Experimental and Theoretical Studies of CO2Absorption Enhancement by Nano-Al2O3and Carbon Nanotube Particles
LU Sumin (盧素敏)*, XING Min (邢敏), SUN Yan (孫燕) and DONG Xiangjun (董相均)
Department of Environmental and Chemical Engineering, Tianjin Polytechnic University, Tianji n 300387, China
Experimental and Theoretical Studies of CO2Absorption Enhancement by Nano-Al2O3and Carbon Nanotube Particles
LU Sumin (盧素敏)*, XING Min (邢敏), SUN Yan (孫燕) and DONG Xiangjun (董相均)
Department of Environmental and Chemical Engineering, Tianjin Polytechnic University, Tianji n 300387, China
The influence of nano-particles on CO2absorption was studied experimentally in a stirred thermostatic reactor. Nano-Al2O3and carbon nanotube (CNT) particles which showed different hydrophobic properties were chosen for the investigation. The experimental results were compared with that of micron-size activated carbon (AC) and Al2O3particles. From the results, no enhancement by micron-size Al2O3was found, and with the increase of Al2O3concentration, the enhancement factor decreased. However, nano-Al2O3showed a weak enhancement for the CO2absorption. AC and CNT particles all intensified the gas-liquid mass transfer effectively, yet the trend of the enhancement factor with stirring speed for the two particles was different. With increasing stirring speed, the enhancement factor of AC particles was decreased, whereas in CNT suspensions it was increased. The experimental phenomena demonstrated a difference in enhancement mechanism for different size particles. For nano-particles, besides the influence of adsorbability and hydrophobicity, the micro-convection caused by Brownian motion should be also taken into account. Considering the micro-convection effect, a theoretical model was developed to shed light on the absorption enhancement by nano-particles.
absorption enhancement, nano-particles, enhancement factor, Brownian motion
1INTRODUCTION
Many researches reported that the absorption of sparingly soluble gas can be enhanced by the addition of a third dispersed phase, e.g. droplets or solid particles [1-5]. An important application example is that in the presence of n-dodecane and perfluorocarbon (F66E), the O2mass-transfer rate was greatly increased [6]. The particles used were mostly in the micron-size range. Various homogeneous and heterogeneous models were proposed to explain the enhancement mechanism [7, 8].
With the development of the synthesis technology of the nanometer materials and the wide application of nanotechnology, the investigation of nanoparticles in the enhancement of mass and heat transfer has received considerable attention in recent years. Especially in the application of the enhancement of heat transfer, many researches showed that the heat transfer performance of the base fluid could be improved significantly by adding nano-particles [9-13]. The study on the enhancement of mass transfer, however, is relatively insufficient. Krishnamurthy et al. [14] firstly observed the diffusion of fluorescein in liquids containing Al2O3nanoparticles by visualization in 2006. The fluorescein diffused much faster in nanofluids than in pure water. A binary nanofluid of Cu and CuO was prepared by Kim and Kang et al. [15, 16], and the change of NH3bubble in nanofluids was recorded by optical visualization. NH3bubbles disappeared much faster in nanofluids than that in water without nanoparticles, indicating an enhanced effect of nanoparitcles. Olle et al. [17] measured the oxygen absorption rate in the presence of colloidal dispersions of magnetite (Fe3O4) nanoparticles coated with oleic acid. They obtained that the nanoparticles improved the O2volumetric mass transfer coefficient kLa up to 6-fold at nanoparticle volume below 1% in an agitated, sparged reactor.
The enhancement mechanism of nanoparticles remains unclear at present. Different from micron-size particles, nanometer particles agglomerate more easily due to the high surface energy, which may have an unfavorable influence on the mass transfer enhancement. Therefore, it is unreasonable to explain the nanoparticle enhancement mechanism with classical macroscopic theories. In this paper, to investigate the mass transfer property of nanoparticles, the absorption of CO2in the presence of carbon nano-tubes (CNT) and nanometer Al2O3particles was investigated and a comparison with micron-particles [activated carbon (AC) and Al2O3particles] was made. A 3-D heterogeneous mathematical model was developed to predict the mass transfer enhancement factor in the presence of nanometer size particles. The model not only considered the adsorption of the particles, but also analyzed the micro-convection caused by Brownian motion.
2EXPERIMENTAL
The absorption experiments were carried out in a thermostatic stainless steel reactor with a volume of 1 L (Fig. 1). The inner diameter of the reactor is 85 mm and the height is 200 mm. Four symmetrical baffles were mounted to prevent the formation of a large vortex around the stirrer shaft.Two blade stirrers with diameters of 30 mm were installed on the same shaft to mix gas and liquid phases respectively. A cooling coil in the vessel is connected to the thermostatic bath to maintain a constant reaction temperature (298 K±0.1 K).
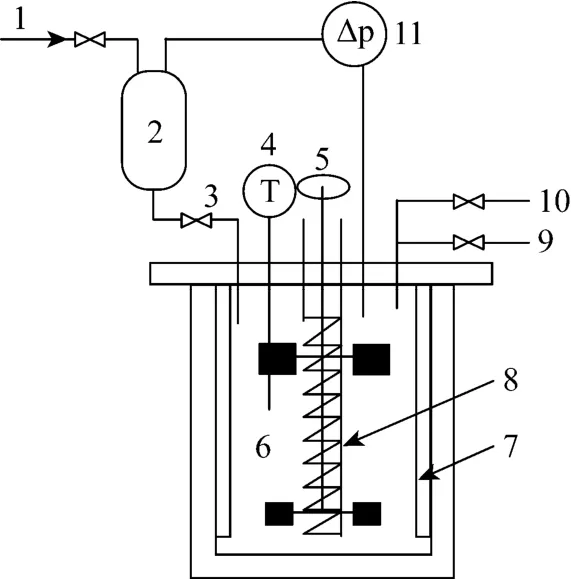
Figure 1Experimental set-up for gas absorption1—inlet gas; 2—balance tank; 3—junction valve; 4—temperature transmitter; 5—magnetic stirrer; 6—stainless steel reactor; 7—baffles; 8—cooling coil; 9—gas outlet; 10—vacuum; 11—pressure difference transmitter (connected with the computer)
The initial pressure has a positive effect on the absorption rate, and 0.1 MPa was used in the experiments. Before each experiment, about 0.6 L liquid is added into the reactor 6, the liquid was vacuum-degassed by opening valve 10 until the slurry is equilibrated under the water vapor pressure, then valve 10 is closed. Open valves 1 and 3 and CO2in gas cylinder is fed into up to a fixed pressure. Valves 1 and 3 are then closed rapidly. Turn on the motor of the magnetic stirrer and the absorption is started. Vessel 2 is a reference vessel, and a pressure difference transducer is connected between vessel 2 and reactor 6 to indicate the growing pressure difference due to the absorption of CO2in the reactor. The transducer signal was transmitted to the computer and recorded. With the value of the recorded pressure difference, the absorption rate can be calculated. The experimental operating conditions were listed in Table 1.
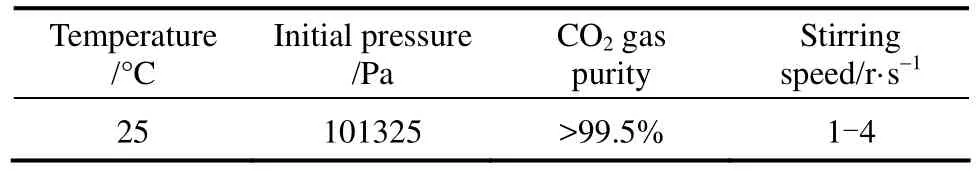
Table 1Experimental conditions
The nano-Al2O3and carbon nanotube used in the study were purchased from Beijing Dk Nano Technology Co. The micron-size activated carbon and Al2O3particles were provided by Dalian Yixiu Catalyst Co. The properties of solid particles were shown in Table 2,and the specific surface area was determined using a Gemini V 2380 instrument from Micrometitics Instrument Corp. (USA).
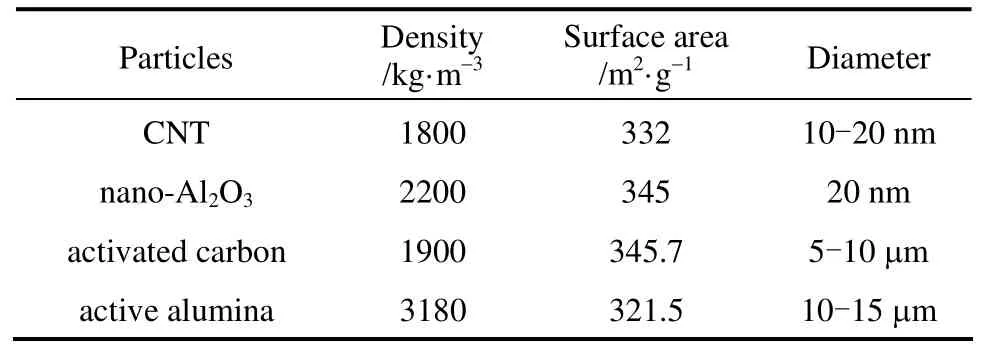
Table 2Properties of solid particles
3THEORY
The addition of nanoparticles changes the rate of mass transfer of the solute across the gas-liquid interface. Some possible mechanisms have been proposed in the literatures. (1) Grazing effect [15, 18]. At the gas-liquid interface, the solid particles with a high adsorption are loaded with the gas solute, leading to an increase of the concentration gradient of the solute in the mass transfer layer. After spending a certain time in the interfacial layer, the particles return to the bulk of the liquid where the solute is desorbed and the particles regenerated. With this so-called “shuttle”between the interface and the bulk, gas absorption rate is enhanced. (2) The Brownian motion of nanometer particles [14, 17, 19]. For very small size particles, the Brownian motion can not be neglected, and hence promotes the micro-convection effect of the particles in the liquid, resulting in an increase of mass transfer rate.
For adsorbed particles, both “grazing” and Brownian motion may be used to explain some phenomena of mass transfer enhancement. However, it is obviously unreasonable to interpret the strengthening behavior with “grazing” mechanism for particles with poor adsorbability to the solute.
3.1Assumptions
(1) Due to the Brownian motion, the velocity gradient around the particle is increased, leading to an increase of diffusion rate of the solute. Therefore, the diffusion coefficient of the solute in the liquid film around the nano-particles should be modified. According to Nagy et al. [19], the diffusion coefficient can be written as

where m=1.7 [20, 21] and B=640 [19]. Parameter ε is the solid volumetric fraction and Re is the Reynolds number of particle Brownian motion:
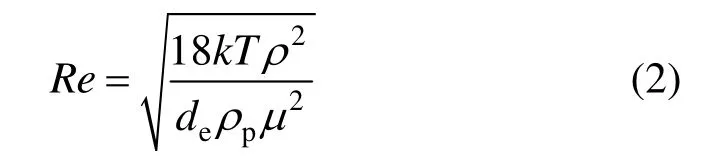
(2) For hydrophobic solid particle, it tends to adhere itself to the gas-liquid interface, the solid concentration at gas-liquid interface is higher than that in the bulk. Assumed that the coverage of the gas-liquid interface can be expressed by Langmuir-type adhesion isotherm [3, 22, 23]:
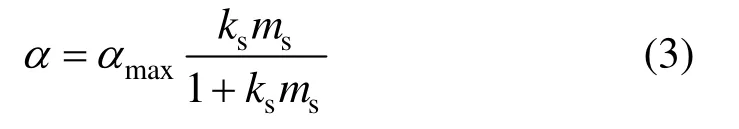
(3) The particles are taken to be spherical and uniformly distributed in the interface layer.
(4) The mass transfer in the penetration film is mainly considered (Fig. 2), and the thickness of the liquid film is calculated as follows [24]:

3.2Model equations
Taking a liquid element with the size of d x, d y, d z near the gas-liquid interface (Fig. 2) and a species balance for the solute in the unit is given by

where D is the diffusion coefficient of the solute, which is increased by the micro-convection effect of the nano-particles. D in Eq. (5) should be calculated according to Eq. (1).
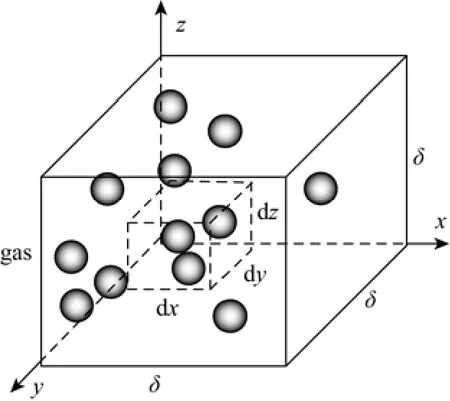
Figure 2Graphical representation of model
Rdin Eq. (5) indicates the influence of the particle adsorption. Based on the “grazing” mechanism, the “shuttle” of the adsorptive particles between the interface and the bulk increases the concentration gradient of the solute in the gas-liquid interface, and thus promote mass transfer. The adsorption rate Rdcan be written as
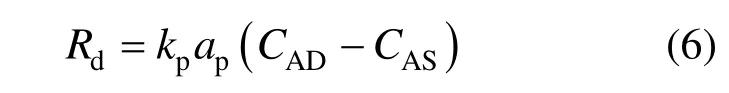
For the adsorbing particle, its adsorption can be given by
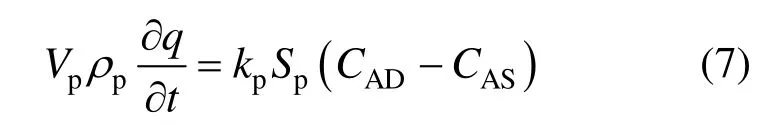
where q is the absorbed amount of the solute on the particles. It is assumed that the adsorption of the solute on the particles can be described by a Langmuir type adsorption isotherm [22].
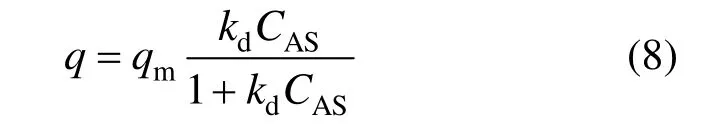
In the solution free from solid particles, mass balance for the solute is
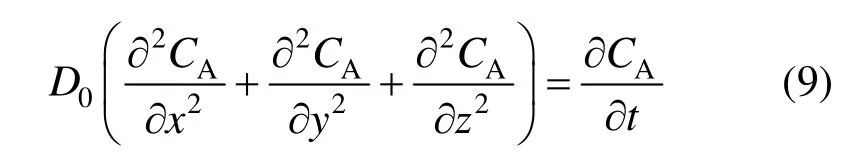
The initial and boundary conditions of Eqs. (5) and (9) are given by

Taking into account the direction perpendicular to gas-liquid interface as the main direction of mass transfer, the specific absorption rate in suspensions is defined as
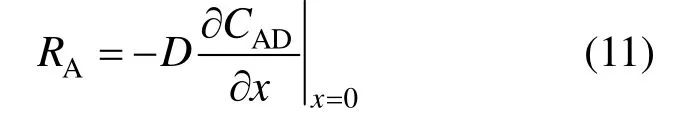
The enhancement factor is calculated according to

where RA0is the absorption rate in the solution free from solid particles. The above equations are solved numerically by gPROMs modeling software (Process System Enterprise Ltd.).
4RESULTS AND DISCUSSION
4.1Experimental results and observation
A typical variation curve of pressure difference with time is shown in Fig. 3. The slope of the curve at a given time is proportional to the absorption rate at that point of time. The experimental enhancement factor is defined as the ratio of the initial absorption rate with and without solid particles added, and the initial absorption rate can be calculated according to the slope of the Δp~t curve obtained at t=0.

Figure 3Pressure difference of CO2with batch time with and without particles added (stirring speed=2 r·s?1; solid concentration: 0.8 kg·m?3)
The influence of nano-particles on CO2absorption at different solid concentrations and stirring speeds for both nano-Al2O3and CNT particles is shown in Figs. 4 and 5. As a comparison, the effect of micron-size activated carbon (AC) (5-10 μm) and Al2O3particles (10-15 μm) was also measured experimentally, shown in Figs. 6 and 7.
From Figs. 4 to 7 it is observed that micron Al2O3particles did not show any enhancement on the CO2absorption, and with increasing particle concentration, the enhancement factor was decreased, however, a weak enhancement by nano-Al2O3was obtained. Also, CNT and AC particles increased the CO2absorption rate, whereas the changing trend with stirring speed was different. These facts indicated a difference in the enhancement mechanism for nanometer and micron particles.
Many researches showed that micron-particles exhibiting an enhancing effect on gas absorption should possess high adsorbability and hydrophobicity [3, 25]. Both active alumina and AC particles have good adsorbing capacity, however, Al2O3particles, which are hydrophilic, did not exhibit any enhancement to CO2absorption. In aqueous solution, the surface active sites of Al2O3are easily covered by water molecules due to the hydrophilicity and lose the adsorption capacity to the solute. Moreover, with increasing Al2O3concentration, the diffusion of the gas solute in the liquid is inhibited due to the increased viscosity of the slurry. On the other hand, AC particles, which are highly hydrophobic, displayed good adsorption capacity to CO2and hence a strong enhancement of CO2absorption.
Similar to active alumina, nanometer Al2O3particles are also hydrophilic, however, a weak enhancement to CO2absorption was found. The reason for this enhancement could not be attributed to the grazing effect, but should be explained by the micro-convection of the liquid around the particles caused by Brownian motion. A “creeping flow” can be created around a slowly moving particle, that is, a laminar boundary layer, in which the flow rate continuously changes as a function of the distance from the particle.
According to Nagy et al. [26], the distance between spherical particles can be given as
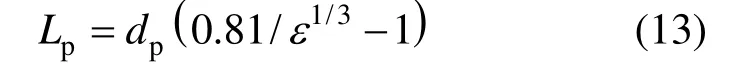
It can be obtained that in the case of dispersed phase holdup ε=0.0004, the particle distance, Lp=9.99dp, which is smaller than the thickness of the moving hydrodynamic boundary layer around the particles [19]. This means that even at very small values of particle concentration, the flow field around nanoparticles will interact, and this increases the mass transfer. Thestrengthening effect of nano-Al2O3particles on CO2absorption should be described by this micro-convection effect.
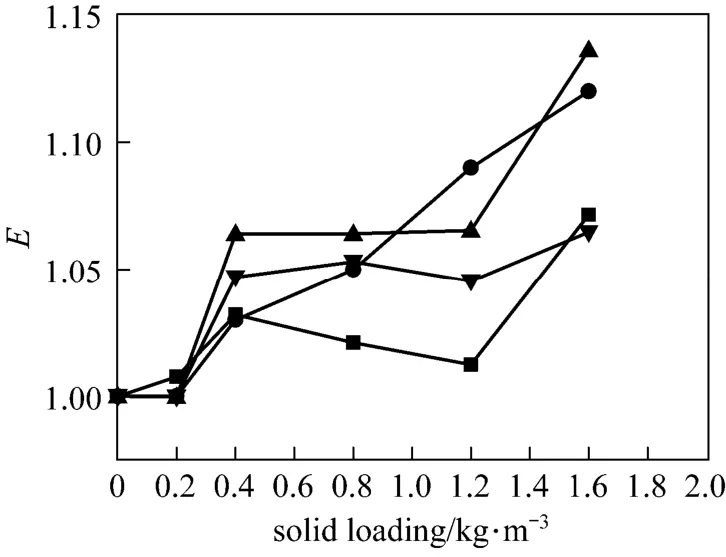
Figure 4The influence of nanometer active alumina on CO2absorptionstirring speed/r·s?1: ■ 1; ● 2; ▲ 3; ▼ 4
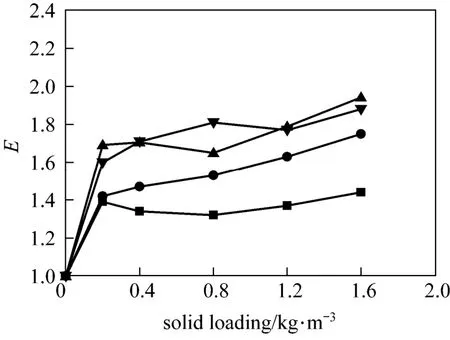
Figure 5The influence of CNT on CO2absorption stirring speed/r·s?1: ■ 1; ● 2; ▲ 3; ▼ 4
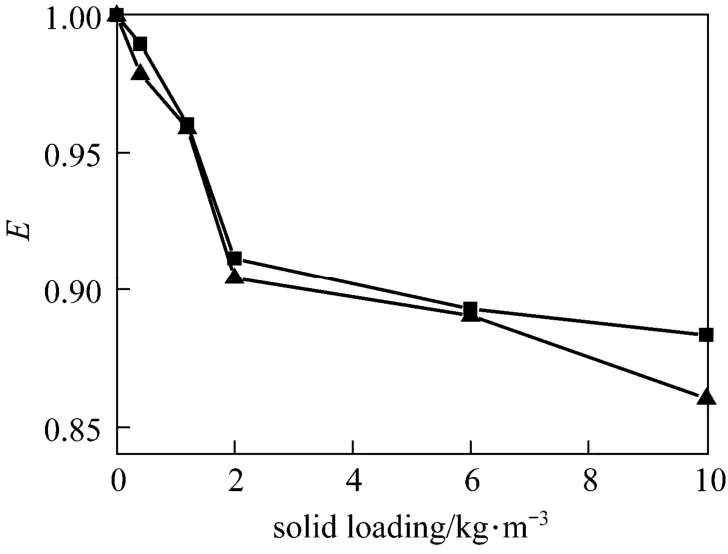
Figure 6The influence of micron Al2O3particles on CO2absorptionstirring speed/r·s?1: ▲ 2; ■ 4
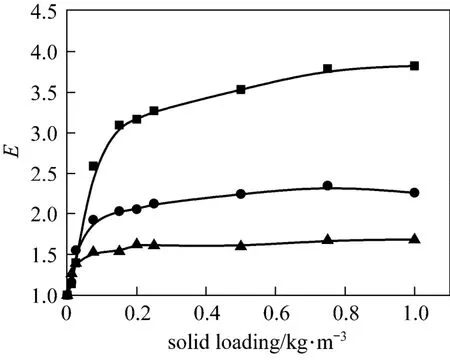
Figure 7The influence of micron activated carbon particles on CO2absorptionstirring speed/r·s?1: ■ 1; ● 2; ▲ 4
Similar to AC particles, CNT also have good adsorbability and hydrophobicity, and hence have a good strengthening effect on gas absorption. However, a different variation of the enhancement factor with stirring speed was observed in the experiments. The influence of stirring on mass transfer enhancement can be analyzed from several aspects. Firstly, it is well known that nanoparticles tend to agglomerate to larger particles and then reduce the intensifying effect on absorption. High speed stirring prevents the agglomeration effectively and the particles can be well dispersed in the slurry, which is advantageous to the absorption enhancement. From the experimental results, the enhancement factor of CNT at low stirring speeds (such as 1 r·s?1) was less than that in the case of high agitating ones. Second, with increasing stirring speed, the residence time of the particles at the gas-liquid interface is reduced rapidly. The particles do not have enough time to play their role before leaving the interface. Moreover, high stirring speeds induce low fractional coverage of the interface by the particles [1, 3], most particles are immersed in the bulk, thus leading to decrease of the intensifying effect on gas absorption. Under the combined action of the above effects, the enhancement factor of CNT was initially increased with stirring speed, and when the agitating speed was higher than 3 r·s?1, no further increase was found.
The experimental volumetric mass transfer coefficients for nano-system can be determined from the variation of pressure pAwith time [1]. A mass balance for CO2during the absorption process can be given as
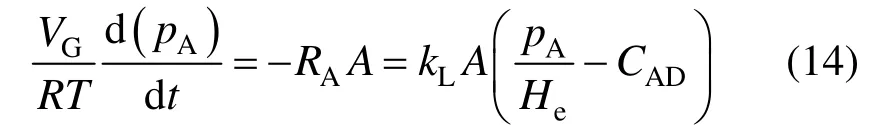
The concentration of CO2in the liquid-phase (CAD) is eliminated by setting up a total mole balance for CO2leading to
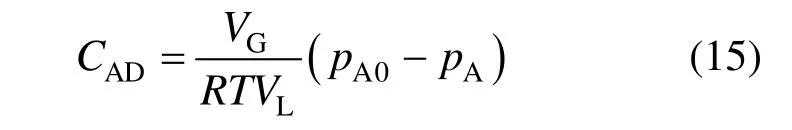
Combination of Eqs. (14) and (15) leads to the following equation:

The experimental results of kLa as a function of the solid concentration with the stirrer speed as a parameter are given in Figs. 8 and 9.

Figure 8Volumetric mass transfer coefficients in nanometer active alumina slurrystirring speed/r·s?1: ■ 1; ● 2; ▲ 3; ▼ 4
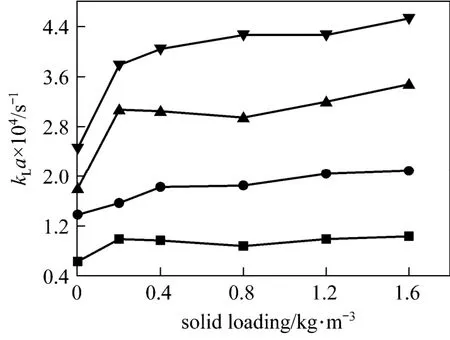
Figure 9Volumetric mass transfer coefficients in CNT slurrystirring speed/r·s?1: ■ 1; ● 2; ▲ 3; ▼ 4
A different variation of kLa in CNT and nano-Al2O3slurries respectively can be observed. In nano-Al2O3system, there is only a weak increase of kLa caused by the micro-convection of the nano-particles, while in CNT slurry, a significant enhancement of kLa can be found. For hydrophobic CNT particles, not only the micro-convection but also the solute “shuttle”by the particles between the interface and the bulk can strenghten the mass transfer.
4.2Theoretical modeling
Using the centered finite difference method, the theoretical model is solved numerically by gPROMs modeling software. Forty grid nodes are constructed respectively in x, y and z directions to ensure the accuracy of the simulation results. In order to evaluate the effect of the particles by the model presented here, the parameters qm, kd, ks, αmax, kL, δ, apand kpneed to be defined previously. The adsorption equilibrium constant kdand maximum adsorption amount qmof solid particles are determined experimentally: q is obtained by measuring the difference of the absorption amount of the solute in the slurry and in blank solution, and then kdand qmare calculated by fitting the curves of the experimental CAand q at different initial pressures. The liquid-to-particle mass transfer coefficient kpis calculated from S h=2 [27]. The adhesion constants of the particles, ksand αmaxrefer to the results of Shen et al [27]. Based on the value of αmax,assumed a uniform distribution of the particles in the interface layer, the solid concentration in the liquid film can be obtained and thus the solid-liquid interfacial area apcan be calculated. The liquid side mass transfer coefficient kLwas determined by Eq. (16) and the simulation thickness of the liquid film can be obtained according to Eq. (4).

Table 3Experimental and estimated parameter values in the model presented (stirring speed=4 r·s?1)
The parameter values of CNT particles used in the simulation are given in Table 3.
Due to the effect of the micro-convection, the flow field around nanoparticles interacts, thus leading to the increase of the diffusion coefficient of the absorbed component. From Eq. (1), the value of the diffusion coefficient increases with increasing solid concentration and decreasing particle size. The aggregation of the particles may easily occur and can alter the mass transfer rate. This effect is not taken into account in Eq. (1), but it is accounted for indirectly through the changed apparent particle size due to particle aggregation. The effect of the diffusion coefficient on the enhancement factor is indicated in Fig. 10. With the increase of the diffusion coefficient, the enhancement factor is increased almost linearly.

Figure 10Influence of the diffusion coefficient on the enhancement factor (qm=0.065 kmol·kg?1, dp=30 nm, kp=2.6×10?3m·s?1, kd=50 m3·kmol?1)
A simulation was made for the absorption of CO2in CNT and nanometer alumina slurries at a stirring speed of 4 r·s?1, as shown in Fig. 11. Due to the hydrophilicity, nanometer alumina particles lose the capacity of adsorption to the solute in aqueous solution. The effect of particle adsorption was not taken into account in the simulation. In the present experiments, a relatively low solid concentration (0-1.6 kg·m?3) was employed, thus only a weak enhancement of mass transfer for nano-alumina was observed in Fig. 11.

Figure 11Measured and the simulated curves using the theoretical model in the paper (stirring speed=4 r·s?1)Curve 1—model prediction for CNT; Curve 2—predicted CNT without considering micro-convection; Curve 3—prediction of nano-Al2O3■ CNT; ▼ nano-Al2O3
On the other hand, CNT particles, which are hydrophobic, exhibit a strong adsorbent effect in aqueous solution. Therefore, both adsorption and micro-convection should be considered when simulating. Curve 2 in Fig. 11 shows the simulation result of CNT without considering the micro-convection. It is obvious that the enhancement factor is significantly lower than the experimental data. The concentration of CNT particles at the gas-liquid interface is higher than that in the bulk due to the hydrophobicity. Hence the influence of Brownian motion of CNT particles on mass transfer is greater than nano-Al2O3. As can be seen in Fig. 11, after considering the effect of micro-convection, curve 1 obtained from the model fits the experimental data very well.
5CONCLUSIONS
Using CNT and nano-Al2O3particles which have different hydrophobicity, experimental investigation of the CO2absorption enhancement was conducted. The results were compared with that of micron AC and active Al2O3particles. From the experimental results, micron-size Al2O3particles do not have any intensifying effect on CO2absorption, while a weak enhancement by nano-Al2O3was achieved. Both AC and CNT particles strengthen the absorption of CO2, however, the trend of the enhancement factor with the increase of stirring speed was different: declining for AC particles, while rising for CNT particles. Therefore, for a reasonable interpretation of the enhancement mechanism of nanoparticles, besides the influence of adsorptivity and hydrophobicity, micro-convection caused by Brownian motion should also be considered. To particles with low adsorbability to the solute (suchas nano-Al2O3), the micro-convection may play a major role in the absorption enhancement. For hydrophobic particles, due to the relatively high solid concentration at the gas-liquid interface, without considering the effect of micro-convection, the enhancement factor will be obviously lower than the experimental results. In CNT suspensions, there is a significant increase of kLa, while in nano-Al2O3slurries, only a weak enhancement of kLa can be found.
A theoretical model based on the penetration theory was developed to predict the variation of enhancement factor of nanoparticles. Taking into account the effect of both micro-convection and adsorption, the present model provides a good prediction of mass transfer enhancement caused by nanoparticles.
NOMENCLATURE
A gas-liquid contact area, m2
a gas-liquid interface area, m2·m?3
apsolid-liquid interfacial area, m2·m?3
CAsolute concentration in pure water, mol·m?3
CADsolute concentration in suspensions, mol·m?3
CASconcentration of the solute on the surface of the particle, mol·m?3
CA0solute concentration at the interface, mol·m?3
D diffusion coefficient in slurry, m2·s?1
D0diffusion coefficient in pure water, m2·s?1
deequivalent diameter of the particles, m
dpparticle diameter, m
E enhancement factor
HeHerry coefficient, m3·Pa·mol?1
k Boltzmann constant (=1.3807×10?23J·K?1)
kdadsorption coefficient of the solute, m3·mol?1
kLgas-liquid mass transfer coefficient, m·s?1
kpliquid-solid mass transfer coefficient, m·s?1
ksparticle adhesion coefficient, m3·kg?1
Lpthe distance between two particles, m
mssolid concentration, kg·m?3
Q parameter in Eq. (14)
q adsorbed amount of the solute on solid per unit mass of particle, mol·kg?1
qmmaximum amount of adsorbed solute, mol·kg?1
RAabsorption rate, mol·m?2·s?1
RA0absorption rate in the liquid free from particles, mol·m?2·s?1
Rdadsorption rate of the particle, mol·m?2·s?1
Re Renolds defined in Eq. (2)
Sc Schmidt number ( =μ/ρD)
Sh Sherwood number ( =kpdp/D)
Spsurface area of the particles, m2
T temperature, K
t time, s
VGgas volume, m?3
VLliquid volume, m?3
Vpvolume of the particles, m3
x, y, z distance in x, y, z direction
α coverage of particles at the gas-liquid interface
αmaxthe maximum coverage of particles at the gas-liquid interface
δ thickness of the liquid film, m
ε solid volumetric fraction
μ viscosity of the liquid, Pa·s
ρ liquid density, kg?m?3
ρpparticle density, kg·m?3
REFERENCES
1 Dagaonkar, M.V., Heeres, H.J., Beenackers, A.A.C.M., Pangarkar, V.G., “The application of fine TiO2particles for enhanced gas absorption”, Chem. Eng. J.,92(1-3), 151-159 (2003).
2 Ruthiya, K.C., van der Schaaf, J., Kuster, B.F.M., Schouten, J.C.,“Mechanisms of physical and reaction enhancement of mass transfer in a gas inducing stirred slurry reactor”, Chem. Eng. J.,96(1-3), 55-69 (2003).
3 Demmink, J.F., Mehra, A., Beenackers, A.A.C.M., “Gas absorption in the presence of particles showing interfacial affinity: case of fine sulfur precipitates”, Chem. Eng. Sci.,53(16), 2885-2902 (1998).
4 Kaya, A., Schumpe, A., “Surfactant adsorption rather than ‘shuttle effect’ ?”, Chem. Eng. Sci.,60(22), 6504-6510 (2005).
5 Shen, S.H, Ma, Y.G., Lu, S.M., Zhu, C.Y., “An unsteady heterogeneous mass transfer model for gas absorption enhanced by dispersed third phase droplets”, Chin. J. Chem. Eng.,17(4), 602-607 (2009).
6 Rols, J.L., Condoret, J.S., Fonade, C., Goma, G., “Mechanism of enhanced oxygen transfer in fermentation using emulsified oxygen-vectors”, Biotechnol. Bioeng.,35(4), 427-435 (1990).
7 Nagy, E., Moser, A., “Three-phase mass transfer: Improved pseudo-homogeneous model”, AIChE J.,41(1), 23-34 (1995).
8 Brilman, D.W.F., Goldschmidt, M.J.V., Versteeg, G.F., van Swaaij, W.P.M., “Heterogeneous mass transfer models for gas absorption in multiphase systems”, Chem. En g. Sci.,55(15), 2793-2812 (2000).
9 Ahn, H.S., Kim, H., Jo, H.J., Kang, S.H., Chang, W.P., Kim, M.H.,“Experimental study of critical heat flux enhancement during forced convective flow boiling of nanofluid on a short heated surface”, Int. J. Multiphase Flow,36(5), 375-384 (2010).
10 Kumar, S., Prasad, S.K., Banerjee, J., “Analysis of flow and thermal field in nanofluid using a single phase thermal dispersion model”, Appl. Math. Model.,34(3), 573-592 (2010).
11 Li, J., Kleinstreuer, C., “Thermal performance of nanofluid flow in microchannels”, Int. J. Heat Fluid Flow,29(4), 1221-1232 (2008).
12 Santra, A.K., Sen, S., Chakraborty, N., “Study of heat transfer due to laminar flow of copper-water nanofluid through two isothermally heated parallel plates”, Int. J. Therm. Sci.,48(2), 391-400 (2009).
13 Noie, S.H., Zeinal, H.S., Kahani, M., Nowee, S.M., “Heat transfer enhancement using Al2O3/water nanofluid in a two-phase closed thermosyphon”, Int. J. Heat Fluid Flow,30(4), 700-705 (2009).
14 Krishnamurthy, S., Bhattacharya, P., Phelan, P.E., Prasher, R.S.,“Enhanced mass transport in nanofluids”, Nano Lett.,6(3), 419-423 (2006).
15 Kim, J.K., Jung, J.Y., Kang, Y.T., “The effect of nano-particles on the bubble absorption performanc in a binary nanofluid”, Int. J. Refrig.,29(1), 22-29 (2006).
16 Kim, J.K., Jung, J.Y., Kang, Y.T., “Mass transfer enhancement of a binary nanofluid for absorption application”, In: The 13th International Heat Conference, Australia, NAN-017 (2006).
17 Olle, B., Bucak, S., Holmes, T.C., Bromberg, L., Hatton, A., Wang, D.I.C., “Enhancement of oxygen mass transfer using functionalized magnetic nanoparticles”, Ind. Eng. Chem. Res.,45(12), 4355-4363 (2006).
18 Kim, J.K., Jung, J.Y., Kang, Y.T., “Absorption performance enhancement by nanoparticles and chemical sufactants in binary nanofluids”, Int. J. Refrig.,30(1), 50-57 (2007).
19 Nagy, E., Feczkó, T., Koroknai, B., “Enhancement of oxygen mass transfer rate in the presence of nanosized particles”, Chem. En g. Sci.,62(24), 7391-7398 (2007).
20 Prasher, R., Phelan, P.E., Bhattacharya, P., “Effect of aggregation kinetics on the thermal conductivity of nanoscale colloidal solutions (nanofluid)”, Nanoletters,6(7), 1529-1534 (2006a).
21 Prasher, R., Bhattacharya, P., Phelan, P.E., “Brownian-motion-based convective-conductive model for the effective thermal conductivity of nanofluids”, Trans. ASME J. Heat Transfer,128(6), 588-595 (2006).
22 Lu, S.M., Ma, Y.G., Zhu, C.Y., Shen, S.H., “The enhancement of CO2chemical absorption by K2CO3aqueous solution in the presence of activated carbon particles”, Chin. J. Chem. Eng.,15(6), 842-846 (2007).
23 Vinke, H, Hamersma, P.J, Fortuin, J.M.H., “Enhancement of the gas-absorption rate in agitated slurry reactors by gas-adsorbing particles adhering to gas bubbles”, Chem. Eng. Sci.,48(12), 2197-2210 (1993).
24 Zhang, D., “Study on mechanism and model of gas-liquid mass transfer enhancement by dispersed particles”, Ph.D. Thesis, Tianjin University, China (2006). (in Chinese)
25 Wimmers, O.J., Fortuin, J.M.H., “The use of adhesion of catalyst particles to gas bubbles to achieve enhancement of gas absorption in slurry reactors. II. Determination of the enhancement in a bubbles containing slurry reactor”, Chem. Eng. Sci.,43(2), 313-319 (1988).
26 Nagy, E., Hadik, P., “Three-phase mass transfer: Effect of the size distribution”, Ind. Eng. Chem. Res.,42(21), 5363-5372 (2003).
27 Shen, S.H., Ma, Y.G., Zhu, C.Y., Lu, S.M., “Absorption enhancement of carbon dioxide in aqueous activated carbon slurries”, CIESC J.,58(4), 835-841 (2007). (in Chinese)
10.1016/S1004-9541(13)60550-9
2012-06-13, accepted 2012-10-20.
* To whom correspondence should be addressed. E-mail: lusumin0720@163.com
 Chinese Journal of Chemical Engineering2013年9期
Chinese Journal of Chemical Engineering2013年9期
- Chinese Journal of Chemical Engineering的其它文章
- Soft Sensor for Inputs and Parameters Using Nonlinear Singular State Observer in Chemical Processes*
- A New Tuning Method for Two-Degree-of-Freedom Internal Model Control under Parametric Uncertainty*
- Halloysite Nanotube Composited Thermo-responsive Hydrogel System for Controlled-release*
- Recent Advances in Separation of Bioactive Natural Products*
- A Novel γ-Alumina Supported Fe-Mo Bimetallic Catalyst for Reverse Water Gas Shift Reaction*
- Volumetric and Transport Properties of Aqueous NaB(OH)4Solutions*
