. stutzeri 1317LF (ph aCAh).
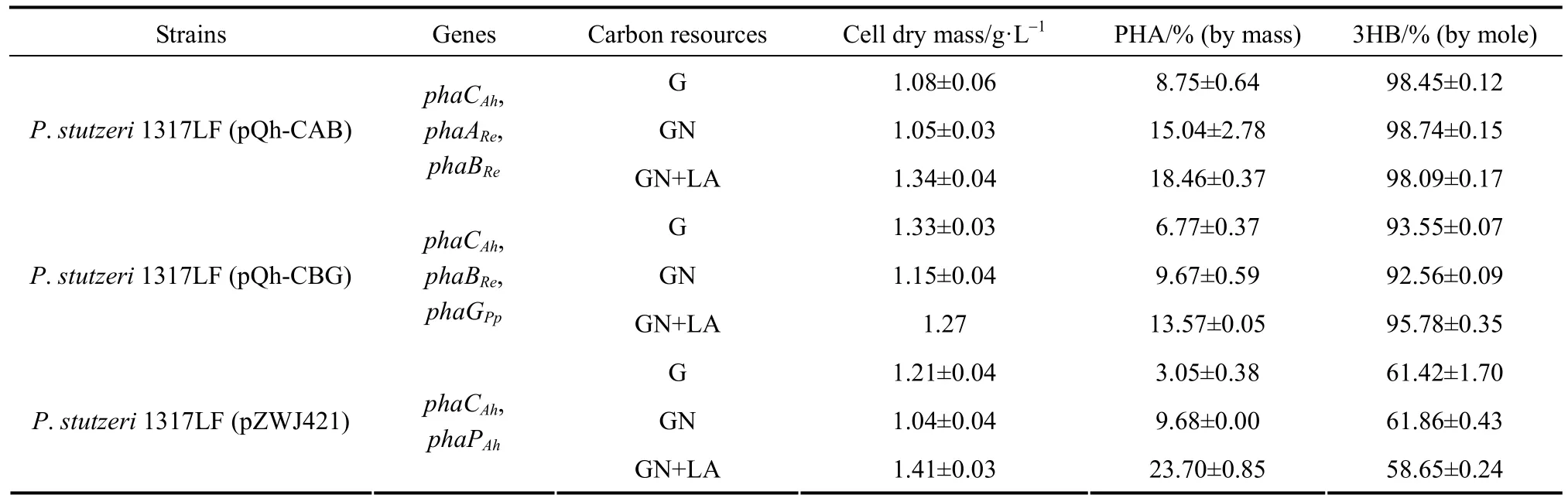
Table 2The PHBHHx production of P. stutzeri 1317LF recombinants from different carbon sources
3.3PHBHHx accumulation in recombinant P. stutzeri 1317LF with different carbon sources
The recombinant P. stutzeri 1317LF strains with different plasmids, P. stutzeri 1317LF (pQh-CAB), P. stutzeri 1317LF (pQh-CBG) and P. stutzeri 1317LF (pZWJ421), were studied for PHBHHx synthesis from glucose. In addition, the utilization of other unrelated carbon sources was further studied in these three strains. The results are given in Table 2. Shake flask results showed that no significant effect was found on the monomer composition of PHBHHx with different carbon sources. Compared with using glucose as the carbon source, using gluconate remarkably increased the production of PHBHHx, though the cell dry mass of these strains decreased slightly. The contents of PHBHHx in P. stutzeri 1317LF (pQh-CAB), P. stutzeri 1317LF (pQh-CBG) and P. stutzeri 1317LF (pZWJ421) achieved 15.04% (by mass), 9.67% (by mass) and 9.68% (by mass), respectively. The amounts of accumulated PHBHHx increased by 67.11%, 23.50% and 172.79%, respectively, as compared with using glucose medium as the carbon source. The results showed that gluconate was a better carbon source candidate for PHBHHx accumulation in the recombinant P. stutzeri. When a small amount of lauric acid (0.5 g·L?1) was added in the culture medium, the intracellular PHBHHx content could be improved greatly. In P. stutzeri 1317LF (phaPCAh), the cellular dry mass and cellular PHBHHx content achieved 1.41 g·L?1and 23.70% (by mass), respectively. This suggests that PHBHHx accumulation in the recombinant P. stutzeri 1317LF can be further improved with low-cost carbon sources such as unrelated carbon sources, providing a higher industrial value.
4CONCLUSIONS
In this study, three modules containing phaCAhAReBRe, phaCAhBReGPpand phaCAhPAhwere constructed and introduced into the phaC1-phaZ-C2 operon deficient mutant strain P. stutzeri 1317LF. The PHBHHx synthesis of all the recombinant strains from unrelated carbon sources such as glucose was studied. The gluconate was found to be a better carbon source for PHBHHx accumulation than glucose, and the lauric acid could significantly increase the PHBHHx content. Through improving the precursors of PHBHHx in recombinant strains and enhancing the PHA synthase activity, the PHBHHx production was increased. The results provide basis for further improvement of PHBHHx production from unrelated carbon sources in P. stutzeri 1317. In summary, the recombinant P. stutzeri 1317LF appears to be a good candidate strain for production of PHBHHx.
ACKNOWLEDGEMENTS
We are grateful to professor Andreas Sch?fer for the generous donation of vector pK18mobsacB. And we also thank QIU Yuanzheng and LU Xiaoyun for the plasmids used in this paper.
REFERENCES
1 Chen, G.Q., “A microbial polyhydroalkanoates (PHA) based bioand materials industry”, Chem. Soc. Rev.,38, 2434-2446 (2009).
2 Dowes, E.A., Senior, P.J., “The role and regulation of energy reserve polymers in microorganisms”, Ad v. Microb. Physiol.,10, 135-266 (1973).
3 Chen, G.Q., Luo, R.C., Xu, J., Wu, Q., Microbial PHA Based Eco-Material Industry, Chemical Industry Press, Beijing, 1-21 (2008). (in Chinese)
4 Chen, G.Q., Wu, Q., “The application of polyhydroxyalkanoates as tissue engineering materials”, Biomaterials,26, 6565-6578 (2005).
5 Li, Z.J., Wei, X.X., Chen, G.Q., “Microbial cell factories for production of polyhydroxyalkanoates”, Chin. J. Biotechnol.,26, 1426-1435 (2010).
6 Steinbüchel, A., “Perspectives for biotechnological production and utilization of biopolymers: metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example”, Macromol. Biosci.,1, 1-24 (2001).
7 Wu, Q., Wang, Y., Chen, G.Q., “Medical application of microbial biopolyesters polyhydroxyalkanoates”, Artif. Cells Blood Substit. Immobil. Biotechnol.,37, 1-12 (2009).
8 Liu, F., Jian, J., Shen, X.W., Chung, A., Chen, J.C., Chen, G.Q.,“Metabolic engineering of Aeromonas hydrophila 4AK4 for production of copolymers of 3-hydroxybutyrate and medium-chain-length 3-hydroxyalkanoate”, Bioresour. Technol.,102, 8123-8129 (2011).
9 Lu, X.Y., Zhang, W.J., Jian, J., Wu, Q., Chen, G.Q., “Molecular cloning and functional analysis of two polyhydroxyalkanoate synthases from two strains of Aeromonas hydrophila spp.”, FEMS Microbiol. Lett.,243, 149-155 (2005).
10 Chen, G.Q., Zhang, G., Park, S.J., Lee, S.Y., “Industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)”, Appl. Microbiol. Biotechnol.,57, 50-55 (2001).
11 He, W.N., Tian, W.D., Zhang, G., Chen, G.Q., Zhang, Z.M., “Production of novel polyhydroxyalkanoates by Pseudomonas stutzeri 1317 from glucose and soybean oil”, FEMS. Microbiol. Lett.,169, 45-49 (1998).
12 Chen, J.Y., Liu, T., Zheng, Z. Chen, J.C., Chen, G.Q., “Polyhydroxyalkanoate synthases phaC1 and phaC2 from Pseudomonas stutzeri 1317 had different substrate specificities”, FE MS. Microbiol. Lett.,234, 231-237 (2004).
13 Chen, G.Q., Xu, J., Wu, Q., Zhang, Z.M. Ho, K.P., “Synthesis of copolyesters consisting of medium-chain-length β-hydroxyalkanoates by Pseudomonas stutzeri 1317”. React. Funct. Poly.,48, 107-112 (2001).
14 Benner, S.A., Sismour, A.M., “Synthetic biology”, Nat. Rev. Genet.,6, 533-543 (2005).
15 Sch?fer, A., Tauch, A., J?ger, W., Kalinowski, J., Thierbach, G., Pühler, A., “Small mobilizable multi-purpose cloning vectors derived from the Escherich ia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum”, Gene.,145, 69-73 (1994).
16 Chen, J.Y., Song, G., Chen, G.Q., “A lower specificity phaC2 synthase from Pseudomonas stutzeri catalyses the production of copolyesters consisting of short-chain-length and medium-chain-length 3-hydroxyalkanoates”, Anton Leeuw. Int. J. G.,89, 157-167 (2006).
17 Cai, L., Yuan, M.Q., Liu, F., Chen, G.Q., “Enhanced production of medium-chain-length polyhydroxyalkanoates (PHA) by PHA depolymerase knockout mutant of Pseudomonas putida KT2442”, Bioresour. Technol.,100, 2265-2270 (2009).
18 Chung, A., Liu, Q., Ouyang, S.P., Wu, Q., Chen, G.Q., “Microbial production of 3-hydroxydodecanoic acid by pha operon and fadBA knockout mutant of Pseudomonas putida KT2442 harboring tesB gene”, Appl. Microbiol. Biotechnol.,83, 513-519 (2009).
19 Wei, X.X., Shi, Z.Y., Yuan, M.Q., Chen, G.Q., “Effect of anaerobic promoters on the microaerobic production of polyhydroxybutyrate (PHB) in recombinant Escherichia coli”, Appl. Microbiol. Biotechnol.,82, 703-712 (2009).
20 Yan, Y.L., Yang, J., Dou, Y.T., Chen, M., Ping, S.Z., Peng, J.P., Zhang, W., Yao, Z.Y., Li, H.Q., Liu, W., He, S., Geng, L.Z., Zhang, X.B., Yang, F., Yu, H.Y., Zhan, Y.H., Li, D.H., Lin, Z.L., Wang, Y.P., Elmerich, C., Lin, M., Jin, Q., “Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501”, Proc. Natl. Acad. Sci. USA,105, 7564-7569 (2008).
21 Rehm, B.H.A., Kruger, N., Steinbüchel, A., “A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis: the phaG gene from Pseudomonas putida KT2440 encodes a 3-hydroxyacyl-acyl carrier protein coenzyme A transferase”, J. Biol. Chem.,273, 24044-24051 (1998).
22 Fukui, T., Kichise, T., Iwata, T., Doi, Y., “Characterization of 13 kDa granule-associated protein in Aeromonas caviae and biosynthesis of polyhydroxy-alkanoates with altered molar composition by recombinant bacteria”, Biomacromolecules,2, 148-153 (2001).
23 Simon, R., Priefer, U., Pühler, A., “A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria”, Nat. Biotechnol.,1, 784-791 (1983).
24 Qiu, Y.Z., Han, J., Guo, J.J., Chen, G.Q., “Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from gluconate and glucose by recombinant Aeromonas hydrophila and Pseudomonas putida”, Biotechnol. Lett.,27, 1381-1386 (2005).
25 Lu, X.Y., Wu, Q., Zhang, W.J., Jian, J., Chen, G.Q., “Studies on synthesis of polyhydroxyalkanoate consisting of 3-hydroxyvalerate by Aeromonas hydrophila”, Chin. J. Biotechnol.,20, 779-783 (2004).
10.1016/S1004-9541(13)60549-2
2012-09-27, accepted 2013-02-28.
* Supported by the National Natural Science Foundation of China (31260015), Natural Science Foundation of Qinghai Province (2012-Z-919Q), the Extramural Project from State Key Laboratory for Agrobiotechnology (2012SKLAB06-5) and the Research Funds for Young Project of Qinghai University (2011-QYY-1).
** To whom correspondence should be addressed. E-mail: chengq@mail.tsinghua.edu.cn
 Chinese Journal of Chemical Engineering2013年9期
Chinese Journal of Chemical Engineering2013年9期
- Chinese Journal of Chemical Engineering的其它文章
- Soft Sensor for Inputs and Parameters Using Nonlinear Singular State Observer in Chemical Processes*
- A New Tuning Method for Two-Degree-of-Freedom Internal Model Control under Parametric Uncertainty*
- Halloysite Nanotube Composited Thermo-responsive Hydrogel System for Controlled-release*
- Recent Advances in Separation of Bioactive Natural Products*
- A Novel γ-Alumina Supported Fe-Mo Bimetallic Catalyst for Reverse Water Gas Shift Reaction*
- Experimental and Theoretical Studies of CO2Absorption Enhancement by Nano-Al2O3and Carbon Nanotube Particles
