Recent Advances in Separation of Bioactive Natural Products*
REN Qilong (任其龍)**, XING Huabin (邢華斌), BAO Zongbi (鮑宗必), SU Baogen (蘇寶根), YANG Qiwei (楊啟煒), YANG Yiwen (楊亦文) and ZHANG Zhiguo (張治國)
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, Department of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
Recent Advances in Separation of Bioactive Natural Products*
REN Qilong (任其龍)**, XING Huabin (邢華斌), BAO Zongbi (鮑宗必), SU Baogen (蘇寶根), YANG Qiwei (楊啟煒), YANG Yiwen (楊亦文) and ZHANG Zhiguo (張治國)
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, Department of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
Bioactive natural products are a main source of new drugs, functional foods and food additives. The separation of bioactive natural products plays an important role in transformation and use of biomass. The isolation and purification of bioactive principle from a complex matrix is often inherent bottleneck for the utilization of natural products, so a series of extraction and separation techniques have been developed. This review covers recent advances in the separation of bioactive natural products with an emphasis on their solubility and diffusion coefficients, recent extraction techniques and isolation techniques. This overview of recent technological advances, discussion of pertinent problems and prospect of current methodologies in the separation of bioactive natural products may provide a driving force for development of novel separation techniques.
biomass, chromatography, extraction, isolation, natural products
1INTRODUCTION
Bioactive natural products are the main source of new drugs, functional foods and food additives. They are secondary metabolites of plants and animals generated through various biological pathways in secondary metabolism processes. Typical features of bioactive natural products include: (1) diverse structures, i.e. flavonoids, alkaloids, sterols, terpenes, quinones and phenylpropanoid, etc.; (2) molecular mass between 200 and 1000, usually with complex structures containing a skeleton of aromatic rings or multi-rings and a number of functional groups; (3) various physiological activities; (4) boiling points mostly above 200 °C, some of which are heat-sensitive. Nowadays, more than 80% food active compounds and more than 30% drugs are produced from bioactive natural products, and the annual growth rate of global natural products-derived drugs is up to 20% [1, 2]. As a result, the research on the separation and purification of bioactive natural products from plants, animals and microorganisms has attracted much attention in academia and industry. In view of tremendous components and low contents of target compounds (0.01%-10%) in a plant, separation is often a complex process, which may last from days to months, depending on the problem being tackled.
Generally, the separation of target products includes two steps, extraction and purification. In the extraction, studies are mainly focused on the application of environmentally friendly solvents and intensification of extraction process by means of physical fields. In the isolation and purification, novel separation technologies such as chromatography techniques, membrane separation techniques and ionic liquids-mediated extraction have been developed and brought a major impact on this respect. Since the separation efficiency is relevant with the solubility and diffusion coefficient, research efforts in measuring and modeling the solubility and diffusion coefficient of bioactive natural products are also included in this review. In the past decades, there has been considerable evolution of separation knowledge, theory, and technology. It is the goal of this review to give an insight into the techniques and some future challenges.
2SOLUBILITY AND DIFFUSION COEFFICIENT OF BIOACTIVE NATURAL PRODUCTS
2.1Solubility
Solubility is of particular importance for the design and optimization of separation processes. Bioactive natural products usually have complex scaffolds bearing a number of functional groups [3]. Hydrophobic and hydrophilic segments are relatively common in bioactive natural products, which render their solubility different from conventional small organic molecules. So far, a large number of solubility data of bioactive natural products in organic solvents have been reported, such as flavonoids [4-7], terpenes [8] and steroids [9-11]. However, due to the highly cohesive energy of some bioactive natural products, it is difficult to select suitable solvents for separation process [12, 13]. Some bioactive natural products have the maximum solubility in binary solvents [14, 15]. Chen et al. [16] have found that the solubility of cholesterol in n-hexane-ethanol binary solvent reaches its peak at the mole fraction of ethanol close to 0.45 (shown in Fig. 1). The rationale is presumably that the synergistic effect of mixed solvents meets the polarity requirements of cholesterol.
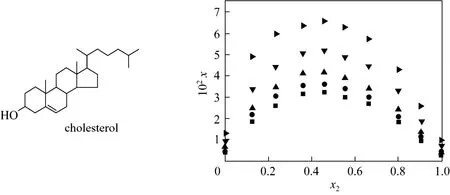
Figure 1Mole fraction solubility of cholesterol in n-hexane (1) + ethanol (2) mixed solvents vs. mole fraction of ethanol on solute-free basis [16]
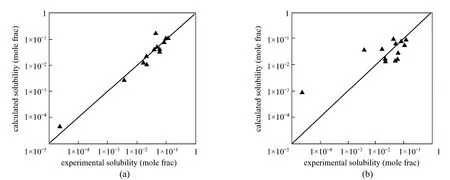
Figure 2NRTL-SAC (a) and UNIFAC (b) results for aspirin solubility at 298.15 K, with solubility data for 14 solvents fitted with NRTL-SAC [23]
Ionic liquid (IL) is a new class of solvent consisting of entirely ionic species. As they consist of at least two components that can be varied (anion and cation), the solvent can be designed to dissolve bioactive natural products that scarcely dissolve in conventional solvents [17-20]. Thus ILs have the potential to replace traditional organic solvents for extraction of bioactive natural compounds from plants. Different from ILs, supercritical carbon dioxide (scCO2) dissolves lipophilic or weak polar bioactive natural products easily, e.g. artemisinin [21], natural vitamin E and carotene [22].
It is highly desirable to establish models for correlation and prediction of solubility, but it is challenging for bioactive natural products owing to the structure complexity. For simple organic molecules, models such as UNIFAC group contribution method, NRTL-SAC and COSMO are in good agreement with experimental data, while those for bioactive natural products are still under investigation.
UNIFAC model has been used to predict the solubility of some bioactive natural products [23]. However, this model is based on the assumption that there is no interaction in the molecule and the interaction between groups is equivalent in different chemical environment, which is obviously not the case in bioactive natural products. As a consequence, the solubility prediction with such model is often in error, especially with several non-alkyl groups in bioactive natural products [23, 24]. Besides, some groups widely present in bioactive natural products such as steroid nucleus are not defined in UNIFAC model, which makes prediction difficult. Thus it is essential to define new groups. With C5H2N+, ArCOOH and steroid nucleus defined [25, 26], the prediction for solubility of berberine chloride, p-toluic acid and cholesterol coincides well with experimental data.
Based on the “virtual site” concept, the NRTL-SAC model describes the surface property of a molecule in a new way and successfully solves the problems resulted from the complexity of structure, especially in bioactive natural products. This model has been used to predict the solubility of drugs with a medium size [27] and other bioactive natural products [28], giving better accuracy than UNIFAC model (Fig. 2). Furthermore, it is possible to use the NRTL-SAC model to describe the solubility variation in binary solvents [27]. COSMO-RS and COSMO-SAC have been used to compute molecular interactions through calculation of electronic density in the molecular surface,improving the prediction accuracy [28, 29]. These models are far more convenient than other models as they can be used without any experimental data. Such models have been successfully used to predict the solubility of artemisinin [30], nitro compounds [31] and other substances [32, 33].
The study on solubility models of bioactive natural products has some progress, but the accuracy is not satisfactory especially to substances with complex structures. Development of new models with better accuracy is the direction of future study. In view of the advantages of COSMO-RS and COSMO-SAC models, they also need further study because of great convenience in solubility prediction and solvent screening.
2.2Diffusion coefficient
Diffusion and mass transfer coefficient are essential for process design, scale-up and optimization. Earlier research efforts were focused on determination of diffusion coefficients of bioactive natural products in organic solvents or in water. In recent years, studies were concentrated on the diffusion behavior of bioactive natural products in scCO2.
Hills et al. [34] summarized the diffusion coefficients of 139 organic compounds with molecular mass ranging from 2 to 235 g·mol?1in water at 298 K, which varies from 0.23×10?5to 4.4×10?5cm2·s?1. For bioactive natural products with higher molecular weights, such as amino acids [35] and sugars [36], their diffusion coefficients in water at 298 K range from 0.602×10?5to 1.040×10?5cm2·s?1and from 0.546×10?5to 0.773×10?5cm2·s?1, respectively. Funazukuri et al. [37, 38] measured the diffusion coefficients of some small organic molecules including acetone, benzene and bioactive natural products, such as vitamin E, vitamin K1 and linoleic acid methyl ester, in organic solvents. The diffusion coefficients of vitamin E are 70% lower than that of acetone. In general, due to their relatively large molecular volumes, bioactive natural substances have smaller diffusion coefficients than small organic molecules.
Because of the low viscosity of scCO2, which is close to that of gas, the self diffusion coefficient and molecular diffusion coefficient of solutes in scCO2are distinctly higher than that in organic solvents. Diffusion coefficients of various bioactive natural products in scCO2have been reported, including C18-C22saturated and unsaturated fatty acids and esters [39-48], terpenes (citral [49], pinene [50], L-carvone [51] and linalool [52]), natural pigments (β-carotene [53] and malvidin 3,5-diglucoside [54]), and other active compounds such as tocopherol [53], CoQ10 [55],indole [56], and vitamin K3 [57]. The diffusion coefficients of bioactive natural substances in pure scCO2are ten times that in organic solvents or water.
The diffusion of bioactive natural compounds in the mixture of scCO2and cosolvent was investigated by Dong et al [51, 58]. The diffusion coefficients of L-menthone and L-earvone in the mixture of scCO2and ethanol increase with temperature, and decrease with the increase of pressure, density or viscosity at constant temperature, and ethanol mole fraction in the mixture due to the chemical association between the two solutes and ethanol [51]. The experimental results show that for cosolvents without hydrogen-bond ability, the dispersion force between solute and cosolvent is the primary factor affecting the diffusion of solute. For cosolvents with only HBA basicity and amphiprotic cosolvents, the hydrogen-bond interaction between solute and cosolvent influences the diffusion of solute significantly [58].
At present, the study on the diffusion coefficient is focused on its determination. The investigation on solute-solvent interaction effect on diffusion coefficients from a microscopic aspect is quite challenging but deserves study.
3EXTRACTION TECHNIQUES
The first step to isolate bioactive natural compounds is generally the extraction of target compound from plant or animal tissues. Recent study on extraction of bioactive natural compounds focused on intensification of extraction process and application of novel solvents, such as scCO2, subcritical water, and ILs.
3.1Multi-stage countercurrent extraction and extraction intensification
Extraction of natural substances using conventional solvents such as water has been a popular method for more than 2000 years. Maceration (batch single pot extraction) [59-62], percolation [63-65] and countercurrent extraction [66, 67] are commonly used solvent extraction methods in industry [68-72]. Multi-stage countercurrent extraction (MCE) technique attracts much attention due to its high efficiency. The comparison in Table 1 [73] shows that the extraction efficiency of MCE for extraction of glycyrrhizic acid from licorice (Glycyrrh izauralensis Fisch) presents better results than other extraction techniques including microwave-assisted extraction, ultrasonic extraction, Soxhlet extraction and room temperature extraction, offering the highest yield, short extraction time, and the least solvent consumption.
For improvement of extraction efficiency, various types of physical field such as microwave and ultrasound have been used in the extraction of bioactive natural products to strengthen the mass transfer [74-77]. Microwave-assisted extraction is a technology developed recently for extracting soluble products into a fluid aided by microwave energy, which can significantly increase the mass transfer rate, reduce the extraction time and extract bioactive natural products more rapidly and selectively in comparison to traditional extraction processes [78]. This technique has been successfully applied to the extraction of natural compounds such as glycosides, alkaloids, carotenoids,terpenes, and essential oils [79-84]. For extracting active components in Chinese quince, with 44.15% ethanol/water as solvent and microwave power of 102.1 W, the product was obtained with the yield more than 77.47% in 5 min [85]. Although the improvement of extraction efficiency via microwave energy is obvious in analytical scale, its wide applications just appear around one decade since the invention of several extraction instruments. The results obtained so far show that microwave radiation does not damage the extracted compounds, unless the temperature in the extraction instrument increases dramatically.
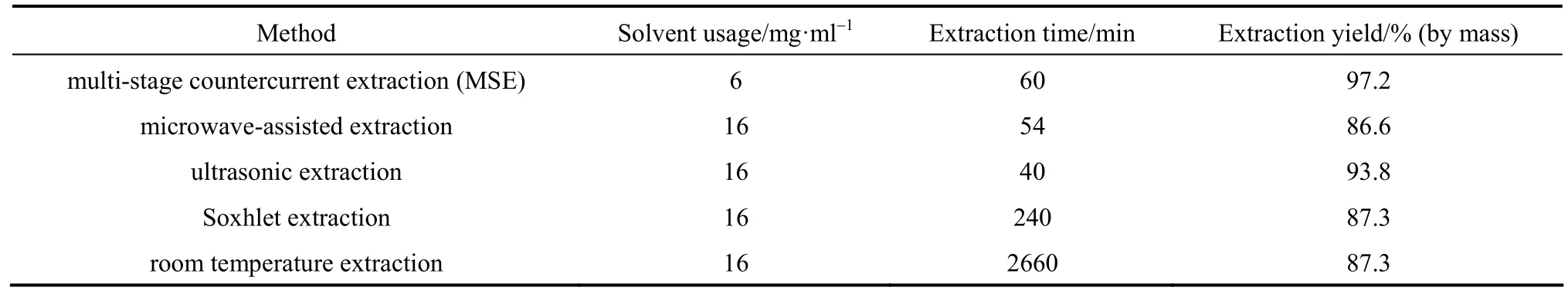
Table 1Comparison of extraction processes for extraction of glycyrrhizic acid from licorice [73]

Figure 3SEM images of defatted soy flakes at high amplitude (84 μmpp) [90] (a) control (0 s); (b) 15 s; (c) 60 s; (d) 120 s; bar: 10 μm
Ultrasonic cavitation is another recently developed technique to improve traditional extraction processes [86-88]. The extraction of saponins, steroids and triterpenoids from chresta spp was three times faster than that with traditional extraction methods [89]. Scanning electron micrographs of sonicated samples showed the structural disruption of soy fl akes (Fig. 3). The particle size decreased nearly 10-fold with ultrasonic treatment at high amplitudes. Sonication at high amplitude for 120 s gave the highest increase in total sugar release (50%) and protein yield (46%) compared with non-sonicated samples (control). The use of ultrasound can significantly improve protein yield and reduce the overall cost in producing soy protein from fl akes [90].
However, microwave and ultrasound-assisted extraction may cause local high temperature, which may not be suitable for heat-sensitive natural substances. In addition, it may not be easy to realize industrial production for lacking of corresponding industrial facilities [76].
3.2Supercritical/subcritical fluid extraction
Supercritical/subcritical fluids are regarded as alternatives to conventional solvents. The properties of supercritical fluids (SCFs) can be simply changed by changing pressure and temperature, rendering them potential applications in a range of industrial and laboratory processes.Carbon dioxide (CO2) and water are the most commonly used supercritical/subcritical fluids. Supercritical CO2is an excellent solvent for the extraction of bioactive compounds, especially heat-sensitive substances because of its mild critical condition, and has been widely used for extraction of essential oils, fatty acids and antioxidants [91-96]. Table 2 is a summary of scCO2extraction of natural substances recently. Lipid-soluble bioactive substances such as essential oil and germ oil can be efficientlyextracted by pure CO2[97-102]. For polar bioactive substances (e.g. flavonoid), modifiers are added to increase the polarity of CO2[103-108]. Another advantage of scCO2extraction is the easy operation and efficient separation of products by simple decompression. With a refrigerator installed to the separator, highly volatile substances are able to be collected without organic solvent residue [109]. However, collection of products by decompression would consume power since CO2needs to be re-pressurized to complete a cycle. Researchers are making their efforts on coupling extraction-absorption and extraction-adsorption. By using selective solvent or adsorbent, separation of products without decompression has been successfully carried out [110]. After thirty years of development, supercritical fluid extraction (SFE) technology has become a mature technique and has been used in a number of large-scale extractions for natural substances, such as the removal of caffeine from coffee [111-115] and industrial extraction of hops [116-119].

Table 2Summary on the extraction of bioactive natural compounds from plant by SFE in recent years
In recent years, SFE model has become a useful tool for design of extraction processes. With appropriate models, one can obtain more useful information about extraction mechanisms and optimize extraction conditions. Many models [119-124], such as diffusion model and hot-ball model, have been developed, among which the simple and practically useful one is the hot-ball model developed by Bartle et al [125]. Fischer and Jefferies used this model to predict extraction of nicotine from tobacco using methanol modified scCO2[120] and the prediction was in accord with experimental data.
Extraction of bioactive compounds from natural substances using subcritical water was in the rapid development over the past decade [126] because it is a cheap and pollution-free extraction solvent [127]. Sereewatthanawut et al. [128] compared the recovery of protein and amino acids by subcritical water extraction at various temperatures and time with those by alkaline method at 30 °C for 45 min. The protein content in the extraction by subcritical water at 160 °C for 20 min or longer and at 220 °C for more than 5 min was higher than that by alkaline extraction. However, subcritical extraction technology usually operates at a high temperature (more than 150 °C), so degradation of bioactive compounds may occur in the extraction process [129].
3.3Ionic liquid extraction
Ionic liquids attract great interest as alternative solvents in recent years, due to their unique features such as ultra-low vapor-pressure, wide liquid range and good thermal stability [130]. Moreover, ILs are“designable” solvents whose physicochemical properties can be well tuned by introducing various functional groups into the structures of cations and anions [131, 132], so they often dissolve many organic materials. Using ILs as solvents in extracting bioactive natural compounds from biomass not only leads to a green process, but also improves the extraction efficiency from a thermodynamic point of view.

Figure 4SEM micrographs of leaves before extraction (left), after extraction by methanol (middle), and after extraction by [bmim]Cl (right) [133]
Usuki et al. utilized [bmim]Cl as solvent to extract shikimic acid from Ginkgo biloba leaves at 423.15 K and obtained an extraction yield of 2.3% (by mass), which was 2.5 times that using methanol (0.93%, by mass) and 2 times that using DMF (1.1%, by mass) [133]. The enhanced yield is ascribed to the great solubility of [bmim]Cl for cellulose, which is the primary component of the wall of plant cells, with the evidence from SEM micrographs showing the change of microscopic morphology of leaves after extraction (Fig. 4). The dissolved shikimic acid was isolated from [bmim]Cl by using anion exchange resin. Chowdhury et al. employed a distillable IL, N,N-dimethylammonium N′N′-dimethylcarbamate (DIMCARB), to extract hydrolysable tannin materials from plant sources [134]. For the samples of catechu (Acacia Catechu) and myrobolan nut (Terminalia Chebula), the content of tannins in DIMCARB extract was 56% and 23%, respectively, much higher than those using water as solvent (27% and 21%). Moreover, the DIMCARB extract showed a better selectivity of ellagic acid than the water extract. Bioniqs Ltd. investigated the extraction of artemisinin from dry solid materials with ILs [135]. In comparison with hexane, ILs were superior because of elevated equilibrium concentration of artemisinin or reduced extraction time. Further purification of artemisinin after the IL extraction was performed by an IL/water distribution-recrystallization method.
The relatively large viscosity of ILs is one of the main problems in IL extraction, which may impede the mixing of ILs with biomass and diffusion of solutes. Lu et al. studied the IL-based microwave-assisted extraction of phenolic alkaloids from medicinal plant Nelumbo nucifera Gaertn [136]. Compared with heat-reflux extraction, this method improved the extraction efficiency by 20%-50% within 90 s. Du et al. utilized the aqueous solution of [bmim]Br in the microwave-assisted extraction of trans-resveratrol from Rhizma Polygoni Cuspidati [137]. With the liquid/solid ratio of 20︰1, an extraction yield of 92.85% was achieved after 10 min. Row et al. employed the aqueous solution of chloride-anion ILs in the ultrasonication-assisted extraction of medicinal products from herb, such as cryptotanshinone, tanshinone I and tanshinone II [138], and found that the extraction efficiency in aqueous ILs was much higher than that in pure water or n-hexane. Among these ILs used, the length of alkyl chain of cation is proportional to the extraction efficiency, probably owing to the increase in hydrophobicity of ILs.
In spite of a series of advantages provided by ILs, efficient recovery methods of bioactive natural compounds from ILs are not yet resolved due to their high-boiling points or heat-sensitive properties, greatly hindering its industrial applications. Therefore, development of highly effective isolation/recovery methods is needed in future researches.
4ISOLATION AND PURIFICATION TECHNIQUES
4.1Chromatography separation techniques
Bioactive natural products are often a mixture of a number of compounds with similar structures and polarities. Chromatography related technologies are the most often used methods for separation of pure natural products. The separation efficiency of targeted compounds is highly dependent on their adsorption affinity to stationary phase. In the past decades, chromatography techniques have seen an explosion of interests and have been successfully applied to the separation of natural products. This part of review mainly focuses on recent progress of high speed counter current chromatography, supercritical fluid chromatography, simulated moving bed and gel permeation chromatography, and their applications in the separation of bioactive natural products are briefly presented.
4.1.1High speed countercurrent chromatography
High speed counter current chromatography (HSCCC) is a support free liquid-liquid partition chromatographic technique, which eliminates irreversible adsorption of samples on solid support in the conventional chromatographic column. Compared with traditional chromatography, HSCCC offers various advantages such as rapid separation, low solvent consumption and high recovery. It has been successfully applied to isolate and purify a number of natural products, especially for polar substances. The crucial factor for the HSCCC is the selection of the two-phase solvent system. With appropriate solvent systems, HSCCC has been widely used for the isolation of flavonoid compounds from natural products. Preparative isolation and purification of flavonoid glycosides from Chinesemedicinal herbs, including Taraxacum mongolicum [139-141], Nelumbo nucifera [142, 143], Radix Astragali [144], Ziziphus jujube [145], sarcandra glabra [146], hedyotis diffusa [147], oroxylum indicum [148], etc., have been broadly reported. It has been proven that n-hexane-ethyl acetate-methanol-water solvent system is effective to separate and purify phenolic acids [149], anthraquinones [150], tetrahydropalmatine [151], gingerols [152], ferulic acid [153] and liensinine homologues [154] from corresponding natural products. The solvent systems such as ethyl acetate/2-propanol/water and ethyl acetate/1-butanol/water were successfully used for the fractionation of dimeric to tetrameric procyanidins [155]. In order to achieve fast and efficient separation, gradient mode in HSCCC was often employed. Peng et al. [156] employed flow rate gradient HSCCC to separate five diterpenoids from Triperygium wilfordii. Niu et al. [157] first demonstrated that preparative isolation of alkaloids from Corydalis bungeana Turcz. was possible with gradient elution. For both development and practical experience with existing HSCCC technology, an expected major area of expansion is the development of new HSCCC instrumentation. In order to bring the numerous benefits of HSCCC to an increasing number of research and application laboratories, factors such as dependability and simplicity, paired with a healthy degree of automation, can be considered key criteria of successful progression.
4.1.2Supercritical fluid chromatography
Supercritical fluid chromatography (SFC) is a chromatographic technique generally using supercritical carbon dioxide (scCO2) instead of organic solvents as mobile phase. Compared to traditional organic solvents, scCO2has higher diffusivity and lower viscosity, which give rise to better separation efficiencies and lower pressure drops. SFC is thus allowed to be operated at higher flow-rate, leading to shorter run time. In addition, SFC significantly reduces solvent consumption and subsequent energy consumption for solvent recovery. Ren et al. [158-160] developed a process for separating DHA-EE and EPA-EE from fish oil using SFC without co-solvent. The separation of arachidonic acid ethyl ester and linoleic acid ethyl ester by SFC on RP C18stationary phase was reported [161]. Because of poor solubility of polar solutes in scCO2, addition of co-solvents such as methanol, ethanol and 2-propanol is a general solution to elute the solutes that interact strongly with stationary phase. Jiang et al. [162] found that baseline resolution of tocopherol homologues could be achieved on C18column using unmodified scCO2in temperature range from 303 K to 343 K and pressure range of 16-22 MPa. In the absence of co-solvents, it was difficult to elute these solutes from silica gel in a reasonable time. Nevertheless, resolutions and peak profiles were significantly improved by adding 5% (by mass) ethanol or 2-propanol to the mobile phase. Recently, SFC was also coupled with SFE to isolate desired ingredient from natural products. Ramírez et al. [163] demonstrated the potential use of preparative-supercritical fluid chromatography to fractionate complex supercritical rosemary extracts. The fractions containing carnosic acid, carnosol and methyl carnosate were obtained by SFC in the presence of 10% of ethanol as the modifier in LC-Diol packed column at 80 °C and 13 MPa. SFC was also employed to fractionate thyme (Thymus vulgaris L.) extracts, which were obtained by supercritical carbon dioxide extraction of thyme leaves [164]. Solanesol [165] and artemisinin [166] were fractionated from the supercritical extracts by preparative SFC. Desmortreux et al. [167] utilize SFC to improve separation of furocoumarins of essential oils. SFC with inert scCO2could effectively prevent some oxygen-sensitive bioactive products from oxidation, which likely occurs in liquid chromatography. SFC has attracted much attention in purification of bioactive natural products as standard of residual solvent is becoming more rigorous in recent years.
4.1.3Simulated moving bed
Simulated moving bed (SMB) is a continuous chromatography, in which the feed entrance and the analyte recovery are simultaneous and continuous by properly simulating the countercurrent movement between stationary phase and mobile phase. The separation efficiency of SMB is much higher than that of batch liquid chromatography, and high purity of product may be obtained even if the selectivity of two solutes is too low to be baseline resolved. The high productivity of SMB has made it practical for industrial applications.
SMB has been widely used to separate racemates since 1990s [168-171]. It also serves to separate bioactive natural products. Lu et al. [172] separated tocopherol homologues using SMB for the first time, in which γ- and δ-tocopherols with purity greater than 98% were obtained. The productivity was as high as 6 g·L?1·h?1, which was three times higher than that of batch chromatography at the same feeding concentration of 28 g·L?1. The SMB was used for separations of capsaicin and dihydrocapsaicin, L-arabinose and D-xylose in xylose mother liquor, mono- and di-d-α-tocopherol polyethylene glycol 1000 succinate [173-175]. Sun et al. [176] separated xylose and xylitol in xylose mother liquor, and obtained xylose with purity of 99.3% and xylitol with purity of 99.8% though the resolution of the two components was only 0.97. Wei and Zhao [177] purified capsaicinoids by combining SMB with adsorption technique with macroporous resin. Cong et al. [178] reported the purification of liquiritin with SMB after suitable post-treatment and the purity of product was around 85%, achieving the purity of 99% by further recrystallization. Long et al. [179] separated D-psicose from D-fructose and obtained product with purity higher than 99%. By optimizing the SMB process using multiple objective optimization method, a maximum productivity reached as high as 103 g·L?1·h?1, and the purities of succinic acid and lactic acid were up to 98% [180]. Although SMB has been proven to be a promising technique forpilot preparation of racemates, isomers and bioactive homologues, the cost of bulk packing materials and equipment limits its industrial applications.
4.1.4Gel permeation chromatography
Gel permeation chromatography (GPC) is a form of liquid chromatography also known as size exclusion chromatography or gel filtration chromatography. Generally, Sephadex is the most widely used stationary phase in GPC because it could filter molecules according to their sizes. While some smaller molecules enter the pores of gel and travel longer distance, larger molecules shows much shorter retention time. In view of its separation mechanism, GPC is often applied to the purification of water soluble macromolecules. Lu et al. [181] tried to remove macromolecular impurities from penicillin G sodium, which may cause allergic reactions. The impurities could be completely separated using HW-40F as stationary phase and 0.005 mol·L?1citrate buffer with pH close to 7.0 as mobile phase. Aryusuk et al. [182] separated rice bran wax and its major impurity triglyceride using 10 nm Phenogel column as stationary phase and isooctane-toluene (volume ratio 65/35) as mobile phase. Du et al. [183] separated theaflavins from the extract of black tea leaves using conventional Sephadex LH-20 column chromatography. Cara et al. [184] reported a separation of olive tree pruning oligomers from olive tree biomass hydrolyzates using preparative gel filtration chromatography. Zhang [185] separated immunoglobulin IgG and IgM in colostrum by combining GPC and ion exchange chromatography and obtained sIgA with purity of 96.88%. The major challenges of GPC technique in practical applications are the scale-up of chromatographic process and the life period of bulk packing because of its relatively weak pressure resistance.
4.2Membrane separation technology
Advances in material science and membrane manufacturing technology have made membrane technique grow to be an important technology for separation of natural products. The general principle of membrane separation is based on the selective permeability of the membrane to allow the target substances to penetrate through the membrane, whilst unwanted substances are normally rejected by the membrane. Membrane-based processes are generally operated at room temperature, and there are no phase changes and chemical reactions in the process, so it is especially suitable for separation and purification of thermal-sensitive bioactive substances.
Nowadays, membrane technologies are still restricted to desalinate, recover or remove protein from extracts of natural products because of poor selectivity of membrane for similar molecules with close size and structures. Cho et al. reported that the extracted pectin solution was concentrated with the cross flow microfiltration using a 0.2 μm regenerated cellulose membrane. The filtration process was effective to remove flavonoids, polyphenols and carotenoids, with the galacturonic acid content of pectin increased from 68.0% to 72.2% [186]. With ultrafiltration, Denis et al. concentrated and pre-purified R-phycoerythrin solution extracted from macro-algae [187], Xu et al. achieve high purity of flavoniods from Ginkgo Biloba leaf extract [188], Goulas et al. obtained the yields of 19% (by mass) for monosaccharides and 88% (by mass) for di- and oligosaccharides [189], and Nabarlatz et al. investigated purification of xylo-oligosaccharides from almond shells [190]. With nanofiltration technology, the refinement of rice bran oil increased the content of γ-oryzanol in oil from 0.95% (by mass) to 4.1% (by mass) [191], and the purity was over 90% in fructooligosaccharides with the yield around 80% [192]. Ion-exchange membrane was used to remove pectinesterase from Marsh grapefruit pulp extract effectively, preventing pectin from esterification [193].
Membrane separations were coupled recently when a single membrane filtration step is not satisfactory. A coupling membrane process was investigated for the separation and concentration of polyphenols in bergamot juice, in order to develop a natural product enriched in polyphenols [194]. Kamada et al. examined the effectiveness of combined membrane process with ultrafiltration and nanofiltration for purification and concentration of oligosaccharides from chicory rootstock [195]. Bazinet et al. evaluated the feasibility of the production of a cranberry juice enriched with natural phenolic antioxidant compounds using an ultrafiltration membrane stacked in an electrodialysis cell [196]. A process for isolating tea polyphenol and caffeine from green tea leaves was developed using extraction followed by ultrafiltration with CA-Ti composite membrane and adsorption with PA resin and a purified product containing more than 90% of tea polyphenol was obtained [197].
In summary, membrane separation technology has demonstrated potential application in the separation of bioactive products with the development of novel membrane materials. The main problem encountered in membrane-based separation is the decay of permeate flux caused by concentration polarization and fouling, increasing operating cost and shortening membrane life. As a result, much effort has been directed to modify the structure and chemistry of membranes and develop new membrane systems with reduced fouling characteristics and enhanced permeate flux. Another important trend in membrane separation is to improve the selectivity for target substances by incorporation of appropriate functional groups onto membrane surface. With these contributions, membrane separation techniques will remain a promising tool in the separation of bioactive natural products.
4.3Ionic liquids-mediated separation of bioactive natural homologues
The most challenging problem in the separation of bioactive natural products from other compounds isthe separation of bioactive homologues [1, 198], which has become a critical procedure in producing highly bioactive products. Current methods for separating bioactive natural homologues mainly include adsorption [199], high-speed countercurrent chromatography and crystallization [200, 201], but they usually suffer from problems such as large consumption of solvent and energy, environmental pollution, and high production cost. The application of liquid-liquid extraction is limited by lacking of appropriate extractants and biphasic systems as well as poor selectivity for different homologues.
In virtue of the unique features of ionic liquids, such as ultralow vapor pressure, non-flammability, designable structure and property, and the facility of forming biphasic systems with other solvents, Ren and co-workers developed a novel method for separating bioactive natural homologues based on IL-mediated liquid-liquid extraction [202-205], showing a very promising method for separating typical bioactive homologues including polyphenols, flavonoids and terpenes. A series of IL/hexane and IL/polar cosolvent/hexane biphasic systems that have low mutability and different microscopic solvent properties were prepared and utilized to separate the mixture of tocopherol homologues, which are the main components of natural vitamin E (Fig. 5) [202, 203, 205]. The selectivity of δ-tocopherol to α-tocopherol (the difference of their structures is in two methyl groups) was remarkably increased to more than 20.0, which is much more superior to common biphasic systems (e.g., selectivities in DMF/hexane and methanol/hexane systems are 1.8 and 1.3, respectively). The use of cosolvent is very important, because it could not only reduce the viscosity of extraction system, but also enhance the tunability of the physicochemical properties of extractant such as dipolarity/polarizability, hydrogenbond basicity and hydrogen-bond acidity. A synergistic effect between IL and cosolvent has been revealed by Ren and his coworkers through the discovery of a maximum point in the distribution coefficient-IL concentration plot (Fig. 6) [203], showing that cosolventmay improve the extraction efficiency of ILs in some extent. With cosolvent, a large separation selectivity, adequate distribution coefficient, low viscosity, and low consumption of IL can be simultaneously achieved in the extractive separation of tocopherol homologues. Based on this strategy, [bmim]Cl-acetonitrile mixture with a molar ratio of 2︰98 as the extractant (with viscosity of about 0.44 m·Pa·s only) at 303.15 K resulted in the selectivity of δ-tocopherol to α-tocopherol of 11.3, four times larger than that using pure acetonitrile, and the distribution coefficients of δ-, β- and γ-, and α-tocopherol were 4.07, 2.02, and 0.36, respectively, at least eighteen times larger than those using pure [bmim]Cl. A correlation between the selectivity of different tocopherol homologues and the Kamlet-Taft hydrogen-bond basicity β of IL-cosolvent mixtures was established, and the essential role of hydrogenbonding recognition in separating homologues was also illustrated by quantum chemical study.
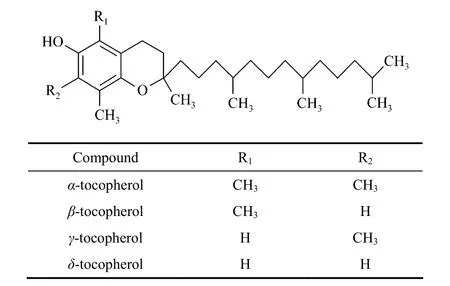
Figure 5Structure of tocopherol homologues [202]
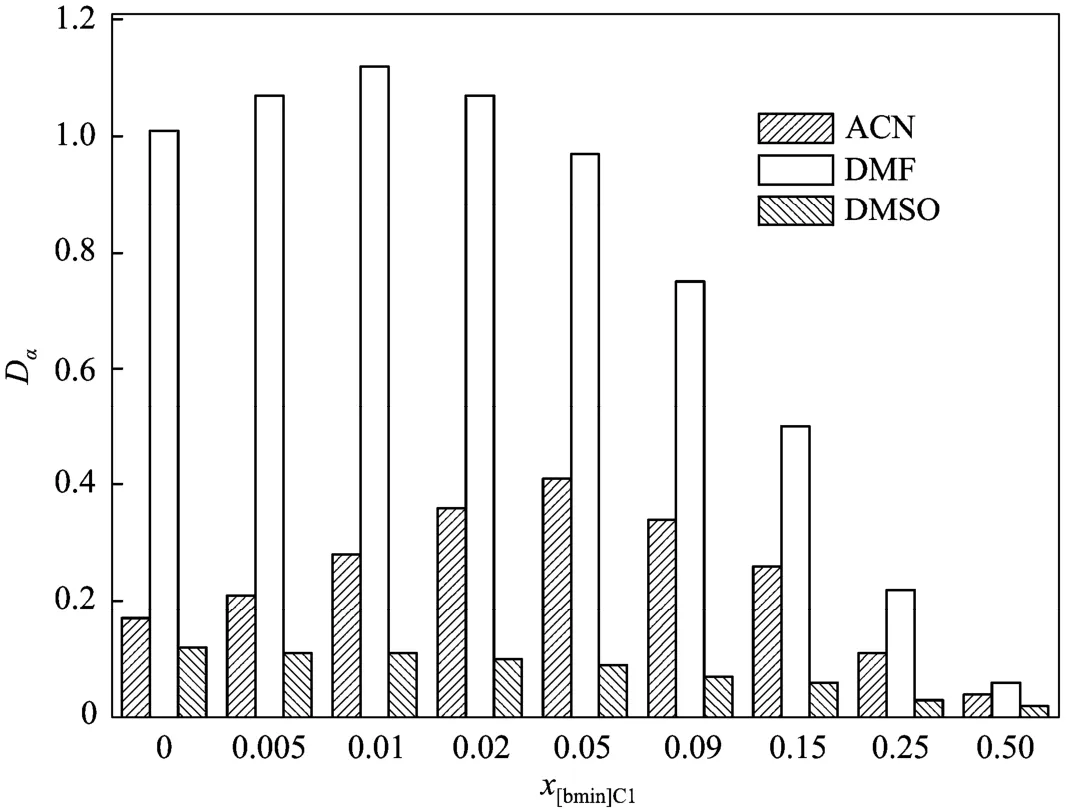
Figure 6Distribution coefficient of α-tocopherol (Dα) using different [bmim]Cl-cosolvent mixtures as extraction solvent at 303.15 K [203]
In order to apply liquid-liquid extract techniques to scarcely soluble natural homologues, Ren’s group developed novel IL + water + ethyl acetate biphasic systems to separate those “sparingly aq-/lipo-soluble”bioactive homologues with high efficiency [204-206]. For ginkgolide homologues and soybean isoflavone homologues, the distribution coefficients of ginkgolides obtained with [emim]Br-water-ethyl acetate system at 303.2 K were about 10-1000 times larger than those obtained with conventional biphasic systems and a selectivity of ginkgolide C to ginkgolide B was up to 12 [206]. With the same biphasic system, adequate distribution coefficients and selectivities over 7.0 were also achieved for the separation of different soybean isoflavone homologues [204]. Through a laboratory-scale simulation of fractional extraction process containing four extraction stages and four scrubbing stages, genistein was separated from other soybean isoflavone homologues with a purity of 95.3% and a recovery >90%.
In general, recent studies have demonstrated that IL-mediated liquid-liquid extraction is a promising method for separation of bioactive natural homologues, which results in low solvent/energy consumption, high selectivity, large throughput capacity and low environment pollution. With the application of this technique, it is expected that the production cost of highly-bioactive natural products will reduce remarkably. IL-mediated liquid-liquid extraction can be extended to the separation of other natural analogues, e.g., Ni et al. prepared several amino acid-functionalized ILs to separate α-tocopherol from its structural analogue methyl linoleate by liquid-liquid extraction [207]. With the 15︰85 (molar ratio) IL-DMF mixture as extractant, the selectivity was as high as 29.0 with an adequate extraction capacity. Future research efforts should be made to promote IL-mediated liquid-liquid extraction as an industrial technology, which include the effective recovery of ILs, design of more efficient IL extractants, study on mass transfer, and intensification of large-scale extraction.
5CONCLUSIONS AND OUTLOOK
Numerous approaches have been developed for efficient separation of bioactive natural products. Crucial breakthroughs in separation technologies have greatly lowered the hurdles in the isolation of structurally complex molecules or natural homologues. However, lacking of systematical thermodynamic theory and effective models, solvent selection and process optimization rely mainly on random screening. Since the majority of separation methods are related with solubility, liquid/liquid, solid/liquid partition equilibrium and other thermodynamic properties, the study on those properties remains to be an important subject in separation of natural products. On the one hand, the collection of a large amount of thermodynamic data is important to establish quantitative structure-property relationships for bioactive natural products. On the other hand, molecular simulation, spectral and other advanced methods should be utilized to study the interactions between bioactive natural products and separation medium (solvent, extractant, adsorbent, etc.) at micro-scale and meso-scale, which may provide an insight into the separation mechanism for bioactive products. Accordingly, a rational optimization of separation process may be achieved.
The advance of separation technologies is highly dependent on the evolution of separation media, e.g. adsorbents and solvents. As a consequence, development of novel adsorbents is the focus of research efforts. Ideal adsorptive materials, such as adsorptive macroporous resins, mesoporous molecular sieves and monolithic continuous beds, are expected to have low cost, specific pore diameters, adjustable pore structures and enriched porous surfaces with functional groups. For alternative solvents, SCF and ILs are increasingly considered to replace conventional solvents, because they are generally regarded as environmental benign solvents and are superior in the reduction of solvent consumption, energy consumption and wastes emission in the separation process. Moreover, in the realm of liquid-liquid extraction, ILs have showed great advantages in the separation of natural homologues, which is generally considered as the most challenging in isolation of natural products.
Some physical fields or methods, such as ultrasonic extraction, microwave-assisted extraction, near-critical water extraction, and supercritical chromatography, have been utilized to improve the separation efficiency of bioactive natural products on laboratory-scale or analytical-scale. However, the application of such techniques in industry still remains to be fully explored. For some bioactive compounds, mutual interplay or combination of different separation techniques are essential for their separation. It is thus of great importance to study the combination ways of different separation techniques in the future.
REFERENCES
1 Sticher, O., “Natural product isolation”, Nat. Prod. Rep.,25(3), 517-554 (2008).
2 Rocha, L.G., Almeida, J., Macedo, R.O., Barbosa-Filho, J.M., “A review of natural products with antileishmanial activity”, Phytomedicine,12(6-7), 514-535 (2005).
3 Iqbal, M., Black, R.J.G., Winn, J., Reeder, A.T., Blake, A.J., Clarke, P.A., “Studies on transannulation reactions across a nine-membered ring: The synthesis of natural product-like structures”, Org. Biomol. Chem., (9), 5062-5078 (2011).
4 Xiao, M., Shao, Y.D., Yan, W.D., Zhang, Z.Z., “Measurement and correlation of solubilities of apigenin and apigenin 7-O-rhamnosylglucoside in seven solvents at different temperatures”, J. Chem. Thermodyn.,43(3), 240-243 (2011).
5 Chebil, L., Chipot, C., Archambault, F., Humeau, C., Engasser, J.M., Ghoul, M., Dehez, F., “Solubilities inferred from the combination of experiment and simulation case study of quercetin in a variety of solvents”, J. Phys. Chem. B,114(38), 12308-12313 (2010).
6 Chebil, L., Humeau, C., Anthoni, J., Dehez, F., Engasser, J.M., Ghoul, M., “Solubility of flavonoids in organic solvents”, J. Chem. En g. Data,52(5), 1552-1556 (2007).
7 Liu, L.X., Chen, J., “Solubility of hesperetin in various solvents from (288.2 to 323.2) K”, J. Chem. Eng. Data,53(7), 1649-1650 (2008).
8 Tamura, K., Li, H.D., “Mutual solubilities of terpene in methanol and water and their multicomponent liquid-liquid equilibria”, J. Chem. Eng. Data,50(6), 2013-2018 (2005).
9 Chen, W., Su, B.G., Xing, H.B., Yang, Y.W., Ren, Q.L., “Solubility of desmosterol in five organic solvents”, J. Chem. Eng. Data,53(11), 2715-2717 (2008).
10 Zheng, Y.L., Liu, X.S., Luan, L.J., Wang, L.H., Wu, Y.J., “Solubility of physalin D in ethanol, methanol, propanone, trichloromethane, ethyl ethanoate, and water at temperatures from (283.2 to 313.2) K E-5232-2011”, J. Chem. Eng. Data,55(9), 3690-3692 (2010).
11 Bakhbakhi, Y., Charpentier, P., Rohani, S., “The solubility of beclomethasone-17, 21-dipropionate in selected organic solvents: Experimental measurement and thermodynamic modeling”, Org. Process Res. Dev.,13(6), 1322-1326 (2009).
12 Shareef, A., Angove, M.J., Wells, J.D., Johnson, B.B., “Aqueous solubilities of estrone, 17 beta-estradiol, 17 alpha-ethynylestradiol, and bisphenol A”, J. Chem. Eng. Data,51(3), 879-881 (2006).
13 Lesser, S., Cermak, R., Wolffram, S., “Bioavailability of quercetin in pigs is influenced by the dietary fat content B-7379-2011”, J. Nutr.,134(6), 1508-1511 (2004).
14 Xiao, M., Yan, W.D., Zhang, Z.Z., “Solubilities of apigenin in ethanol plus water at different temperatures”, J. Chem. Eng. Data,55(9), 3346-3348 (2010).
15 Peng, B., Yan, W.D., “Solubility of luteolin in ethanol plus water mixed solvents at different temperatures”, J. Chem. En g. Data,55(1), 583-585 (2010).
16 Chen, W., Su, B.G., Xing, H.B., Yang, Y.W., Ren, Q.L., “Solubilities of cholesterol and desmosterol in binary solvent mixtures of n-hexane plus ethanol”, Fluid Phase Equilibr.,287(1), 1-6 (2009).
17 Kahlen, J., Masuch, K., Leonhard, K., “Modelling cellulose solubilities in ionic liquids using COSMO-RS”, Green Ch em.,12(12), 2172-2181 (2010).
18 Guo, Z., Lue, B.M., Thomasen, K., Meyer, A.S., Xu, X.B., “Predictions of flavonoid solubility in ionic liquids by COSMO-RS: Experimental verification, structural elucidation, and solvation characterization”, Green Chem.,9(12), 1362-1373 (2007).
19 Smith, K.B., Bridson, R.H., Leeke, G.A., “Solubilities of pharmaceutical compounds in ionic liquids”, J. Chem. Eng. Data,56(5), 2039-2043 (2011).
20 Zakrzewska, M.E., Bogel-Lukasik, E., Bogel-Lukasik, R., “Solubility of carbohydrates in ionic liquids A-6315-2011”, Energ. Fuel.,24, 737-745 (2010).
21 Xing, H.B., Yang, Y.W., Su, B.G., Huang, M., Ren, Q.L., “Solubility of artemisinin in supercritical carbon dioxide”, J. Chem. En g. Data,48(2), 330-332 (2003).
22 Talansier, E., Braga, M., Rosa, P., Paolucci-Jeanjean, D., Meireles, M., “Supercritical fluid extraction of vetiver roots: A study of SFE kineticsC-3777-2008 C-5479-2008”, J. Supercrit. Fluid.,47(2), 200-208 (2008).
23 Chen, C.C., Song, Y.H., “Solubility modeling with a nonrandom two-liquid segment activity coefficient model C-3054-2008”, Ind. Eng. Chem. R es.,43(26), 8354-8362 (2004).
24 Gracin, S., Brinck, T., Rasmuson, A.C., “Prediction of solubility of solid organic compounds in solvents by UNIFAC”, Ind. Eng. Chem. Res.,41(20), 5114-5124 (2002).
25 Lu, Y.C., Lin, Q., Luo, G.S., Dai, Y.Y., “Solubility of berberine chloride in various solvents”, J. Chem. Eng. Data,51(2), 642-644 (2006).
26 Luo, W.P., Wang, Q.B., Fu, L.Q., Deng, W., Zhang, X.Y., Guo, C.C., “New group-interaction parameters of the UNIFAC model: Aromatic carboxyl binaries”, Ind. En g. Chem. Res.,50(7), 4099-4105 (2011).
27 Chen, C.C., Crafts, P.A., “Correlation and prediction of drug molecule solubility in mixed solvent systems with the nonrandom two-liquid segment activity coefficient (NRTL-SAC) model C-3054-2008”, Ind. Eng. Chem. Res.,45(13), 4816-4824 (2006).
28 Klamt, A., Eckert, F., “COSMO-RS: A novel and efficient method for the a priori prediction of thermophysical data of liquids”, Fluid Phase Equilibr.,172(1), 43-72 (2000).
29 Klamt, A., Eckert, F.Hornig, M., Beck, M.E., Burger, T., “Prediction of aqueous solubility of drugs and pesticides with COSMO-RS”, J. Comput. Chem.,23(2), 275-281 (2002).
30 Lapkin, A.A., Peters, M., Greiner, L., Chemat, S., Leonhard, K., Liauw, M.A., Leitner, W., “Screening of new solvents for artemisinin extraction process using ab initio methodology A-8577-2008 B-2127-2009 A-7194-2008”, Green Chem.,12(2), 241-251 (2010). 31 Kholod, Y.A., Grynova, G., Gorb, L., Hill, F.C., Leszczynski, J.,“Evaluation of the dependence of aqueous solubility of nitro compounds on temperature and salinity: A COSMO-RS simulation”, Chemosphere,83(3), 287-294 (2011).
32 Shu, C.C., Lin, S.T., “Prediction of drug solubility in mixed solvent systems using the COSMO-SAC activity coefficient model”, Ind. Eng. Chem. R es.,50(1), 142–147 (2011).
33 Tung, H.H., Tabora, J., Variankaval, N., Bakken, D., Chen, C.C.,“Prediction of pharmaceutical solubility via NRTL-SAC and COSMO-SAC”, J. Pharm. Sci.,97(5), 1813-1820 (2008).
34 Hills, E.E., Abraham, M.H., Hersey, A., Bevan, C.D., “Diffusion coefficients in ethanol and in water at 298 K: Linear free energy relationships”, Fluid Phase Equilibr.,303(1), 45-55 (2011).
35 Ma, Y.G., Zhu, C.Y., Ma, P.S., Yu, K.T., “Studies on the diffusion coefficients of amino acids in aqueous solutions”, J. Chem. En g. Data,50(4), 1192-1196 (2005).
36 Mogi, N., Sugai, E., Fuse, Y., Funazukuri, T., “Infinite dilution binary diffusion coefficients for six sugars at 0.1 MPa and temperatures from (273.2 to 353.2) K”, J. Chem. Eng. Data,52(1), 40-43 (2007).
37 Funazukuri, T., Nishimoto, N., Wakao, N., “Binary diffusion-coefficients of organic-compounds in hexane, dodecane, and cyclohexane at 303.2-333.2 K and 16.0 MPa”, J. Chem. Eng. Data,39(4), 911-915 (1994).
38 Funazukuri, T., Fukuda, Y., Nishimoto, N., Wakao, N., “Measurement of binary diffusion-coefficients of organic-compounds in n-hexane, n-dodecane and cyclohexane at 16.0 MPa”, Kag. Kog. Ronbunshu,19(6), 1157-1164 (1993).
39 Rezaei, K.A., Temelli, F., “Using supercritical fluid chromatography to determine diffusion coefficients of lipids in supercritical CO2”, J. Supercrit. Fluids,17(1), 35-44 (2000).
40 Funazukuri, T., Kong, C.Y., Kagei, S., “Binary diffusion coefficient,partition ratio, and partial molar volume for docosahexaenoic acid, eicosapentaenoic acid and alpha-linolenic acid at infinite dilution in supercritical carbon dioxide”, Fluid Phase Equilibr.,206, 163-178 (2003).
41 Funazukuri, T., Kong, C.Y., Kikuchi, T., Kagei, S., “Measurements of binary diffusion coefficient and partition ratio at infinite dilution for linoleic acid and arachidonic acid in supercritical carbon dioxide”, J. Chem. Eng. Data,48(3), 684-688 (2003).
42 Funazukuri, T., Kong, C.Y., Kagei, S., “Effects of molecular weight and degree of unsaturation on binary diffusion coefficients for lipids in supercritical carbon dioxide”, Fluid Phase Equilib r.,219(1), 67-73 (2004).
43 Kong, C.Y., Withanage, N., Funazukuri, T., Kagei, S., “Binary diffusion coefficients and retention factors for long-chain triglycerides in supercritical carbon dioxide by the chromatographic impulse response method”, J. Chem. Eng. Data,50(5), 1635-1640 (2005).
44 Kong, C.Y., Withanage, N., Funazukuri, T., Kagei, S., “Binary diffusion coefficients and retention factors for gamma-linolenic acid and its methyl and ethyl esters in supercritical carbon dioxide”, J. Supercrit. Fluids,37(1), 63-71 (2006).
45 Han, Y.S., Yang, Y.W., Wu, P.D., “Binary diffusion coefficients of arachidonic acid ethyl esthers, cis-5,8,11,14,17-eicosapentaenoic acid ethyl esthers, and cis-4,7,10,13,16,19-docosahexanenoic acid ethyl esthers in supercritical carbon dioxide”, J. Chem. Eng. Data,52(2), 555-559 (2007).
46 Kong, C.Y., Mori, M., Funazukuri, T., Kagei, S., “Measurements of binary diffusion coefficients, retention factors and partial molar volumes for myristoleic acid and its methyl ester in supercritical carbon dioxide”, Anal. Sci.,11(22), 1431-1436 (2006).
47 Scott, A., Smith, V.S.M.A., “Diffusion in supercritical mixtures: CO2+ cosolvent + solutes”, J. Supercrit. Fluids, (3), 175-179 (1990). 48 Suarez-Iglesias, O., Medina, I., Pizarro, C., Bueno, J.L., “Diffusion of benzyl acetate, 2-phenylethyl acetate, 3-phenylpropyl acetate, and dibenzyl ether in mixtures of carbon dioxide and ethanol”, Ind. Eng. Chem. Res.,46(11), 3810-3819 (2007).
49 Filho, C.A., Silva, C.M., Quadri, M.B., Macedo, E.A., “Tracer diffusion coefficients of citral and D-limonene in supercritical carbon dioxide”, Fluid Phase Equilibr.,204(1), 65-73 (2003).
50 Silva, C.M., Filho, C.A., Quadri, M.B., Macedo, E.A., “Binary diffusion coefficients of alpha-pinene and beta-pinene in supercritical carbon dioxide”, J. Supercrit. Fluids,32, 167-175 (2004).
51 Dong, X.Y., Su, B.G., Xing, H.B., Yang, Y.W., Ren, Q.L., “Diffusion coefficients of L-menthone and L-carvone in mixtures of carbon dioxide and ethanol”, J. Supercrit. Fluids,55(1), 86-95 (2010).
52 Filho, C.A., Silva, C.M., Quadri, M.B., Macedo, E.A., “Infinite dilution diffusion coefficients of linalool and benzene in supercritical carbon dioxide”, J. Chem. Eng. Data,47(6), 1351-1354 (2002).
53 Funazukuri, T., Kong, C.Y., Murooka, N., Kagei, S., “Measurements of binary diffusion coefficients and partition ratios for acetone, phenol, alpha-tocopherol, and beta-carotene in supercritical carbon dioxide with a poly(ethylene glycol)-coated capillary column”, Ind. Eng. Chem. Res.,39(12), 4462-4469 (2000).
54 Mantell, C., Rodriguez, M., Ossa, E.M., “Measurement of the diffusion coefficient of a model food dye (malvidin 3,5-diglucoside) in a high pressure CO2plus methanol system by the chromatographic peak-broadening technique”, J. Supercrit. Fluids,25(1), 57-68 (2003).
55 Funazukuri, T., Kong, C.Y., Kagei, S., “Infinite-dilution binary diffusion coefficient, partition ratio, and partial molar volume for ubiquinone CoQ10 in supercritical carbon dioxide”, Ind. En g. Chem. Res.,41(11), 2812-2818 (2002).
56 Funazukuri, T., Ishiwata, Y., “Diffusion coefficients of linoleic acid methyl ester, vitamin K3 and indole in mixtures of carbon dioxide and n-hexane at 313.2 K, and 16.0 MPa and 25.0 MPa”, Fluid Phase Equilibr.,164(1), 117-129 (1999).
57 Funazukuri, T., Ishiwata, Y.,Wakao, N., “Molecular-diffusion coefficients of vitamin-K3 in mixtures of CO2and normal-hexane at temperature of 313.2 K and total pressure of 16.0 MPa”, J. Chem. Eng. Jpn,24(3), 387-388 (1991).
58 Dong, X.Y., Su, B.G., Xing, H.B., Bao, Z.B., Yang, Y.W., Ren, Q.L.,“Cosolvent effects on the diffusions of 1,3-dichlorobenzene, L-carvone, geraniol and 3-fluorophenol in supercritical carbon dioxide”, J. Supercrit. Fluids,58(2), 216-225 (2011).
59 Gasik, A., Mitek, M., Kalisz, S., “Impact of the maceration process and storage conditions on the antioxidant capacity and content of some selected components in the cornelian cherry juice”, Zywn-Nauk Technol. Ja.,15(5), 161-167 (2008).
60 Puertolas, E., Saldana, G., Alvarez, I., Raso, J., “Experimental design approach for the evaluation of anthocyanin content of rose wines obtained by pulsed electric fields. Influence of temperature and time of maceration”, Food Chem.,126(3), 1482-1487 (2011).
61 Dobreva, A., Kovatcheva, N., Astatkie, T., Zheljazkov, V.D., “Improvement of essential oil yield of oil-bearing (Rosa damascena Mill.) due to surfactant and maceration”, Ind. Cro p. Prod.,34(3), 1649-1651 (2011).
62 Romero-Cascales, I., Ros-Garcia, J.M., Lopez-Roca, J.M., Gomez-Plaza, E., “The effect of a commercial pectolytic enzyme on grape skin cell wall degradation and colour evolution during the maceration process”, Food Chem.,130(3), 626-631 (2012).
63 Ranalli, A., De Mattia, G., Ferrante, M.L., “The characteristics of percolation olive oils produced with a new processing enzyme aid”, Int. J. Food Sci. Tech.,33(3), 247-258 (1998).
64 Blumberg, S., Frank, O., Hofmann, T., “Quantitative studies on the influence of the bean roasting parameters and hot water percolation on the concentrations of bitter compounds in coffee brew”, J. Agr. Food Chem.,58(6), 3720-3728 (2010).
65 Yi, Y.N., Yang, H., Zhao, Y., Bai, Z.G., “Extracting flavonoids from Choerospondias axillaris by percolation”, China Journal of Chinese Materia Medica,35(14), 1806-1808 (2010). (in Chinese)
66 Kim, K.H., Tucker, M.P., Nguyen, Q.A., “Effects of operating parameters on countercurrent extraction of hemicellulosic sugars from pretreated softwood”, Appl. Biochem. Biotech.,98, 147-159 (2002). 67 Li, H.B., Chen, F., “Simultaneous separation and purification of five bioactive coumarins from the Chinese medicinal plant Cnidium monnieri by high-speed countercurrent chromatography”, J. Sep. Sci.,28(3), 268-272 (2005).
68 Kassing, M., Jenelten, U., Schenk, J., Strube, J., “A new approach for process development of plant-based extraction processes”, Chem. Eng. Technol.,33(3), 377-387 (2010).
69 Yoon, K.D., Chin, Y.W., Yang, M.H., Kim, J., “Separation of anti-ulcer flavonoids from Artemisia extracts by high-speed countercurrent chromatography”, Food Chem.,129(2), 679-683 (2011).
70 Regalado, E.L., Tolle, S., Pino, J.A., Winterhalter, P., Menendez, R., Morales, A.R., Rodriguez, J.L., “Isolation and identification of phenolic compounds from rum aged in oak barrels by high-speed countercurrent chromatography/high-performance liquid chromatography-diode array detection-electrospray ionization mass spectrometry and screening for antioxidant activity”, J. Chromatogr. A,1218(41), 7358-7364 (2011).
71 Fang, L., Liu, Y.Q., Yang, B., Wang, X., Huang, L.Q., “Separation of alkaloids from herbs using high-speed counter-current chromatography”, J. Sep. Sci.,34(19), 2545-2558 (2011).
72 Ito, Y., “Spiral column configuration for protein separation by high-speed countercurrent chromatography”, Chem. Eng. Process.,49(7SI), 782-792 (2010). (in Chinese)
73 Wang, Q.E., Ma, S.M., Fu, B.Q., Lee, F., Wang, X.R., “Development of multi-stage countercurrent extraction technology for the extraction of glycyrrhizic acid (GA) from licorice (Glycyrrhiza uralensis Fisch)”, J. Biochem. Eng.,21(3), 285-292 (2004).
74 Wang, L.J., Weller, C.L., “Recent advances in extraction of nutraceuticals from plants”, Trends Food Sci. Tech.,17(6), 300-312 (2006).
75 Lu, S.P., Sun, Q., Wang, J.H., Sun, B.Q., “Survey of study on the extraction, purification and determination methods of glycyrrhizicacid in licorice”, C hina Journal of Chinese Materia Medica,31(5), 357-360 (2006). (in Chinese)
76 Alupului, A., Calinescu, I., Lavric, V., “Ultrasonic vs. microwave extraction intensification of active principles from medicinal plants”, Chem. Eng. Trans.,17, 1023-1028 (2009).
77 Garcia-Salas, P., Morales-Soto, A., Segura-Carretero, A., Fernandez-Gutierrez, A., “Phenolic-compound-extraction systems for fruit and vegetable samples”, Molecules,15(12), 8813-8826 (2010).
78 Li, W., Zheng, C., Zhao, J., Ning, Z.X., “Microwave assisted multi-stage countercurrent extraction of dihydromyricetin from Ampelopsis grossedentata”, Int. J. Food Eng.,7(4), DOI: 10.2202/1556-3758.2173 (2011).
79 Kaufmann, B., Christen, P., “Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction”, Phytochem. Analysis,13(2), 105-113 (2002).
80 Gonzalez-Nunez, L.N., Canizares-Macias, M.P., “Focused microwaves-assisted extraction of theobromine and caffeine from cacao”, Food Chem.,129(4), 1819-1824 (2011).
81 Ma, C.H., Liu, T.T., Yang, L., Zu, Y.G., Chen, X.Q., Zhang, L., Zhang, Y., Zhao, C.J., “Ionic liquid-based microwave-assisted extraction of essential oil and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits”, J. Chromatogr. A,1218(48), 8573-8580 (2011).
82 Liu, T.T., Sui, X.Y., Zhang, R.R., Yang, L., Zu, Y.G., Zhang, L., Zhang, Y., Zhang, Z.H., “Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from Rosmarinus officinalis”, J. Chromatogr. A,1218(47), 8480-8489 (2011).
83 Li, H.Y., Deng, Z.Y., Wu, T., Liu, R.H., Loewen, S., Tsao, R., “Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes”, Food Chem.,130(4), 928-936 (2012).
84 Upadhyay, R., Ramalakshmi, K., Rao, L.J.M., “Microwave-assisted extraction of chlorogenic acids from green coffee beans”, Food Chem.,130(1), 184-188 (2012).
85 Teng, H., Ghafoor, K., Choi, Y.H., “Optimization of microwave-assisted extraction of active components from Chinese Quince using response surface methodology”, J. Korean Soc. Appl. Bi.,52(6), 694-701 (2009).
86 Li, T., Qu, X.Y., Zhang, Q.A., Wang, Z.Z., “Ultrasound-assisted extraction and profile characteristics of seed oil from Isatis indigotica fort”, Ind. Crop. Prod.,35(1), 98-104 (2012).
87 Li, M.F., Sun, S.N., Xu, F., Sun, R.C., “Ultrasound-enhanced extraction of lignin from bamboo (Neosinocalamus affinis): Characterization of the ethanol-soluble fractions”, Ultrason. Sonochem.,19(2), 243-249 (2012).
88 Pan, Z.L., Qu, W.J., Ma, H.L., Atungulu, G.G., Mchugh, T.H.,“Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel”, Ultrason. Sonoch em.,19(2), 365-372 (2012).
89 Schinor, E.C., Salvador, M.J., Turatti, I., Zucchi, O., Dias, D.A.,“Comparison of classical and ultrasound-assisted extractions of steroids and triterpenoids from three Chresta spp”, Ultrason. Sonochem.,11(6), 415-421 (2004).
90 Karki, B., Lamsal, B.P., Jung, S., van Leeuwen, J., Pometto, A.L., Grewell, D., Khanal, S.K., “Enhancing protein and sugar release from defatted soy flakes using ultrasound technology”, J. Food Eng.,96(2), 270-278 (2010).
91 Pereira, C.G., Meireles, M.A.A., “Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives”, Food Bioprocess Tech.,3(3), 340-372 (2010).
92 Liza, M.S., Rahman, R.A., Mandana, B., Jinap, S., Rahmat, A., Zaidul, I.S.M., Hamid, A., “Supercritical carbon dioxide extraction of bioactive flavonoid from Strobilanthes crispus (Pecah Kaca)”, Food Bioprod. Process.,88(C2-3), 319-326 (2010).
93 Serra, A.T., Seabra, I.J., Braga, M.E.M., Bronze, M.R., de Sousa, H.C., Duarte, C.M.M., “Processing cherries (Prunus avium) using supercritical fluid technology. Part 1: Recovery of extract fractions rich in bioactive compounds”, J. Supercrit. Fluid.,55(1), 184-191 (2010).
94 Babovic, N.V., Petrovic, S.D., “Obtaining of the antioxidants by supercritical fluid extraction”, Hem. Ind.,65(1), 79-86 (2011).
95 Ganan, N., Brignole, E.A., “Fractionation of essential oils with biocidal activity using supercritical CO2-experiments and modeling”, J. Supercrit. Fluid.,58(1), 58-67 (2011).
96 Vidovic, S., Mujic, I., Zekovic, Z., Lepojevic, Z., Milosevic, S., Jokic, S., “Extraction of fatty acids from boletus edulis by subcritical and supercritical carbon dioxide”, J. Am. Oil Chem. Soc.,88(8), 1189-1196 (2011).
97 Langa, E., Cacho, J., Palavra, A., Burillo, J., Mainar, A.M., Urieta, J.S., “The evolution of hyssop oil composition in the supercritical extraction curve modelling of the oil extraction process”, J. Supercrit. Fluid.,49(1), 37-44 (2009).
98 Li, J.L., Zhang, M., Zheng, T.S., “The in vitro antioxidant activity of lotus germ oil from supercritical fluid carbon dioxide extraction”, Food Chem.,115(3), 939-944 (2009).
99 Upadhyay, N.K., Kumar, R., Mandotra, S.K., Meena, R.N., Siddiqui, M.S., Sawhney, R.C., Gupta, A., “Safety and healing efficacy of sea buckthorn (Hippophae rhamnoides L.) seed oil on burn wounds in rats”, Food Chem. Toxicol.,47(6), 1146-1153 (2009).
100 Chan, K.W., Ismail, M., “Supercritical carbon dioxide fluid extraction of Hibiscus cannabinus L. seed oil: A potential solvent-free and high antioxidative edible oil”, Food Chem.,114(3), 970-975 (2009).
101 Amiguet, V.T., Kramp, K.L., Mao, J.Q., Mcrae, C., Goulah, A., Kimpe, L.E., Blais, J.M., Arnason, J.T., “Supercritical carbon dioxide extraction of polyunsaturated fatty acids from northern shrimp (Pandalus borealis Kreyer) processing by-products”, Food Chem.,130(4), 853-858 (2012).
102 Tello, J., Viguera, M., Calvo, L., “Extraction of caffeine from robusta coffee (Coffea canephora var. robusta) husks using supercritical carbon dioxide”, J. Supercrit. Fluid.,59, 53-60 (2011).
103 Iheozor-Ejidor, P., Dey, E.S., “Extraction of rosavin from Rhodiola rosea root using supercritical carbon dioxide with water”, J. Supercrit. Fluid.,50(1), 29-32 (2009).
104 Yang, Z.N., Luo, S.Q., Peng, Q.C., Zhao, C., Yu, Z.W., “GC-MS analysis of the essential oil of coral ginger (Zingiber corallinum hance) rrhizome obtained by supercritical fluid extraction and steam distillation extraction”, Chromatogr.,69, 785-790 (2009).
105 Zarena, A.S., Sankar, K.U., “Supercritical carbon dioxide extraction of xanthones with antioxidant activity from garcinia mangostana: Characterization by HPLC/LC-ESI-MS”, J. Supercrit. Fluid.,49(3), 330-337 (2009).
106 Yesil-Celiktas, O., Otto, F., Parlar, H., “A comparative study of flavonoid contents and antioxidant activities of supercritical CO2extracted pine barks grown in different regions of Turkey and Germany”, Eur. Food Res. Technol.,229, 671 (2009).
107 Park, H.S., Im, N.G., Kim, K.H., “Extraction behaviors of caffeine and chlorophylls in supercritical decaffeination of green tea leaves”, Lwt-Food Sci. Technol.,45(1), 73-78 (2012).
108 Tzeng, T.C., Lin, Y.L., Jong, T.T., Chang, C., “Ethanol modified supercritical fluids extraction of scopoletin and artemisinin from Artemisia annua L.”, Sep. Purif. Technol.,56(1), 18-24 (2007).
109 Shi, J., Nawaz, H., Pohorly, J., Mittal, G., Kakuda, Y., Jiang, Y.M.,“Extraction of polyphenolics from plant material for functional foods-Engineering and technology”, Food Res. Int.,21(1), 139-166 (2005).
110 Mohamed, R.S., Saldana, M., Socantaype, F.H., Kieckbusch, T.G.,“Reduction in the cholesterol content of butter oil using supercritical ethane extraction and adsorption on alumina”, J. Supercrit. Fluid.,16(3), 225-233 (2000).
111 Huang, K.J., Wu, J.J., Chiu, Y.H., Lai, C.Y., Chang, C., “Designed polar cosolvent-modified supercritical CO2removing caffeine from and retaining catechins in green tea powder using response surface methodology”, J. Agr. Food Chem.,55(22), 9014-9020 (2007).
112 De Azevedo, A., Kieckbush, T.G., Tashima, A.K., Mohamed, R.S., Mazzafera, P., de Melo, S., “Extraction of green coffee oil using supercritical carbon dioxide”, J. Supercrit. Fluid.,44(2), 186-192 (2008).
113 Icen, H., Guru, M., “Extraction of caffeine from tea stalk and fiber wastes using supercritical carbon dioxide”, J. Supercrit. Fluid.,50(3), 225-228 (2009).
114 Tang, W.Q., Li, D.C., Lv, Y.X., Jiang, J.G., “Extraction and removal of caffeine from green tea by ultrasonic-enhanced supercritical fluid”, J. Food Sci.,75(4), C363-C368 (2010).
115 Icen, H., Guru, M., “Effect of ethanol content on supercritical carbon dioxide extraction of caffeine from tea stalk and fiber wastes”, J. Supercrit. Fluid.,55(1), 156-160 (2010).
116 Del Valle, J.M., Rivera, O., Teuber, O., Palma, M.T., “Supercritical CO2extraction of Chilean hop (Humulus lupulus) ecotypes”, J. Sci. Food Agr.,83(13), 1349-1356 (2003).
117 Brunner, G., “Supercritical fluids: Technology and application to food processing”, J. Food Eng.,67, 21-33 (2005).
118 Roj, E., Skowronski, B., “Modeling of hop extraction under supercritical conditions”, Przem. Chem.,85(8-9Part 2), 1140-1141 (2006).
119 Zekovic, Z., Pfaf-Sovljanski, I., Grujic, O., “Supercritical fluid extraction of hops”, J. Serb. Chem. Soc.,72(1), 81-87 (2007).
120 Fischer, M., Jefferies, T.M., “Optimization of nicotine extraction from tobacco using supercritical fluid technology with dynamic extraction modeling”, J. Agr. Food Chem.,44(5), 1258-1264 (1996).
121 Pilorz, K., Bjorklund, E., Bowadt, S., Mathiasson, L., Hawthorne, S.B., “Determining PCB sorption desorption behavior on sediments using selective supercritical fluid ertraction. 1. Describing PCB extraction with simple diffusion models”, Environ. Sci. Technol.,33(13), 2204-2212 (1999).
122 Reverchon, E., Donsi, G., Osseo, L.S., “Modeling of supercritical-fluid extraction from herbaceous matrices”, Ind. Eng. Chem. Res.,32(11), 2721-2726 (1993).
123 Kim, K.H., Hong, J., “A mass transfer model for super- and near-critical CO2extraction of spearmint leaf oil”, Sep. Sci. Technol.,37(10), 2271-2288 (2002).
124 Poletto, M., Reverchon, E., “Comparison of models for supercritical fluid extraction of seed and essential oils in relation to the masstransfer rate”, Ind. Eng. Chem. Res.,35(10), 3680-3686 (1996).
125 Bartle, K.D., Clifford, A.A., Hawthorne, S.B., Langenfeld, J.J., Miller, D.J., Robinson, R., “A model for dynamic extraction using a supercritical fluid”, J. Supercrit. Fluid.,3(3), 143-149 (1990).
126 Herrero, M., Cifuentes, A., Ibanez, E., “Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae - A review”, Food Chem.,98(1), 136-148 (2006).
127 Ibanez, E., Kubatova, A., Senorans, F.J., Cavero, S., Reglero, G., Hawthorne, S.B., “Subcritical water extraction of antioxidant compounds from rosemary plants”, J. Agr. Food Chem.,51(2), 375-382 (2003).
128 Sereewatthanawut, I., Prapintip, S., Watchiraruji, K., Goto, M., Sasaki, M., Shotipruk, A., “Extraction of protein and amino acids from deoiled rice bran by subcritical water hydrolysis”, Bioresource Technol.,99(3), 555-561 (2008).
129 Wiboonsirikul, J., Hata, S., Tsuno, T., Kimura, Y., Adachi, S.,“Production of functional substances from black rice bran by its treatment in subcritical water”, Lwt-Food Sci. Technol.,40(10), 1732-1740 (2007).
130 Welton, T., “Room-temperature ionic liquids. solvents for synthesis and catalysis”, Chem. Rev.,99(8), 2071-2083 (1999).
131 Giernoth, R., “Task-specific ionic liquids”, Angew. Chem. Int. Edit.,49(16), 2834-2839 (2010).
132 Ohno, H., “Functional design of ionic liquids”, B. Chem. Soc. Jpn,79(11), 1665-1680 (2006).
133 Usuki, T., Yasuda, N., Yoshizawa-Fujita, M., Rikukawa, M., “Extraction and isolation of shikimic acid from ginkgo biloba leaves utilizing an ionic liquid that dissolves cellulose”, Chem. Commun.,47(38), 10560-10562 (2011).
134 Chowdhury, S.A., Vijayaraghavan, R., Macfarlane, D.R., “Distillable ionic liquid extraction of tannins from plant materials”, Green Chem.,12(6), 1023-1028 (2010).
135 Lapkin, A.A., Plucinski, P.K., Cutler, M., “Comparative assessment of technologies for extraction of artemisinin”, J. Nat. Prod.,69(11), 1653-1664 (2006).
136 Lu, Y.B., Ma, W.Y., Hu, R.L., Dai, X.J., Pan, Y.J., “Ionic liquid-based microwave-assisted extraction of phenolic alkaloids from the medicinal plant Nelumbo nucifera Gaertn”, J. Chromatogr. A,1208, 42-46 (2008).
137 Du, F.Y., Mao, X.H., Li, G.K., “Application of ionic liquids in the microwave-assisted extraction of trans-resveratrol from rhizma polygoni cuspidati”, J. Chromato gr. A,1140, 56-62 (2007).
138 Bi, W., Tian, M., Row, K.H., “Ultrasonication-assisted extraction and preconcentration of medicinal products from herb by ionic liquids”, Talanta,85(1), 701-706 (2011).
139 Shi, S.Y., Huang, K.L., Zhang, Y.P., Liu, S.Q., “Preparative isolation and purification of two flavonoid glycosides from taraxacum mongolicum by high-speed counter-current chromatography”, Sep. Purif. Technol.,60(1), 81-85 (2008).
140 Shi, S.Y., Zhang, Y.P., Zhao, Y., Huang, K.L., “Preparative isolation and purification of three flavonoid glycosides from taraxacum mongolicum by high-speed counter-current chromatography”, J. Sep. Sci.,31(4), 683-688 (2008).
141 Jiang, X.Y., Shi, S.Y., Zhang, Y.P., Chen, X.Q., “Activity-guided isolation and purification of three flavonoid glycosides from neo-taraxacum siphonanthum by high-speed counter-current chromatography”, Sep. Sci. Technol.,45(6), 839-843 (2010).
142 Deng, S.G., Deng, Z.Y., Fan, Y.W., Peng, Y., Li, J., Xiong, D.M., Liu, R., “Isolation and purification of three flavonoid glycosides from the leaves of Nelumbo nucifera (Lotus) by high-speed counter-current chromatography”, J. Chromatogr. B.,877(24), 2487-2492 (2009).
143 Guo, X.F., Wang, D.J., Duan, W.J., Du, J.H., Wang, X., “Preparative isolation and purification of four flavonoids from the petals of Nelumbo nucifera by high-speed counter-current chromatography”, Phytochem. Analysis,21(3), 268-272 (2010).
144 Xiao, W.H., Han, L.J., Shi, B., “Isolation and purification of flavonoid glucosides from radix astragali by high-speed counter-current chromatography”, J. Chromatogr. B,877, 697-702 (2009).
145 Bai, H.L., Wang, J., Liu, C.M., Li, L., “Isolation and purification of flavonoids from ziziphus jujuba by high-speed counter-current chromatography”, J. Chin. Chem. Soc. -Taip,57(5A), 1071-1076 (2010).
146 Xiao, X.H., Guo, Z.N., Deng, J.C., Li, G.K., “Separation and purification of isofraxidin from sarcandra glabra by microwave-assisted extraction coupled with high-speed counter-current chromatography”, Sep. Purif. Technol.,68(2), 250-254 (2009).
147 Tang, Q.F., Yang, C.H., Chen, F.L., Xin, X.F., Zeng, Y.C., “Separation and purification of bioactive flavonol glycosides from hedyotis diffusa willd by high-speed counter-current chromatography”, Sep. Sci. Technol.,46(7), 1184-1188 (2011).
148 Liu, R.M., Xu, L.L., Li, A.F., Sun, A.L., “Preparative isolation of flavonoid compounds from oroxylum indicum by high-speed counter-current chromatography by using ionic liquids as the modifier of two-phase solvent system”, J. Sep. Sci.,33(8), 1058-1063 (2010).
149 Yang, C.H., Tang, Q.F., Liu, J.H., Zhang, Z.J., Liu, W.Y., “Preparative isolation and purification of phenolic acids from smilax china by high-speed counter-current chromatography”, Sep. Purif. Technol.,61(3), 474-478 (2008).
150 Zhu, L.C., Yu, S.J., Zeng, X.A., Fu, X., Zhao, M.M., “Preparative separation and purification of five anthraquinones from Cassia tora L. by high-speed counter-current chromatography”, Sep. Purif. Technol.,63(3), 665-669 (2008).
151 Liu, Z.L., Yu, Y., Shen, P.N., Wang, J., Wang, C.Y., Shen, Y.J.,“Separation and purification of DL-tetrahydropalmatine from corydalis yanhusuo by high-speed counter-current chromatography”, Sep. Purif. Tech nol.,58(3), 343-346 (2008).
152 Zhan, K.Y., Xu, K., Yin, H.Z., “Preparative separation and purification of gingerols from ginger (Zingiber officinale Roscoe) by high-speed counter-current chromatography”, Food Chem.,126(4), 1959-1963 (2011).
153 Liu, Z.L., Wang, J., Shen, P.N., Wang, C.Y., Shen, Y.J., “Microwave-assisted extraction and high-speed counter-current chromatography purification of ferulic acid from radix angelicae sinensis”, Sep. Purif. Tech nol.,52(1), 18-21 (2006).
154 Liu, S., Wang, B., Li, X.Z., Qi, L.F., Liang, Y.Z., “Preparative separation and purification of liensinine, isolliensinine and neferine from seed embryo of nelumbo nucifera gaertn using high-speed counter-current chromatography”, J. Sep. Sci.,32(14), 2476-2481 (2009).
155 Kohler, N., Wray, V., Winterhalter, P., “Preparative isolation of procyanidins from grape seed extracts by high-speed counter-current chromatography”, J. Chromatogr. A,1177(1), 114-125 (2008).
156 Peng, A.H., Li, R., Hua, J., Chen, L.J., Zhao, X., Luo, H.D., Ye, H.Y., Yuan, Y., Wei, Y.Q., “Flow rate gradient high-speed counter-current chromatography separation of five diterpenoids from triperygium wilfordii and scale-up”, J. Chromatogr. A,1200(2), 129-135 (2008).
157 Niu, L.L., Xie, Z.S., Cai, T.X., Wu, P., Xue, P., Chen, X.L., Wu, Z.Y., Ito, Y., Li, F.M., Yang, F.Q., “Preparative isolation of alkaloids from Corydalis bungeana Turcz. by high-speed counter-current chromatography using stepwise elution”, J. Sep. Sci.,34(9), 987-994 (2011).
158 Wang, X.D., Huang, M., Yang, Y.W., Ren, Q.L., “Separation of EPA-EE and DHA-EE with supercritical fluid chromatography”, J. Chem. Ind. Eng.,54(1), 1558-1562 (2003). (in Chinese)
159 Han, Y.S., Ren, Q.L., Yang, Y.W., Zhang, H., Wu, P.D., “Effects of loading and flow rate on the preparation of EPA-EE and DHA-EE by supercritical fluid chromatography”, J. Chem. Eng. Chinese U.,21(2), 189-193 (2007). (in Chinese)
160 Yang, Y.W., Wu, C.J., Wang, X.D., Huang, M., Ren, Q.L., “Purification of EPA-EE and DHA-EE with supercritical fluid chromatography”, J. Ch em. Engd. Chinese U.,18(3), 293-296 (2004). (in Chinese)
161 Yang, X., “Preparation of arachidonic acid ethyl ester by supercritical fluid chromatography”, Master Thesis, Zhejiang Univ., Hangzhou (2006). (in Chinese)
162 Jiang, C.W., Ren, Q.L., Wu, P.D., “Study on retention factor and resolution of tocopherols by supercritical fluid chromatography”, J. Chromatogr. A,1005, 155-164 (2003).
163 Ramirez, P., Garcia-Risco, M.R., Santoyo, S., Senorans, F.J., Ibanez, E., Reglero, G., “Isolation of functional ingredients from rosemary by preparative-supercritical fluid chromatography (Prep-SFC) B-8465-2011”, J. Pharmaceut. Biomed.,41(5), 1606-1613 (2006).
164 Garcia-Risco, M.R., Vicente, G., Reglero, G., Fornari, T., “Fractionation of thyme (Thymus vulgaris L.) by supercritical fluid extraction and chromatography”, J. Supercrit. Fluid.,55(3), 949-954 (2011).
165 Geng, Z.F., Lu, H.S., Zhang, M.H., “Separation of solanesol by preparative-scale supercritical fluid chromatography”, Chem. Ind. En g. Prog.,25(10), 1479-1483 (2007). (in Chinese)
166 Pang, F., Lu, H.S., Liu, Y., Zhang, M.H., “The study on purification of artemisinin by supercritical fluid chromatography”, J. Chem. Eng. Chinese U,24(4), 569-573 (2010). (in Chinese)
167 Desmortreux, C., Rothaupt, M., West, C., Lesellier, E., “Improved separation of furocoumarins of essential oils by supercritical fluid chromatography”, J. Chromatogr. A,1216(42), 7088-7095 (2009).
168 Seidel-Morgenstern, A., Kessler, L.C., Kaspereit, M., “New developments in simulated moving bed chromatography”, Chem. Eng. Tech nol.,31(6), 826-837 (2008).
169 Rajendran, A., Paredes, G., Mazzotti, M., “Simulated moving bed chromatography for the separation of enantiomers”, J. Chromatogr. A,1216(4), 709-738 (2009).
170 Park, J.S., Kim, W.S., Kim, J.M., Kim, I.H., “Ibuprofen racemate separation by simulated moving bed”, J. Chem. En g. Jpn,41(7), 624-626 (2008).
171 Araujo, J., Rodrigues, R., Eusebio, M., Mota, J., “Chiral separation by two-column, semi-continuous, open-loop simulated moving-bed chromatography”, J. Chromatogr. A,1217(33), 5407-5419 (2010).
172 Lu, Y.B., Su, B.G., Yang, Y.W., Ren, Q.L., Wu, P.D., “Simulated moving bed separation of tocopherol homologues: Simulation and experiments”, J Zhejiang Univ-sc A,10(5), 758-766 (2009).
173 Ai, X.Z., “The extraction and purification of capsaicinoids and capsaicin from capsicum oleoresin”, Master Thesis, Zhejiang Univ., Hangzhou (2007). (in Chinese)
174 Lei, H.J., “Process research on separating of L-arabinose from xylose mother liquor”, Master Thesis, Zhejiang Univ., Hangzhou (2010). (in Chinese)
175 Kong, L.Y., Su, B.G., Bao, Z.B., Xing, H.B., Yang, Y.W., Ren, Q.L.,“Direct quantification of mono- and di-d-α-tocopherol polyethylene glycol 1000 succinate by high performance liquid chromatography”, J. Chromatogr. A,1218(48), 8664-8671 (2011).
176 Sun, P., Cai, Y.J., Peng, Q.J., “Optimizing conditions of separating xylitol mother liquid with simulated moving bed chromatography”, China Food Additives,26(6), 73-75 (2003). (in Chinese)
177 Wei, F., Zhao, Y.X., “Separation of capsaicin from capsaicinoids by simulated moving bed chromatography”, J. Chromatogr. A,1187, 281-284 (2008).
178 Cong, J.X., Lin, B.C., “Separation of liquiritin by simulated moving bed chromatography”, J. Chromatogr. A,1145(1-2), 190-194 (2007).
179 Long, N., Le, T.H., Kim, J.L., Lee, J.W., Koo, Y.M., “Separation of D-psicose and D-fructose using simulated moving bed chromatography”, J. Sep. Sci.,32(11), 1987-1995 (2009).
180 Nam, H.G., Han, M.G., Yi, S.C., Chang, Y.K., Mun, S., Kim, J.H.,“Optimization of productivity in a four-zone simulated moving bed process for separation of succinic acid and lactic acid”, Ch em. Eng. J.,171(1), 92-103 (2011).
181 Lu, X.B., Xing, H.B., Su, B.G., Yang, Y.W., Ren, Q.L., “Separation of macromolecular impurities in penicillin G sodium by gel filtration chromatography”, J. Liq. Chromatogr. R. T.,32(7), 985-999 (2009).
182 Aryusuk, K., Chumsantea, S., Sombatsuwan, P., Lilitchan, S., Krisnangkura, K., “Separation and determination of wax content using 100-phenogel column”, J. Am. Oil Chem. Soc.,88(10), 1497-1501 (2011).
183 Du, Q.Z., Jiang, H.Y., Ito, Y., “Separation of theaflavins of black tea. high-speed countercurrent chromatography vs. sephadex LH-20 gel column chromatography”, J. Liq. Chromatogr. R. T.,24(15), 2363-2369 (2001).
184 Cara, C., Ruiz, E., Castro, E., Carvalheiro, F., Moura, P., Girio, F.,“Separation of olive tree pruning oligomers from liquid hot water hydrolyzates using preparative gel filtration chromatography”, New Biotech nol.,251, S249 (2009).
185 Zhang, F., “Purification of immunolglobulin a from bovin colostrum”, Master Thesis, Zhejiang Univ., Hangzhou (2010). (in Chinese)
186 Cho, C.W., Lee, D.Y., Kim, C.W., “Concentration and purification of soluble pectin from mandarin peels using crossflow microfiltration system”, Carbohydr. Polym.,54(1), 21-26 (2003).
187 Denis, C., Masse, A., Fleurence, J., Jaouen, P., “Concentration and pre-purification with ultrafiltration of a R-phycoerythrin solution extracted from macro-algae grateloupia turuturu: Process definition and up-scaling”, Sep. Purif. Technol.,69(1), 37-42 (2009).
188 Xu, Z.H., Li, L., Wu, F.W., Tan, S.J., Zhang, Z.B., “The application of the modified PVDF ultrafiltration membranes in further purification of ginkgo biloba extraction”, J. Membr. Sci.,255, 125-131 (2005).
189 Goulas, A.K., Grandison, A.S., Rastall, R.A., “Fractionation of oligosaccharides by nanofiltration”, J. Sci. Food Agric.,83, 675-680 (2003).
190 Nabarlatz, D., Torras, C., Garcia-Valls, R., Montane, D., “Purification of xylo-oligosaccharides from almond shells by ultrafiltration”, Sep. Purif. Technol.,53(3), 235-243 (2007).
191 Sereewatthanawut, I., Baptista, I.I., Boam, A.T., Hodgson, A., Livingston, A.G., “Nanofiltration process for the nutritional enrichment and refining of rice bran oil”, J. Food Eng.,102(1), 16-24 (2011).
192 Kuhn, R.C., Filho, F.M., Silva, V., Palacio, L., Hernández, A., Prádanos, P., “Mass transfer and transport during purification of fructooligosaccharides by nanofiltration”, J. Membr. Sci.,365, 356-365 (2010).
193 Chen, R.W., Sims, K.A., Wicker, L., “Pectinesterase and pectin complexes inhibit ion exchange membrane separation”, J. Agric. Food Chem.,46(5), 1777-1782 (1998).
194 Conidi, C., Cassano, A., Drioli, E., “A membrane-based study for the recovery of polyphenols from bergamot juice”, J. Membr. Sci.,375, 182-190 (2011).
195 Kamada, T., Nakajima, M., Nabetani, H., Saglam, N., Iwamoto, S.,“Availability of membrane technology for purifying and concentrating oligosaccharides”, Eur. Food Res. Technol.,214(5), 435-440 (2002).
196 Bazinet, L., Cossec, C., Gaudreau, H., Desjardins, Y., “Production of a phenolic antioxidant enriched cranberry juice by electrodialysis with filtration membrane”, J. Agric. Food Chem.,57(21), 10245-10251 (2009).
197 Li, P., Wang, Y.H., Ma, R.Y., Zhang, X.L., “Separation of tea polyphenol from green tea leaves by a combined CATUFM-adsorption resin process”, J. Food Eng.,67(3), 253-260 (2005).
198 Hosomi, A., Arita, M., Sato, Y., Kiyose, C., Ueda, T., Igarashi, O., Arai, H., Inoue, K., “Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs”, Febs Lett.,409(1), 105-108 (1997).
199 Pyka, A., Sliwoik, J., “Chromatographic separation of tocopherols”, J. Chromatogr. A,935, 71-76 (2001).
200 Gutzeit, D., Winterhalter, P., Jerz, G., “Application of preparative high-speed counter-current chromatography/electrospray ionization mass spectrometry for a fast screening and fractionation of polyphenols”, J. Ch romatogr. A,1172(1), 40-46 (2007).
201 Harjo, B., Wibowo, C., Zhang, E., Luo, K.Q., Ng, K.M., “Development of process alternatives for separation and purification of isoflavones”, Ind. Eng. Chem. Res.,46(1), 181-189 (2007).
202 Yang, Q.W., Xing, H.B., Cao, Y.F., Su, B.G., Yang, Y.W., Ren, Q.L., “Selective separation of tocopherol homologues by liquid-liquid extraction using ionic liquids”, Ind. Eng. Chem. Res.,48(13), 6417-6422 (2009).
203 Yang, Q.W., Xing, H.B., Su, B.G., Yu, K., Bao, Z.B., Yang, Y.W., Ren, Q.L., “Improved separation efficiency using ionic liquidcosolvent mixtures as the extractant in liquid-liquid extraction: A multiple adjustment and synergistic effect”, Chem. Eng. J.,181, 334-342 (2012).
204 Cao, Y., Xing, H., Yang, Q., Bao, Z., Su, B., Yang, Y., Ren, Q.,“Separation of soybean isoflavone aglycone homologues by ionic liquid-based extraction”, J. Agr. Food Chem.,60(13), 3432-3440 (2012).
205 Yang, Q.W., Xing, H.B., Yang, Y.W., Su, B.G., Ren, Q.L., “Selective separation of tocopherol homologues by liquid-liquid extraction using ionic liquids: Effect of diluent”, In: AIChE Annual Meeting, Salt Lake City, USA (2009).
206 Cao, Y.F., Xing, H.B., Yang, Q.W., Su, B.G., “Liquid-liquid extractive separation of ginkgolide homologues using ionic liquid as extractant”, In: AIChE Annual Meeting, Minneapolis (2011).
207 Ni, X.L., Xing, H.B., Yang, Q.W., Su, B.G., Bao, Z.B., Yang, Y., Ren, Q.L., “Selective liquid-liquid extraction of natural phenolic compounds using amino acid ionic liquids: A case of α-tocopherol and methyl linoleate separation”, Ind. Eng. Chem. Res.,51(18), 6480-6488 (2012)
10.1016/S1004-9541(13)60560-1
2013-03-17, accepted 2013-05-20.
* Supported by the National Natural Science Foundation of China (20936005, 21076175 and 21076178), the National High Technology Research and Development Program of China (2012AA040211), and the Program for Zhejiang Leading Team of S&T Innovation (2011R50002).
** To whom correspondence should be addressed. E-mail: renql@zju.edu.cn
 Chinese Journal of Chemical Engineering2013年9期
Chinese Journal of Chemical Engineering2013年9期
- Chinese Journal of Chemical Engineering的其它文章
- Soft Sensor for Inputs and Parameters Using Nonlinear Singular State Observer in Chemical Processes*
- A New Tuning Method for Two-Degree-of-Freedom Internal Model Control under Parametric Uncertainty*
- Halloysite Nanotube Composited Thermo-responsive Hydrogel System for Controlled-release*
- A Novel γ-Alumina Supported Fe-Mo Bimetallic Catalyst for Reverse Water Gas Shift Reaction*
- Experimental and Theoretical Studies of CO2Absorption Enhancement by Nano-Al2O3and Carbon Nanotube Particles
- Volumetric and Transport Properties of Aqueous NaB(OH)4Solutions*
