A Novel γ-Alumina Supported Fe-Mo Bimetallic Catalyst for Reverse Water Gas Shift Reaction*
Abolfazl Gharibi Kharaji, Ahmad Shariati,** and Mohammad Ali Takassi
1Petroleum University of Technology, Ahwaz Faculty of Petroleum Engineering, Chemical Engineering Department, Ahwaz, Iran
2Petroleum University of Technology, Ahwaz Faculty of Petroleum Engineering, Science Department, Ahwaz, Iran
A Novel γ-Alumina Supported Fe-Mo Bimetallic Catalyst for Reverse Water Gas Shift Reaction*
Abolfazl Gharibi Kharaji1, Ahmad Shariati1,** and Mohammad Ali Takassi2
1Petroleum University of Technology, Ahwaz Faculty of Petroleum Engineering, Chemical Engineering Department, Ahwaz, Iran
2Petroleum University of Technology, Ahwaz Faculty of Petroleum Engineering, Science Department, Ahwaz, Iran
In reverse water gas shift (RWGS) reaction CO2is converted to CO which in turn can be used to produce beneficial chemicals such as methanol. In the present study, Mo/Al2O3, Fe/Al2O3and Fe-Mo/Al2O3catalysts were synthesised using impregnation method. The structures of catalysts were studied using X-ray diffraction (XRD), Brunauer-Emmett-Teller (BET) method, inductively coupled plasma atomic emission spectrometer (ICP-AES), temperature programmed reduction (H2-TPR), CO chemisorption, energy dispersive X-ray (EDX) and scanning electron microscopy (SEM) techniques. Kinetic properties of all catalysts were investigated in a batch reactor for RWGS reaction. The results indicated that Mo existence in structure of Fe-Mo/Al2O3catalyst enhances its activity as compared to Fe/Al2O3. This enhancement is probably due to better Fe dispersion and smaller particle size of Fe species. Stability test of Fe-Mo/Al2O3catalyst was carried out in a fixed bed reactor and a high CO yield for 60 h of time on stream was demonstrated. Fe2(MoO4)3phase was found in the structures of fresh and used catalysts. TPR results also indicate that Fe2(MoO4)3phase has low reducibility, therefore the Fe2(MoO4)3phase signi fi cantly inhibits the reduction of the remaining Fe oxides in the catalyst, resulted in high stability of Fe-Mo/Al2O3catalyst. Overall, this study introduces Fe-Mo/Al2O3as a novel catalyst with high CO yield, almost no by-products and fairly stable for RWGS reaction.
reverse water gas shift reaction, Fe-Mo/Al2O3catalyst, selectivity, stability, reducibility
1INTRODUCTION
The anthropogenic emissions of greenhouse gases (GHGs) are due mainly to the utilization of fossil fuels. The major challenges in the 21st century are to supply clean fuels and eliminate environmental pollution problems and stabilize GHG emissions due to fossil fuel exploitation. The mitigation of emission of GHGs, particularly CO2, and their effective utilization present both a challenge and an opportunity for sustainable development in energy and environment to the world. CO2is not just a greenhouse gas, but also an important source of carbon for producing organic chemicals, materials, carbohydrates, and fuels such as methanol, carboxylic acids and ethylene [1-5].
It is important to note that the amount of CO2emitted as the concentrated CO2-rich fl ue gases from electric power plants and effluent gases from industrial manufacturing plants have become much higher than the amount of carbon used for making most chemicals, organic materials and liquid transportation fuels [6].
In recent years, most of the authors [7-10] considered carbon dioxide to be the direct source of methanol production but the yield of methanol produced from CO2is lower than CO [11]. The reverse water gas shift (RWGS) reaction,
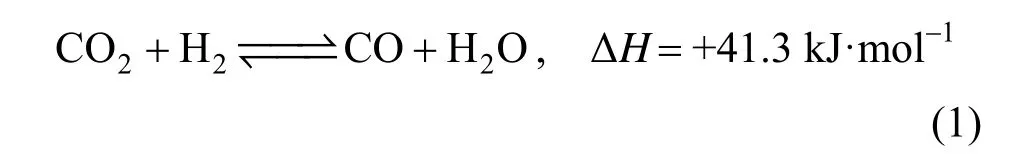
produces CO from CO2, therefore, the yield of methanol production increases when RWGS reaction takes place upstream of methanol synthesis in a chemical plant. The commercial catalyst, Fe2O3/Cr2O3, is a logical candidate for the RWGS reaction for the purpose of methanol production. But, Fe2O3in this catalyst is easily reduced into iron metal under high reaction temperature for the RWGS reaction [12-14].
The endothermic RWGS reaction takes place at high temperatures over Fe, FeSi, Fe3O4, Fe-Cu, ZnO, Pt, Pt-Ca and Pt-Mg based catalysts [3, 15-18] and at low temperatures (below 623 K) over Cu, Cu-ZnO and Pd based catalysts [17, 19, 20]. In the study by Joo and Jung [14] at temperature range of 673 K to 973 K, it is shown that addition of ZnO to Al2O3support would enhance the activity of the catalyst as well as its deactivation rate. The origin of deactivation is the conversion of zinc oxide molecules to zinc ions.
The products of RWGS reaction are CO and H2O. However, at some level of CO, it may react with hydrogen in reaction medium to form methane according to the following side reaction:
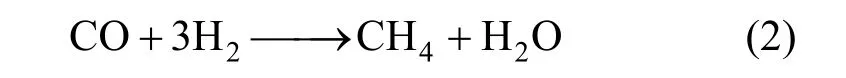
Using impregnation method instead of precipitation method in catalyst synthesis enhances CO selectivity in RWGS [21]. Also, addition of metals such as Fe, K and Pd supported on Al, Si and Ce can change CO selectivity in RWGS reaction [20, 22].
It has been reported [23-26] that CO2adsorbed on metal surface is present in two states: a physisorbed linear CO2state and a chemisorbed bent2CO?state.The chemisorbed anionic species turns out to represent an intrinsic precursor for CO2dissociation into CO and oxygen on iron surface.
Molybdenum has electronic and dispersing effects on Fe-Mo structure [Fe2(MoO4)3phase] of catalysts. The electronic effect transfers electrons from Fe to Mo (via a Fe OMo structure) and leads to an electron-de fi cient state of the Fe species and it would remain after the reduction process [27]. FeOMo existence in catalyst structure increases electronde fi cient state of Fe on the surface of catalyst. Thus, CO2is strongly chemisorbed on the Fe surface (in FeOMo structure) and undergoes sequential carbon-oxygen bond cleavage.
The study of Qin et al. [27] shows that the crystal size of iron oxide decreases continually with increasing Mo content, indicating that the dispersion of Fe species is improved by Mo addition. So aside from the electronic effect, Mo species also has a strong dispersion effect on the Fe species in Fe-Mo promoted catalysts. The Mo dispersing effect inhibits the aggregation of active iron particles in the heat treatment [27].
Light hydrocarbons are by-products of RWGS reaction over many catalysts, and development of catalyst for this reaction also concerns on its catalyst selectivity. In the present study, all catalyst systems (Mo/Al2O3, Fe/Al2O3and Fe-Mo/Al2O3) were compared in terms of their activity and CO selectivity for RWGS reaction in a batch reactor. Three series of catalysts are evaluated using both physical methods (XRD, BET, ICP-AES, H2-TPR, CO chemisorption, EDX and SEM techniques), and kinetic methods. Specifically, a batch reactor is applied for kinetics properties and a fixed-bed reactor for testing the catalyst stability.
2EXPERIMENTAL
2.1Catalyst synthesis
3.2 g of molybdenum complex (NH4)6Mo7O24·4H2O (Merck) was dissolved in 200 ml of distilled water, then this solution was added to a slurry of alumina that consist of 10 g of γ-Al2O3(Merck Co., 170 m2·g?1, 99% pure, porosity=1.3 cm3·g?1, pore diameter=18 nm) and 300 ml of distilled water. The solution was stirred by a high speed mechanical stirrer for 10 h at 308 K. In this process, molybdate anion was chemisorbed on the surface of γ-Al2O3particles, and the Mo/Al2O3catalyst was obtained after filtration, rinse and drying. The Fe/Al2O3catalyst was prepared by slowly addition of iron solution [4.4 g of Fe(NO3)2·6H2O (Merck) in 200 ml of distilled water] to the slurry of 10 g γ-Al2O3(Merck, ibid) in 300 ml water. The Fe-Mo/Al2O3catalyst also was synthesised by slowly addition of iron solution [4.4 g of Fe(NO3)2·6H2O in 200 ml of distilled water] to slurry of above Mo/Al2O3catalyst (10 g in 300 ml water). The stirring conditions for all catalysts were similar, and after that impregnated samples were subsequently air-dried at 323 K for 10 h and they were calcined in air at 923 K for 5 h.
2.2Catalyst characterization
The structures of these catalysts were studied using X-ray diffraction (XRD), which were obtained by a PW1840 X-ray powder diffractometer (Phillips, Netherland) using Cu tube anode operated at 40 kV and 30 mA with step size 0.02 from 5° to 90°.
The speci fi c surface areas of the samples were determined using the Brunauer-Emmett-Teller (BET) method with adsorption of nitrogen at liquid nitrogen temperature and subsequent desorption at room temperature after initial pretreatment of the samples by degassing at 573 K for 1 h. The BET surface area was obtained with a Quanta Chrome Quantasorb surface area analyzer (USA).
Temperature programmed reduction (TPR) was conducted on a U-shaped quartz tube embedded in a programmable furnace. 80 mg of the catalyst was pre-treated with pure He flow at 573 K for 1 h and then reduced with a gas mixture flow (5% H2, 95% Ar, 40 ml·min?1). TPR patterns were obtained by using a recorder connected to a gas chromatography (GC) equipped with TCD in a temperature range 430-1250 K at a heating rate of 10 K·min?1.
The metal dispersion and particle size of the reduced catalysts were determined by irreversible CO adsorption at 303 K by BELCAT-B chemisorption analyzer (BEL Inc., Japan). The CO uptake was measured by injecting successive 0.5 ml CO samples via a calibrated loop into 25 ml·min?1He carrier until constant peak area was reached.
Energy dispersive X-ray (EDX), which analyzes the chemical composition of the catalysts, was determined using a scanning electron microscope (SEM) equipped with an energy dispersive X-ray (EDX) analyzer at 17 kV and an acquisition lifetime of 160 s. The chemical composition of bulk medium were determined with ICP-AES emission spectroscope (Perkin Elmer, Optima 2000DV).
Scanning electron microscopy (SEM) images were recorded with an XL300 SEM (Philips, Netherland). The samples were coated with gold before the measurement. In this study, SEM tests were carried out with 10000 times amplification.
2.3RWGS reactor systems
Selectivity tests of the catalysts in RWGS reaction were carried out in a batch reactor. It consisted of a stainless steel cylinder, 15 cm tall and 7 cm inner diameter, with a flanged lid. This reactor was equipped with electrical heater and magnetic stirrer. For temperature adjustment, a thermocouple connected to a proportional integral derivative (PID) temperature controller was used. Reactants were charged via two holes in one side of the tank and while reaction was carried out, nothing else was put in or taken out. In allexperiments the magnetic stirrer was operated at 700 r·min?1to provide agitation. The sketch of the batch reactor is shown in Fig. 1 (a).
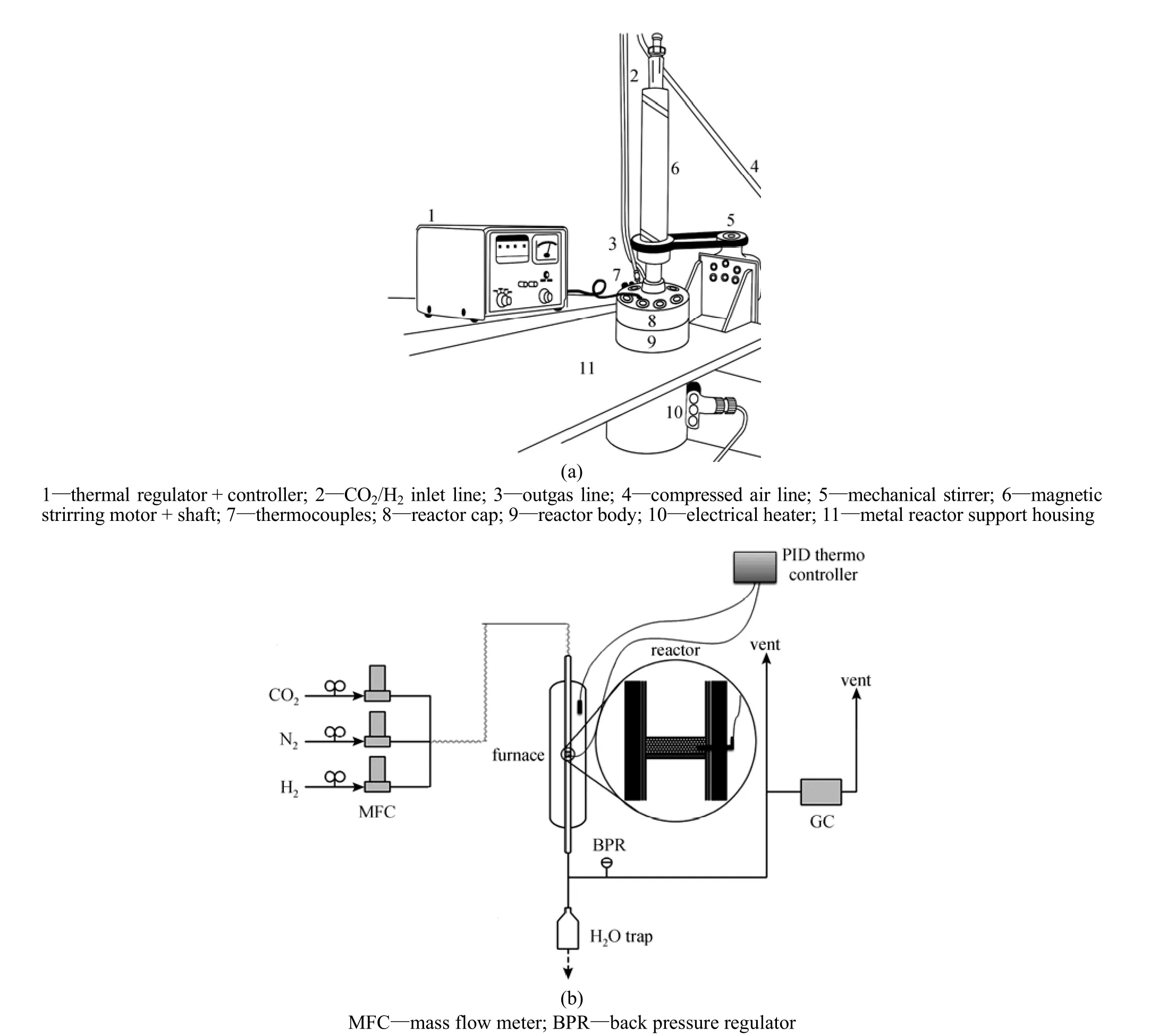
Figure 1A schematic diagram of the batch reactor (a) and the fixed-bed reactor (b)
All catalysts were reduced for 5 h with hydrogen gas at a temperature of 873 K and a pressure of 2.5 MPa before their use in the batch reactor. The reaction was performed with hydrogen to carbon dioxide ratio of 1 under 1 MPa of pressure. The catalyst loading was 5 g for each catalytic test. The activities of all catalysts were studied at a temperature range of 573-973 K. An ice-water cold trap was placed at the outlet of the reactor to condense out any water from the gas product stream. This stream was directed to a gas sampler. To analyze the produced gas, it was injected to a gas chromatography.
Stability test of Fe-Mo/Al2O3catalyst was then conducted in a fixed-bed reactor. Schematic diagrams of the experimental apparatus and the configuration of the fixed-bed reactor is shown in Fig. 1 (b). The reaction tube (20 mm ID and 150 mm length) of fixed bed reactor was made of stainless steel. The mass flow rate was controlled and measured using mass flow controllers (Hitachi). 0.8 g of catalyst was packed in the middle section of the bed. The stability of the Fe-Mo/Al2O3catalyst was investigated under the reaction condition of 873 K and GHSV (gas hourly space velocity) of 30000 [ml·h?1?(g cat.)?1], and the feed stream consists of CO2and H2with molar ratio of 1 under atmospheric pressure. Prior to reaction, the catalyst was reduced in situ at 873 K for 4 h in 200 ml·min?1flow of hydrogen and nitrogen with hydrogen to nitrogen ratio of 1/5. A cold trap at the outlet ofthe reactor was used to condense out any water from the product gas stream.
All products were analyzed by gas chromatography (YL6100, Young Lin, Korea) equipped with Q and MS capillary columns and an helium ionization detector (HID). CO, H2, CO2, and CH4were detected by gas chromatography (GC) and their respective mole fractions were calculated from peak area with a third order calibration function. The initial yield of CO and CH4were repeated five times for each catalyst. The results led to an estimated accuracy of ±3% in experimental measurements.
The CO and CH4yields were determined as follows:

3RESULTS
3.1Textural properties of catalysts
Figure 2 shows the X-ray diffraction patterns for Mo/Al2O3, Fe/Al2O3and Fe-Mo/Al2O3catalysts. The phases Al2O3[process flow diagram (PFD) 073-1512], MoO3(PFD 01-0706), Fe2(MoO4)3(PFD 035-0183), FeAl3(PFD 01-1265) and AlFe(MoO4)3(PFD 079-0193) were identi fi ed in the X-ray diffraction pattern of the Fe-Mo/Al2O3catalyst. Fe2(MoO4)3phase is consistent with the suggestion that Fe2O3and MoO3can easily form Fe2(MoO4)3after heat treatment [28, 29]. Al2O3(PFD 073-1512) is found in both Mo/Al2O3and Fe/Al2O3catalysts. Mo/Al2O3and Fe/Al2O3catalysts also contain of MoO3(PFD 01-0706) and Fe2O3, respectively.
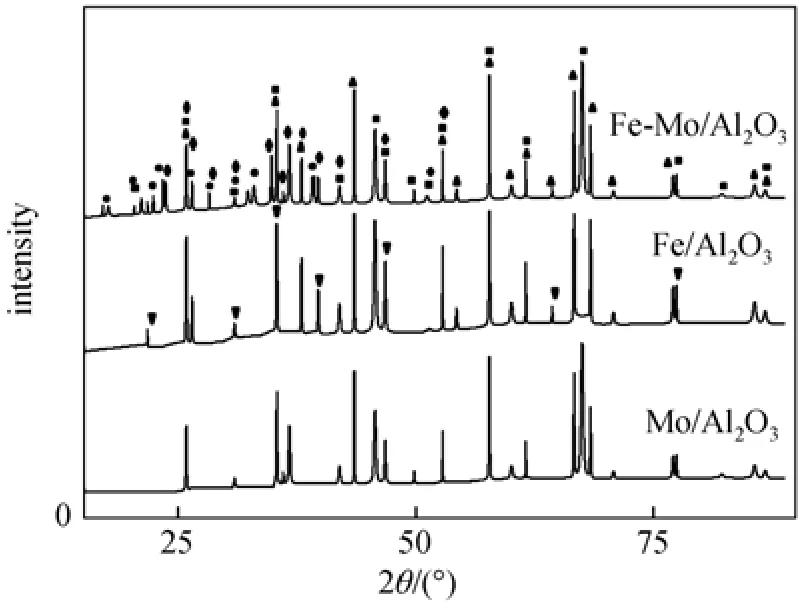
Figure 2The XRD patterns for calcined Mo/Al2O3, Fe/Al2O3and Fe-Mo/Al2O3catalysts▲ Al2O3; ■ MoO3; ◆ Fe2(MoO4)3; ● AlFe(MoO4)3; ▼ Fe2O3
The metals EDX concentrations, ICP-AES concentrations, BET surface areas, Fe dispersion and particles sizes of all catalyst systems are shown in Table 1. Fe and Mo promoter incorporations decreased total surface area of the catalysts. The compositions of the catalyst systems were studied using EDX and ICP-AES technique. The metals (Mo and Fe) concentrations from EDX spectroscopy were signi fi cantly greater than that determined from the ICP-AES. This implies that the metals (Mo and Fe) concentrations on the surface were higher than those of the bulk medium for all catalysts.
The metal (Fe) dispersions of the Fe-Mo/Al2O3and Fe/Al2O3catalysts given in Table 1 denote the dispersion of the active metal (Fe) which participates in the reaction and were formed by irreversible CO adsorption. Adsorption of CO on monometallic Mo/Al2O3catalyst is negligible, that has also been reported in the literature [30, 31]. Fe species in Fe-Mo/Al2O3catalyst has higher extent of dispersion than Fe/Al2O3, so Mo existence in structure of Fe-Mo/Al2O3increases Fe dispersion and at the same time decreases specific surface area of the catalyst. In addition, the results of Table 1 indicate that Fe particles size decreases with Fe dispersion.
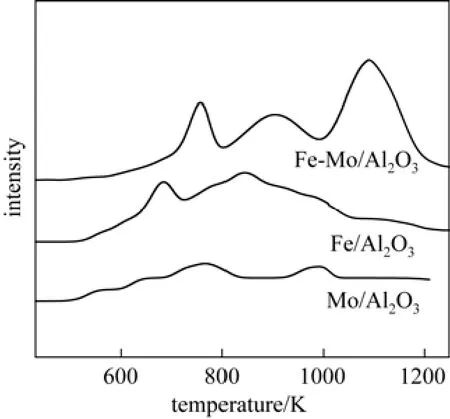
Figure 3The H2-TPR patterns for calcined Mo/Al2O3, Fe/Al2O3and Fe-Mo/Al2O3catalysts
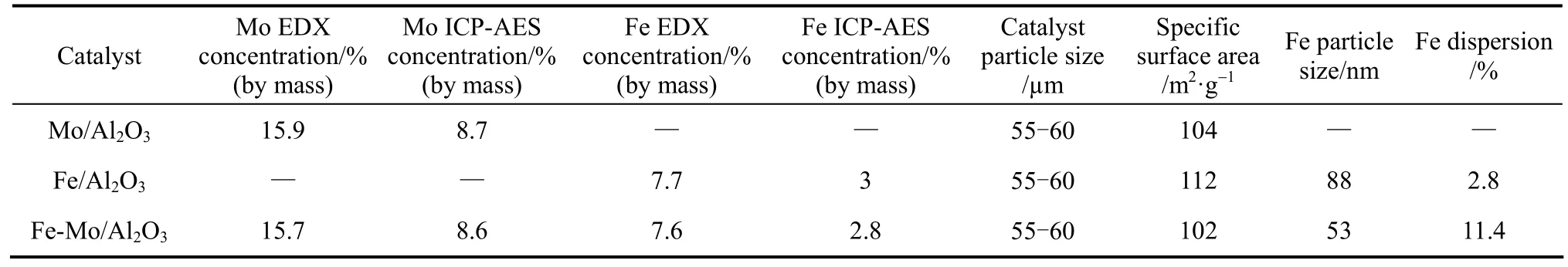
Table 1Specific surface area and mass percent of Fe and Mo for each catalyst
H2-TPR pro fi les of catalysts are shown in Fig. 3. In the case of the H2-TPR, no H2consumption was observed in any case below 600 K. The TPR pattern of Mo/Al2O3catalyst shows two peak approximately at765 and 990 K, The first peak is ascribed to polymeric Mo species which corresponds to Mo4O11formed during reduction of MoO3[32, 33], and the second peak attributes to reduction of Mo4O11to form MoO2[34]. In the pro fi le of the Fe/Al2O3catalyst, two well-separated H2consumption peaks can be observed, which are characteristic of the two-step reduction from Fe2O3to Fe3O4(680 K) and Fe3O4to Fe (840 K) [35, 27].
It is clear that Mo incorporation signi fi cantly affects the reduction behaviour of the Fe-Mo/Al2O3catalyst. Mo Existence in structure of Fe-Mo/Al2O3catalyst adds a peak at the higher temperature side of the second peak. According to XRD results, this peak (the third peak) could be attributed to the reductionresistant Fe-Mo composite oxides (possibly as the Fe2(MoO4)3phase). Therefore, in the pro fi le of Fe-Mo/Al2O3catalyst, three reduction peaks appear. According to the literature [36], these peaks correspond to the following three reduction steps: Fe2(MoO4)3→FeMoO4+ Mo4O11→ Fe2Mo3O8+ Fe3O4→ FeMo alloy. The results of Fig. 4 also indicate that the Fe2(MoO4)3phase inhibits signi fi cantly the reduction of the remaining Fe oxides in the catalyst.
3.2Catalytic activity and selectivity in the batch reactor
Figure 4 shows CO yield of all catalyst systems after 15 min of RWGS reaction at 5 different temperatures in the batch reactor. As it is expected, CO yield increases as temperature increases. It is also apparent that the CO yield for Fe-Mo/Al2O3catalyst is higher than those of Mo/Al2O3and Fe/Al2O3catalysts. The difference in the CO yield is more pronounced at low temperature. Fe-Mo/Al2O3catalyst is more active than Mo/Al2O3, therefore iron can improve catalytic activity in RWGS reaction. Even though Mo existence decreases the surface area of Fe-Mo/Al2O3catalyst compared with the Fe/Al2O3, it increases catalytic activity. According to the results in Table 1, this higher catalytic activity is mainly attributed to higher metal dispersion and also lower iron particle size over Fe-Mo/Al2O3catalyst.

Figure 4CO yield versus temperature for all catalyst systems (reaction time=15 min, H2/CO2=1, total pressure= 1 MPa)▼ Fe/Al2O3; ▲ Mo/Al2O3; ■ Fe-Mo/Al2O3
Figure 5 demonstrates the CO yield versus time for catalyst systems at 873 K in the batch reactor. Upon the start of the reaction, all catalysts had nearly the same CO yields. It takes 5 min for the reaction to reach the maximum yield on Fe-Mo/Al2O3catalyst, and after that a small decrease in CO yield is seen, which may be due to production of by-products such as methane.
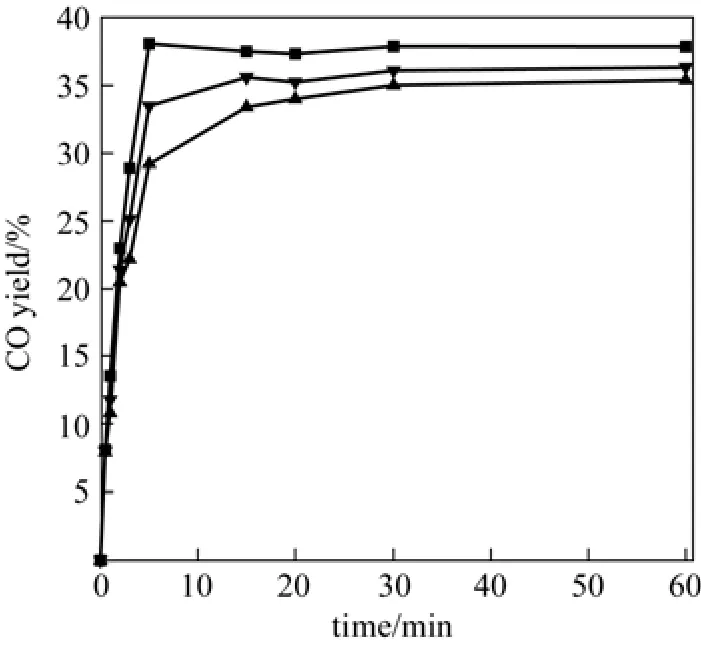
Figure 5CO yield versus time for 3 catalysts (reaction temperature=873 K, H2/CO2=1, total pressure=1 MPa)▼ Fe/Al2O3; ▲ Mo/Al2O3; ■ Fe-Mo/Al2O3
Mo/Al2O3and Fe/Al2O3catalysts also reach maximum CO yields after 5 min and then no significant change in the CO yield is observed. Provision of constant concentration of CO is the equality in the rate of CO production and consumption. Stoichiometric coefficient of CO in reactions 1 and 2 is equal, so the rate of CO production and CO consumption is equal.
Figure 6 shows CH4yield versus time at 873 K for all catalysts in the batch reactor. As is shown, the methane yield for Mo/Al2O3and Fe/Al2O3catalysts are higher than that of Fe-Mo/Al2O3throughout 60min of reaction time. This difference indicates that CO selectivity of Fe-Mo/Al2O3catalyst is higher than that of Mo/Al2O3and Fe/Al2O3.
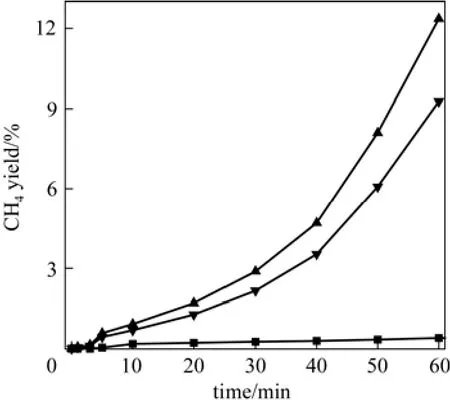
Figure 6CH4yield versus time for 3 catalysts (reaction temperature=873 K, H2/CO2=1, total pressure=1 MPa)▼ Fe/Al2O3; ▲ Mo/Al2O3; ■ Fe-Mo/Al2O3
The CO formation rates versus time at 873 K for all catalysts in the batch reactor are shown in Fig. 7. As it has been demonstrated, the trend of CO formation for all catalysts is the same. However, the initial rate of Fe-Mo/Al2O3catalyst is higher than those of Fe/Al2O3and Mo/Al2O3catalysts. After 30 min of reaction, no CO formation was observed for all catalysts.
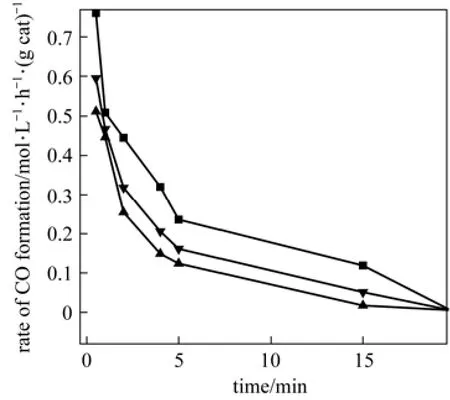
Figure 7CO formation rate versus time for all catalyst systems (reaction temperature=873 K, H2/CO2=1, total pressure=1 MPa)▼ Fe/Al2O3; ▲ Mo/Al2O3; ■ Fe-Mo/Al2O3
3.3Stability test in the fixed-bed reactor
Stability test of the catalyst is done under harsh operational conditions in the fixed bed reactor. The stability of Fe-Mo/Al2O3catalyst was investigated at 873 K and GHSV of 30000 ml·h?1·(g cat)?1. The time-on-stream (TOS) analysis of its activity in the RWGS reaction is shown in Fig. 8. TOS analysis was carried out for a continuous period of 60 h. This catalyst showed a considerable CO yield of 35% while the equilibrium CO yield is 38%. It is evident that this catalyst exhibits good stability within a period of 60 h.
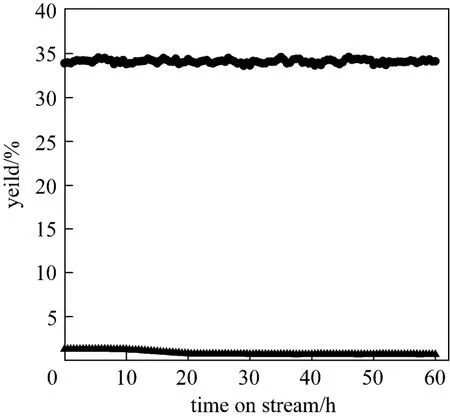
Figure 8Stability of Fe-Mo/Al2O3catalyst for RWGS reaction with respect to time on stream [reaction condition: 873 K, H2/CO2=1, and GHSV=30000 ml·h?1·(g cat)?1]● CO; ▲ CH4
SEM analysis was performed on the fresh Fe-Mo/ Al2O3catalyst (after reduction) and the used catalyst [after 60 h on stream under 873 K and GHSV of 30000 ml·h?1·(g cat)?1]. The SEM backscattered composition images of the fresh and used Fe-Mo/Al2O3catalyst are given in Figs. 9 and 10, respectively. Since the molecular mass of Mo is higher than Fe, its metallic form should be brighter in SEM micrographs, and consequently, brighter Mo species are expected if they are present in their reduced metallic form. XRD patterns confirm that Mo is present in its oxidized form (MoO3), therefore not seen as bright spots in SEM images. It is concluded that the bright spots observed on the particles in Fig. 8 are Fe crystallites that are irregularly spread on the surface. The Fe-Mo/Al2O3catalyst exhibits an agglomerated/mixed structure which indicates that the Fe crystallites reside either on top of or near to MoO3species that were previously impregnated on the support surface.

Figure 9SEM image for fresh Fe-Mo/Al2O3catalyst (×10000)
It is well known that the type and amount of carbon deposits are crucial factors that affect activity and stability of the catalysts. Fig. 10 shows a little carbon deposits formed during reaction on the used Fe-Mo/ Al2O3catalyst surface. Although a small amount of carbon deposition occurred, the catalyst remained stable, since the deactivation appears only at spots of very high surface carbon coverage [37-39].

Figure 10SEM image for used Fe-Mo/Al2O3catalyst (×10000)
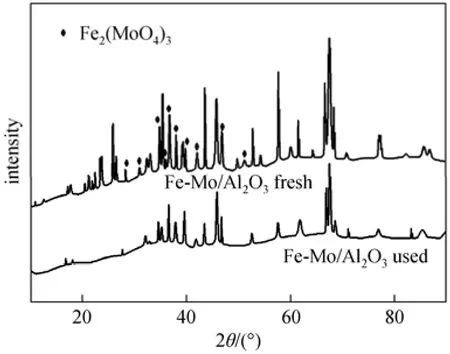
Figure 11XRD patterns of fresh and used Fe-Mo/Al2O3catalysts
XRD patterns of fresh and used Fe-Mo/Al2O3catalysts are illustrated in Fig. 11. Fe2(MoO4)3phase was observed on fresh and used catalysts. Therefore, it is concluded that this phase remained in structure of Fe-Mo/Al2O3catalyst during RWGS reaction. On the other hands, TPR results indicated that Fe2(MoO4)3phase has low reducibility, so that Fe-Mo/Al2O3catalyst was stable throughout the RWGS reaction.
4DISCUSSIONS
Figures 5 and 6 are compared to have a better understanding regarding the catalytic behaviour of all catalysts. According to the results, as long as the CH4yield is low, a high CO yield is observed and as CH4formation for each catalyst increases, the CO production shows a decrease. For all catalysts after 5 min of reaction time, the CO yield curve decreases when the CH4yield increases. At this time, CO yield for Mo/Al2O3, Fe/Al2O3and Fe-Mo/Al2O3catalysts reach 76%, 84% and 99% of the equilibrium production, respectively.
The XRD results indicate that the Fe OMo structure crystallizes into the Fe2(MoO4)3phase. Fe2(MoO4)3is monoclinic, with a structure containing isolated MoO4tetrahedron and FeO6octahedron that share corners, in which each oxygen atom is bound only to one Fe atom and one Mo atom [40, 41]. According to H2-TPR results Fe2(MoO4)3is not a easily reducible phase and it has a higher reduction temperature than iron oxides. It is conceivable that the FeOMo structure would inhibit the reduction of catalysts [27].
In the Fe Mo bimetallic system, there are obvious interactions between Fe and Mo species, which affect not only the properties of catalysts but also the RWGS reaction characteristics. The electronic effect, which transfers electrons from Fe to Mo and leads to an electron-de fi cient state of the Fe species, is the cause to produce the surface positive charge. The surface charge state of Fe species has an essential in fl uence on the adsorption of reactants, catalytic activity and product selectivity. Increasing surface positive charge is not helpful for CO adsorption and hence inhibits CO consumption and hydrocarbon formation. The study of Korner et al. [42] shows that the initial heat of adsorption of CO2on iron is higher than that of CO, the adsorption of CO2on the iron surface is dissociative, and the adsorption speed of CO2in chemisorption class is more than that of CO. It implies that adsorption of CO2on iron as chemisorption increases initial heat of adsorption and consequently its dissociation. On the other hand, oxygen existence can deplete the d-electrons and inhibit the adsorption of CO on transition metal surfaces, especially for the CO dissociation [43, 44]. Electron-de fi cient state of the Fe species (electrons transferred from Fe to Mo) and the existence of Fe2(MoO4)3phase make it reasonable for Fe-Mo/Al2O3to have high activity and CO selectivity in RWGS reaction. In this structure, the negative charge on Fe is transferred to the Mo species, thereby strengthening Fe O bonds and inhibiting the reduction and deactivation of Fe-Mo/Al2O3catalyst.
5CONCLUSIONS
By comparing all catalysts, Fe-Mo/Al2O3showed the best activity and CO selectivity for RWGS reaction at normal operating conditions. The surface charge state of the catalyst has an essential in fl uence on the adsorption of reactants, catalytic activity and product selectivity. Existence of FeOMo structure in Fe-Mo/Al2O3catalyst increases electron-de fi cient state of Fe on the surface of catalyst and cause to increase catalytic activity and CO selectivity for RWGS reaction.
The stability of Fe-Mo/Al2O3catalyst was examined at harsh operating condition which showed fairly stable catalyst. This study introduces Fe-Mo/Al2O3as a novel catalyst with high CO yield, almost no by-products and fairly stable for RWGS reaction.
REFERENCES
1 Melillo, J.M., Mcguire, A.D., Kicklighter, D.W., Moore, B., Voros marty, C.J., Schloss, A.L., “Global climate-change and terrestrial net primary production”, Nature,363, 234-240 (1993).
2 Halmann, M.M., Steinberg, M., Greenhouse Gas Carbon Dioxide Mitigation: Science and Technology, Lewis Publishers, Boca Raton (1999).
3 Park, S.W., Joo, O.S., Jung, K.D., Kim, H., Han, S. H., “Development of ZnO/Al2O3catalyst for reverse-water-gas-shiftreaction of CAMERE process”, Appl. Catal. A,211, 81-90 (2001).
4 Maroto, V.M.M., Song, C.S., Soong, Y., Environmental Challenges and Greenhouse Gas Control for Fossil Fuel Utilization in the 21st Century, Kluwer Academic/Plenum Publishers, New York (2002).
5 Song, C., “Global challenges and strategies for control, conversion and utilization of CO2for sustainable development involving energy, catalysis, adsorption and chemical processing”, Catal. Today,115, 2-32 (2006).
6 Song, C.S., Gaffney, A.M., Fujimoto, K., “CO2conversion and utilization”, ACS Symp. Ser.,809, 2-30 (2002).
7 Ostrovskii, V.E., “Mechanisms of methanol synthesis from hydrogen and carbon oxides at Cu-Zn-containing catalysts in the context of some fundamental problems of heterogeneous catalysis”, Catal. Toda y,15,141-160 (2002).
8 Bussche, K.M.V., Froment, G.F., “A steady-state kinetic model for methanol synthesis and the water gas shift reaction on a commercial Cu/ZnO/Al2O3catalyst”, J. Catal.,161, 1-3 (1996).
9 Sun, Q., Zhang, Y.L., Chen, H.Y., Deng, J.F., Wu, D., Chen, S.Y., “A novel process for the preparation of Cu/ZnO and Cu/ZnO/Al2O3ultrafine catalyst: Structure, surface properties, and activity for methanolsynthesis from CO2+ H2”, J. Catal.,167, 92-105 (1997).
10 Sahibzada, M., Metalfe, L.S., Chadwick, D., “Methanol synthesis from CO/CO2/H2over Cu/ZnO/Al2O3at differential and finite conversions”, J. Catal.,174, 111-118 (1998).
11 Edwards, J.H., “Potential sources of CO2and the options for its large-scale utilisation now and in the future”, Catal. Today,23, 59-66 (1995).
12 Twigg, M.V., Catalyst Handbook, Wolfe Publication, London (1989).
13 Joo, O.S., Jung, K.D., Moon, I., Rozovskii, A.Y., Lin, G.I., Han, S.H., Uhm, S.J., “Carbon dioxide hydrogenation to form methanol via a reverse-water-gas-shift reaction”, Ind. Eng. Chem. Res.,38, 1808-1812 (1999).
14 Joo, O.S., Jung, K.D., “Stability of ZnAl2O4catalyst for reverse-water-gas-shift reaction”, Bull. Korean Chem. Soc.,24, 86-90 (2003).
15 Roman, M.C., Cazorla, A.D., Linares, S.A., Calinas, M.C., “Carbon dioxide hydrogenation catalyzed by alkaline earth and platinum-based catalysts supported on carbon”, Appl. Catal. A,116, 187-204 (1994).
16 Kaspar, J., Graziani, M., Rahman, A.H., Trovarelli, A., Vichi, E.J.S., da Silva, E.C., “Carbon dioxide hydrogenation over iron containing catalysts”, Ap pl. Catal. A,117, 125-137 (1994).
17 Spencer, M.S., “On the activation energies of the forward and reverse water-gas shift reaction”, Catal. Lett,32, 9-13 (1995).
18 Chen, C.S., Cheng, W.H., Lin, S.S., “Study of iron-promoted Cu/SiO2catalyst on high temperature reverse water gas shift reaction”, Ap pl. Catal. A,257, 97-106 (2004).
19 Koeppel, R.A., Baiker, A., Wokaun, A., “Copper/zirconia catalysts for the synthesis of methanol from carbon dioxide: Influence of preparation variables on structural and catalytic properties of catalysts”, Appl. Catal. A,84, 77-79 (1992).
20 Pettigew, D.J., Trimm, D.L., Cant, N.W., “The effects of rare earth oxides on the reverse water-gas-shift reaction on palladium/alumina”, Catal. Lett,28, 313-315 (1994).
21 Yan, S.R., Jun, K.W., Hong, J.S., Choi, M.J., Lee, K.W., “Promotion effect of Fe-Cu catalyst for the hydrogenation of CO2and application to slurry reactor”, Appl. Catal. A,194, 63-70 (2000).
22 Perez-Alonso, F.J., Ojeda, M., Herranz, T., Rojas, S., González-Carballo, J.M., Terreros, P., Fierro, J.L.G., “Carbon dioxide hydrogenation over Fe-Ce catalysts”, Catal. Commun,9, 1945-1948 (2008).
23 Freund, H.J., Messmer, R.P., “On the bonding and reactivity of CO2 on metal surfaces”, Surf. Sci,172, 1-3 (1986).
24 Freund, H.J., Behner, H., Bartos, B., Wedler, G., Kahlen-beck, H., Neumann, M., “CO2adsorption and reaction on Fe(III): An angle resolved photoemission (ARUPS) study”, Surf. Sci,180, 550 (1987).
25 Choe, S.J., Park, D.H., Huh, D.S., “Adsorption and dissociation reaction of carbon dioxide on Pt and Fe surface: MO- study”, Bull. Korean Chem. Soc.,21, 779-784 (2000).
26 Nassir, M.H., Dwyer, D.J.J., “Sequential carbon oxygen bond cleavage in chemisorption of CO2on Fe”, Vac. Sci. Technol,11, 2104-2110 (1993).
27 Qin, S., Zhang, C., Xu, J., Yang, Y., Xiang, H., Li, Y., “Fe-Mo interactions and their influence on Fischer–Tropsch synthesis performance”, Appl. Catal. A,392, 118-126 (2011).
28 Maiti, G.C., Malessa, R., Baerns, M., “Studies on the reduction of the Fe2O3-MoO3system and its interaction with synthesis gas (CO + H2)”, Thermochim. Acta,80, 11-21 (1984).
29 Lin, D., Chen, K. D., Chen, Y., “Study of the Interactions between MoO3and α-Fe2O3”, J. Solid State Ch em,129, 30-36 (1997).
30 Scott, C.E., Romero, T., Lopero, E., Arruebarrena, M., Betancourt, P., Bolivar, C., Perez-Zurita, M. J., Marcano, P., Goldwasser, J., “Reaction between ruthenium and molybdenum in RuMo/Al2O3catalysts”, Appl. Catal. A,125, 71-79 (1995).
31 Erhan Aksoylu, A., Misrli, Z., I?lsen Onsan, Z., “Interaction between nickel and molybdenum in Ni-Mo/Al2O3catalysts (I) CO2methanation and SEM-TEM studies”, Appl. Catal. A, 168, 385-397 (1998).
32 Kaluza, L., Gulkova, D., V?t, Z., Zdrazil, M., “Effect of support type on the magnitude of synergism and promotion in CoMo sulphide hydrodesulphurisation catalyst”, Appl. Catal. A,324, 30-35 (2007).
33 Chary, K.V.R., Bhaskar, T., Kishan, G., Reddy, K.R., “Characterization and reactivity of molybdenum oxide catalysts supported on niobia”, J Phys. Chem. B,105, 4392-4398 (2001).
34 Ressler, T., Wienold, J., Jentoft, R. E., “Formation of bronzes during temperature-programmed reduction of MoO3with hydrogen—An in situ XRD and XAFS study”, Solid State Ionics,141-142, 243-251 (2001).
35 Bukur, D.B., Okabe, K., Rosynek, M.P., Li, C.P., Wang, D.Y., Rao, K.R.P.M., Huffman, G.P., “Activation studies with a precipitated iron catalyst for Fischer-Tropsch synthesis (I) Characterization studies”, J. Catal.,155, 353-365 (1995).
36 Zhang, H., Shen, J., Ge, X., ”The Reduction behavior of Fe-Mo-O catalysts studied by temperature-programmed reduction combined with in situ M?ssbauer spectroscopy and X-ray diffraction”, J. Solid State Chem,117, 127-135 (1995).
37 Wang, S., Lu, G.Q., “Role of CeO2in Ni/CeO2-Al2O3catalysts for carbon dioxide reforming of methane”, Appl. Catal. B,19, 267-277 (1998).
38 Jozwiak, W.K., Nowosielska, M., Rynkowski, J., “Reforming of methane with carbon dioxide over supported bimetallic catalysts containing Ni and noble metal (I) Characterization and activity of SiO2supported Ni-Rh catalysts”, Appl. Catal. A,280, 233-244 (2005).
39 Pawelec, B., Damyanova, S., Arishtirova, K., Fierro, J.L.G., Petrov, L., “Structural and surface features of PtNi catalysts for reforming of methane with CO2”, Appl. Catal. A,323, 188-201 (2007).
40 Chen, H.Y., “The crystal structure and twinning behavior of ferric molybdate, Fe2(MoO4)3”, Mater. Res. Bull.,14, 1583-1590 (1979).
41 Zhang, H., Shen, J., Ge, X., “The reduction behavior of Fe-Mo-O catalysts studied by temperature-programmed reduction combined with in situ M?ssbauer spectroscopy and X-Ray diffraction”, J. Solid State Chem.,117, 127-135 (1995).
42 Korner, H., Linder, H., Welder, G., Kreuzer, H.J., “The effect of carburization and oxygen exposure on the reaction of carbon monoxide on iron films at 573 K under a pressure between 5 and 10 mbar”, Appl. Surface Sci.,18, 361-365 (1984).
43 Benziger, J., Madix, R.J., “The effects of carbon, oxygen, sulfur and potassium adlayers on CO and H2adsorption on Fe(100)”, Surf. Sci.,94, 119-153 (1980).
44 Vink, T.J., Gijzeman, L.L.J., Geus, J.W., “CO interaction with Fe(100): Effects of carbon and oxygen adlayers on co adsorption isotherms”, Surf. Sci.,150, 14-23 (1985).
10.1016/S1004-9541(13)60573-X
2012-01-21, accepted 2012-06-03.
* Supported by the Iranian Nano Technology Initiative Council and Petroleum University of Technology.
** To whom correspondence should be addressed. E-mail: shariati@put.ac.ir
 Chinese Journal of Chemical Engineering2013年9期
Chinese Journal of Chemical Engineering2013年9期
- Chinese Journal of Chemical Engineering的其它文章
- Soft Sensor for Inputs and Parameters Using Nonlinear Singular State Observer in Chemical Processes*
- A New Tuning Method for Two-Degree-of-Freedom Internal Model Control under Parametric Uncertainty*
- Halloysite Nanotube Composited Thermo-responsive Hydrogel System for Controlled-release*
- Recent Advances in Separation of Bioactive Natural Products*
- Experimental and Theoretical Studies of CO2Absorption Enhancement by Nano-Al2O3and Carbon Nanotube Particles
- Volumetric and Transport Properties of Aqueous NaB(OH)4Solutions*
