The Short-term Effects of Temperature and Free Ammonia onAmmonium Oxidization in Granular and Floccular Nitrifying System*
WU Lei (吳蕾), PENG Yongzhen (彭永臻)**, MA Yong (馬勇), LIU Xu (劉旭), LI Lingyun(李凌云) and WANG Shuying (王淑瑩)
Key Laboratory of Beijing for Water Quality Science and Water Environment Recovery Engineering, Beijing University of Technology, Beijing 100124, China
1 INTRODUCTION
Biological processes have been widely applied to remove nitrogen from wastewater in preference to physicochemical processes [1, 2]. Ammonium is first oxidized to nitrite or/and nitrate by aerobic autotrophic nitrifiers and then reduced to nitrogen gas by heterotrophic denitrifiers [1, 2]. Unfortunately, nitrification as a key technology always becomes a rate-limiting step due to the low growth rate of autotrophic nitrifying bacteria. Thus, granulation without any external support has been proposed for immobilizing nitrifying bacteria in wastewater treatment processes. The aerobic granules have the advantages of excellent settling ability, good effluent quality, better resistibility to chemical shock loading and toxicity [3-5] due to its strong and complex structure [6]. Niet al. [7] and Moyet al. [8] showed a better resilience of shock loading with COD concentration fluctuated from 2000 to 5000 mg·L-1. Zhouet al. [9] reported that granular sludge appeared to be more tolerant to FNA inhibition than floccular sludge on anoxic phosphate uptaken because of its higherer resistence for nitrite to transfer into the bacterial aggregates.
There are several reports on temperature effects upon the respiratory activities of ammonia-oxidizing bacteria [2, 10, 11]. FA, the protonated species of ammonia, increases the total ammonia concentration,especially in nitrogen-rich wastewaters, such as the supernatant of the anaerobic sludge digesters, livestock wastewaters, or landfill leachates. Investigations on the effect of FA on nitrification showed that an excessively high FA level above 10-150 mg·L-1NH3-N may lead to an ammonium oxidation inhibition [12-14].Recently, the effect of various environmental factors on the ammonia oxidization was investigated using pure ammonia oxidizers and mixed cultures separately.So far, the report about the effect of environmental factors on aerobic granules is rare. Therefore, the effects of various environmental parameters on nitrification may be different from those in conventional activated sludge and, which is significantly important for a clearer understanding of newly developed biological nitrogen removal (BNR) process.
Our group has successfully produced nitritation granules using a sequencing batch reactor (SBR) with synthesis wastewater [15]. The objectives of this study are: (1) to evaluate the nitrification performance of nitritation granules and flocs within a relatively broad temperature range (10-30 °C); (2) to investigate the dual effect of temperature and FA concentration on nitrification process and compare inhibition degrees with different sludges. The knowledge of tolerance of granules for particular bacterial aggregates is helpful to the application study of aerobic granular sludge in wastewater treatment.
2 MATERIALS AND METHOD
2.1 Sludge sources
Granular sludge was withdrawn from a granular SBR treating synthetic wastewater. It was operated with a 4 h cycle consisting of 5 min feeding, 210 min aerobic, 1 min settling and 24 min idle periods. At the beginning, 6 L of synthetic wastewater was fed into the reactor resulting a hydraulic retention time of 6 h.The ratio of bicarbonate to ammonium-nitrogen was kept over 8.0. It was concluded that the associated inhibition of FA at the beginning of each cycle and free nitrous acid during the later phase of aeration is the key factor to maintain the stable nitritation. More details about the reactor operation and its performance can be found in Liet al[15]. The SBR displayed excellent4NH+-N removal performance (>99%) and nitrite accumulation rate (>95%) in the batch experiments described below. The granules had a compact structure and clear boundary with an average diameter of 1.2 mm. Fig. 1 shows the scanning electron microscopy(SEM)images of the granules.
Floccular sludge was taken from a large-scale pilot-plant SBR with a working volume of 8.8 m3treating real municipal wastewater. Each cycle of the SBR consisted of 0.25 h feeding, 6-10 h aerobic reaction, 2 h anoxic reaction (adding ethanol), 60 min settling, 0.5 h decanting, and 10-14 h idling. The time for aeration reaction was not fixed and the aeration was stopped when the characteristic point (ammonia valley) was detected [11, 16]. The ammonia valley on pH profile was detected by the real-time aeration duration control built before. Because the granules and flocs were taken from different cultivation substrates,the floccular sludge has been operated with synthetic wastewater for three months to decrease the effect of this selection and nitrogen removalvianitrite was successfully and stably achieved for a long period with average nitrite accumulation rate above 95% in the batch experiments described.
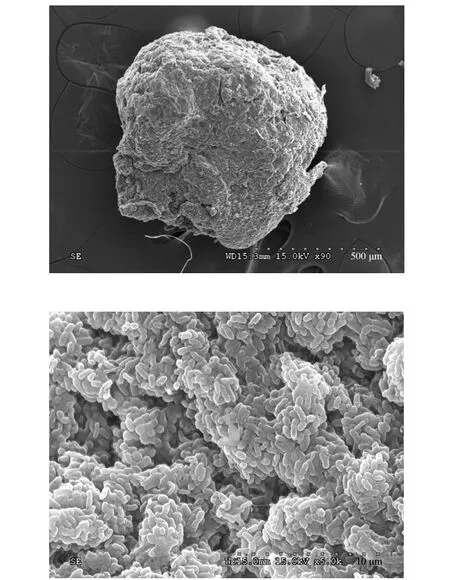
Figure 1 Observation of granular sludge using SEM technology
2.2 Batch experiments
Eighteen batch experiments with inorganic wastewater were performed using two types of sludge.In each test, 1 L of sludge was transferred to the batch reactor. Phosphate and ammonium were then added.The initial phosphate concentration was 5 mg·L-1. The separate ammonium oxidation tests were determined at temperatures of 10, 15, 20, 25 and 30 °C with the ammonium concentration of 50 mg·L-1. The combined effect of temperature and FA on nitrification process was studied with different initial ammonium concentrations varied between 12.7 and 2546 mg·L-1, as shown in Table 1. The concentration of FA, the key factor tested in this study, ranged between 1.76 and 90 mg·L-1(Table 1). The initial FA concentration was calculated using Eq. (1). The desired temperature was kept constant by placing the batch reactor in a water bath. The DO concentration was measured by a DO analyzer and its value was higher than 5 mg·L-1. Each aerobic period lasted for 3 h, during which the pH was regulated at 8.3 by addition of NaHCO3. Mixed liquor samples were taken every 30 min using a syringe and immediately filtered through disposable Millipore filter units (0.45 mm pore size) for the analyses of ammonium, nitrite and nitrate (with methods given below). The rates of ammonium oxidation were determined from the measured ammonia profiles using linear regression. The activation energy was measured using the methods described in the next section.

where TAN is total ammonium nitrogen.

Table 1 Concentrations of temperature and FA used in the batch experiments
2.3 Activation energy
The activation energy of a reaction is determined graphically by taking the natural logarithm of Arrhenius equation that describes the relationship between the reaction rate and temperature,

whereAis the frequency factor for the reaction,Ris the universal gas constant,Tis the temperature (K),Eais the activation energy.
2.4 Analyses
2.5 Fluorescence in situ hybridization (FISH)
Sample fixation and hybridization steps were carried out according to methods previously described by Amann [18]. Sludge samples were analyzed for ammonia oxidizing bacteria (AOB) and nitrite oxidizing bacteria (NOB). The probes applied include EUBMix(EUB338, EUB338-II and EUB338-III, specific for all bacteria), NSO190 (specific for Betaproteobacterial AOB), NIT3 (specific forNitrobactersp.) and Ntspa662 (specific forNitrospiragenera). All probes were commercially synthesised with 5′ FITC (fluorosceinisothiocyanate), or one of the sulfoindocyanine dyes, indocarboncyanine (Cy3) or indodicarbocyanine(Cy5) by ThermoHybaid (Interactiva Division, Ulm,Germany). Sample images were collected using OLYMPUSBX52 fluorescence microscope. FISH quantification was performed by Image-pro plus 6.0 Software, where the relative abundance of the interested bacteria was determined as the mean percentage of all bacteria.
3 RESULTS AND DISCUSSION
3.1 Microbial communities
Fluorescencein situhybridization (FISH) technology was used to investigate the population distribution of nitrifiers in both granular and floccular systems. The results demonstrated that the population of AOB (NSO190) in granular and floccular systems was(14.9±0.5)% and (8.3±1.1)% of the total bacteria, respectively and NOB population (Ntspa662 + NIT3) in both systems was lower than 0.5%. It can be seen that aerobic granulation is a simpler and more effective immobilization method for retaining nitrifying bacteria with high density in a reactor.
3.2 Ammonium oxidation at different temperatures
Ammonium oxidization by granular and floccular nitrifying sludge was studied using ammonia as the nitrogen source at five different temperatures from 10 to 30 °C (Fig.2). The initial nitrogen concentration was set at 50 mg·L-1. It was observed that4NH+-N decreased with time linearly in both systems. Since the coefficients of determination (R2) were above 0.98 in all sets of experiment, the reaction was virtually zeroth order. Since low temperature affected biochemistry reaction rate, the ammonium oxidation processes could not complete at 10 and 15 °C. As temperature rose from 20 to 30 °C,4NH+-N concentration decreased sharply. At the highest temperature of 30 °C applied, aerobic phase was terminated as soon as ammonium oxidation completed at 120 min in the floccular system, while complete depletion could take place within 60 min in the granular system, needing much less time for effective ammonium oxidation.

Figure 2 Ammonia oxidization in granular (a) and floccular (b) systems□ 10 °C; ■ 15 °C; △ 20 °C; ★25 °C; 30 °C

Figure 3 Specific ammonia oxidation rates at different temperatures□ granules; ■ flocs
Figure 3 shows the comparison for specific ammonium oxidation rates. Granular and floccular biomass presents the same trend that the ammonium oxidation rate increased with temperature between 10 and 30 °C. At 10 °C, the ammonium oxidation rates of granular and floccular biomass were both very slow.At 20 °C, the value increased to 0.011 and 0.004 mg·(mg VSS)-1·h-1. At 30 °C, the ammonium consumption rate was increased by a factor of 3-4 with the ammonium oxidation rate of 0.020 and 0.009 mg·(mg VSS)-1·h-1for granular and floccular biomass,respectively. The increase of ammonium oxidation rate with temperature was consistent with those observations reported [2, 19, 20]. Kimet al. [2] found that with temperature increased from 10 to 30 °C, the specific ammonium oxidization rate increased from 0.253 to 1.33 g·(g VSS)-1·d-1. Wang and Yang [19] observed the increase in ammonium oxidation rate by a factor of 4.5 when temperature increased from 12 to 30 °C with a maximum ammonium oxidation rate of 2.76 g·(g VSS)-1·d-1using the enriched ammonium oxidizers. Baeet al. [20] reported that the specific substrate-utilization rate increased by 3.7 times as the temperature rose from 10 to 30 °C and the maximum ammonium oxidization rate was 0.55 g·(g VSS)-1·d-1using a mixed culture at 30 °C. Our results show that nitrifying organism in the granular system is in good accordance with the classical temperature effect. The difference in the specific ammonium oxidation rate is probably due to different biomass compositions and concentrations used in the studies. Since the specific ammonium oxidation rate with granules is 2-3 times higher than that in floccular system, it is obvious that ammonia oxidizing bacteria (AOB) in the granular system presents better nitrification than conventional nitrification systems.
3.3 Activation energy
Figure 4 shows the Arrhenius plot for ammonium oxidization rates at different temperatures. The activation energy displays a break at 20 °C. In 10-20 °C, the activation energies of granules and flocs are 69.8 and 119.7 kJ·mol-1, while in 20-30 °C they are 47.4 and 54.3 kJ·mol-1, respectively. The activation energy of ammonium oxidation in 10-20 °C is larger by a factor of 2.2 than that in 20-30 °C for the flocs system,while a factor of 1.5 is found in the granular system.

Figure 4 Arrhenius plots analyses at different temperatures
Table 2 gives the comparison for activation energies reported in literature. Similar to our observation,two distinct activation energies in ammonium oxidation by AOB in two temperature ranges were reported[2, 10, 21, 22]. Our activation energy values at high and low temperatures are similar to those reported by Benyahia and Polomarkaki [10] and Guoet al. [11], but the activation energy of granules is somewhat lower than that from the floc system. As suggested by Guoet al. [11], activation energies are relatively lower for pure culture or enriched AOB than those for mixed culture. The main reason for different activation energy values may be the different biomass used as well as different nitrifying community population and structure. From the above results it is suggested that rate-limiting step for nitrification using conventional nitrifying floccular sludge may be applied to low temperatures.
3.4 Effect of temperature and initial free ammonia concentration on ammonium oxidation process
Initial FA concentrations were set at 1.76, 8.23,14.68, 26.57, 46.23 and 90.00 mg·L-1in this study to investigate the effect of substrate on the nitrificationperformance at temperatures of 10, 20 and 30 °C.Fig. 5 shows linear relationship between substrate and time. The ammonium oxidization in floccular system was determined in a similar way (data not given). It should be mentioned that initial 3 to 5 data points with a relatively high substrate concentration were selected for linear regression while 1 to 3 points were deleted when ammonia was depleted. Thus the depletion of ammonia is zeroth order with the determination coefficient (R2) higher than 0.9 in all of the experimental batch studies.

Table 2 Comparison of the activation energies for AOB reported in literature
The specific ammonium oxidization rates were determined from the slope of ammonia removal as depicted in Fig. 6. Consistent with the results in section of 3.1, the specific ammonium oxidation rate at six FA concentrations varied between 1.76 and 90.0 mg·L-1, increased by a factor of 3-5 when temperature increased from 10 to 30 °C in both systems. At a given temperature, the specific ammonium oxidation rate remained almost constant at FA concentrations ranging from 1.76 to 46.23 mg·L-1, but the ammonia oxidization inhibition was obvious when FA concentration was increased to 90 mg·L-1. However, the specific ammonium oxidation rate increased significantly as the temperature increased from 10 to 30 °C even at the inhibition concentration of 90 mg·L-1. When temperature dropped from 30 to 10 °C, specific ammonia oxidization rate declined 52.5% with granules. Severely, high concentration of ammonia in effluent corresponds to the absence of nitrite and nitrate production,i.e., no nitrification in floccular system at temperature of 10 °C. It indicates that temperature has potential influence on ammonia oxidation. Higher temperature is helpful to reduce the toxic effect of FA.
Several studies reported that an excessively high FA level may result in ammonia oxidation inhibition at room temperature [1, 2, 13, 20, 26-30]. Baeet al. [20]reported the inhibition of ammonia oxidation was observed at 10-150 mg·L-1of FA. Yanget al. [27] reported that nitrification was completely inhibited when free ammonia concentration was higher than 10 mg·L-1.Efficient and high-rate nitritation was developed recently in the lab and pilot scale for the treatment of ammonium-rich wastewater. Kimet al. [2] suggested that the sludge showing high resistance to FA concentration was usually acclimated to high ammonium concentrations in advance, while significant inhibition of nitrification often occurred in those systems without substrate-rich acclimation procedure. However, in this study, it appears that a relatively higher temperature could provide appropriate bacterial activity in the solution with high strength of ammonia, showing good performance in nitrification. The detectable effect of FA concentration on the ammonium oxidization demonstrates the feasibility of high-temperature strategy at high concentrations to facilitate the ammonia oxidization. As seen in Fig. 6, under the same temperature, the increase in the ammonia oxidation rate with the increase in FA concentration was observed. At FA concentration of 26 mg·L-1, the specific ammonium oxidization rates were increased from 0.0046 to 0.0167 mg. (mg VSS)-1·h-1in the temperature range of 10-30 °C in granular system. And, it increased from 0.001 to 0.012 mg·(mg VSS)-1·h-1.The same trend can be seen in other experiments. The detectable effect of FA concentration on the ammonium oxidization did demonstrate the feasibility of high-temperature strategy. So to obtain a successful nitrification, the use of high temperature concentration may be perceived as a strategy to facilitate the ammonia oxidization under the high FA condition.

Figure 5 Ammonia oxidation profiles in granular system at different initial free ammonia concentrations and temperatures□ 10 °C; ■ 20 °C;30 °C
3.5 Mechanisms for more tolerance to FA inhibition with granules
In order to demonstrate the ability to stand FA inhibition with granules, different inhibition degree of granular and floccular sludge at FA of 90 mg·L-1is shown in Table 3. The specific substrate utilization rate of ammonia is decreased by 7.5% and 10% with granules compared to 18% and 25% with flocs at 30 and 20 °C, respectively. The difference in ammonium oxidization is more significant at 10 °C. The results show that granules have better tolerance on FA inhibition than flocs, which exhibits a clear effect of FA on the ammonia oxidization. This observation is of great importance in the biological nitrogen removal (BNR)with granules, but the reason is not clear yet. Three hypotheses are proposed as follows.
Firstly, granulation without any carriers is one of the effective techniques for immobilizing nitrifying bacteria. Nitrifying bacteria are selectively cultivated and retained in the granules in which heterotrophic bacteria are present. In this study, the fraction of AOB in the granules is much higher than that in the flocs(see Section 3.1 with FISH analysis), so the granules present a better ammonium oxidation capacity as well as lower inhibition by FA per unit of VSS.

Figure 6 Specific ammonia oxidation rate under different initial free ammonia concentrations and temperatures1.76 mg·L-1;8.23 mg·L-1;14.86 mg·L-1;26 mg·L-1;46 mg·L-1;90 mg·L-1

Table 3 Inhibition degree for ammonia oxidization at FA of 90 mg·L-1
Secondly, the more tolerance to FA inhibition may be due to the mass transfer barrier in granules.Aerobic granules are larger and more compact than flocs. The FA concentration gradient within granules may be quite steep and the bulk concentration of substrate does not reasonably reflect the true situation [31].Additionally, the nitrifying population mainly locate on the surface of granules, a depth of 70 to 100 μm as reported by Tayet al[32]. In this study, the flocs size is in the range of 100-200 μm, while the granules are in 750-1200 μm. FA must diffuse into the granules to be available to the bacteria inside. The internal mass transfer resistance increases with the aggregate size,so the FA concentration in the granules is lower than that in the bulk.
Thirdly, compared to the loose, fluffy, and irregular conventional activated sludge flocs, the aerobic granular sludge has denser and stronger microbial structure. Much of the biomass in the granules does not expose to the same high concentration as present in the wastewater, while in the floc system, the bacteria distribute throughout the entire aggregate.
4 CONCLUSIONS
Temperature had obvious effect on ammonia oxidation and the specific ammonium oxidation rate increased as the temperature increased from 10 to 30 °C. The granules had an effective and better nitrification performance, with ammonium oxidation rate 2-3 times higher than that of flocs at the same temperature. Larger activation energy (69.8 and 119.7 kJ·mol-1) was found at lower temperatures of 10-20 °C,whereas smaller value (47.4 and 54.3 kJ·mol-1) was obtained at higher temperatures of 20-30 °C in both granular and floccular systems.
The combination of appropriate temperatures and free ammonia concentrations avoided obvious decrease in ammonium oxidation rate unless the FA concentration was up to 90.0 mg·L-1and the temperature dropped to 10 °C in the two systems. The specific ammonium oxidation rate increased significantly when temperature rose to 30 °C. It indicates that higher reaction temperature is helpful to reduce the toxic effect of FA.
Granules appeared to be more tolerant to FA at the same temperature and FA concentration. Higher fraction of AOB in granules and transfer barrier provided by a granule matrix that produced lower local FA concentrations on cells than in the bulk may be a critical reason.
1 Chung, J., Shim, H., Park, S.J., Kim, S.J., Bae, W., “Optimization of free ammonia concentration for nitrite accumulation in shortcut biological nitrogen removal process”,Bioprocess.Biosyst.Eng., 28,275-282 (2006).
2 Kim, J.H., Guo, X.J., Park, H.S., “Comparison study of the effects of temperature and free ammonia concentration on nitrification and nitrite accumulation”,Process.Biochem., 43, 154-160 (2008).
3 Adav, S.S., Chen, M.Y., Lee, D.J., Ren, N.Q., “Degradation of phenol by aerobic granules and isolated yeastCandida tropicalis”,Biotechnol.Bioeng., 96, 844-852 (2007).
4 Liu, Y., Tay, J.H., “State of the art of biogranulation technology for wastewater treatment”,Biotechnol.Adv., 22, 533-563 (2004).
5 Adav, S.S., Lee, D.J., Show, K.Y., Tay, J.H., “Aerobic granular sludge: Recent advances”,Biotechnol.Adv., 26, 411-423 (2008).
6 Shi, X.Y., Sheng, G.P., Li, X.Y., Yu, H.Q., “Operation of a sequencing batch reactor for cultivating autotrophic nitrifying granules”,Bioresour.Technol. 101, 2960-2964 (2010).
7 Ni, B.J., Xie, W.M., Liu, S.G., Yu, H.Q., Wang, Y.Z., Wang, G., Dai,X.L., “Granulation of activated sludge in a pilot-scale sequencing batch reactor for the treatment of low-strength municipal wastewater”,Water Res., 43, 751-761 (2009).
8 Moy, B.Y.P., Tay, J.H., Toh, S.K., Liu, Y., Tay, S.T.L., “High organic loading influences the physical characteristics of aerobic sludge granules”,Lett.Appl.Microbiol., 34, 407-412 (2002).
9 Zhou, Y., Pijuan, M., Yuan, Z.G., “Free nitrous acid inhibition on anoxic phosphorus uptake and denitrification by poly-phosphate accumulating organisms”,Biotechnol.Bioeng., 98, 903-912 (2007).
10 Benyahia, F., Polomarkaki, R., “Mass transfer and kinetic studies under no cell growth conditions in nitrification using alginate gel immobilizedNitrosomonas”,ProcessBiochem., 40, 1251-1262(2005).
11 Guo, J.H., Peng, Y.Z., Huang, H.J., Wang, S.Y., Ge, S.J., Zhang, J.R.,Wang, Z.W., “Short- and long-term effects of temperature on partial nitrification in a sequencing batch reactor treating domestic wastewater”,J.Hazard.Mater., 179, 471-479 (2010).
12 Turk, O., Mavinic, D.S., “Maintaining nitrite buildup in a system acclimated to free ammonia”,Water Res., 23, 1383-1388 (1989).
13 Anthonisen, A.C., Loehr, R.C., Prakasam, T.B.S., Srinath, E.G., “Inhibition of nitrification by ammonia and nitrous-acid”,Journal Water Pollution Control Federation, 48, 835-852 (1976).
14 Neufeld, R.D., Hill, A.J., Adekoya, D.O., “Phenol and free ammonia inhibition tonitrosomonasactivity”,Water Res., 14, 1695-1703(1980).
15 Li, L.Y., Peng, Y. Z., Wu, L., Wang, S.Y., Ma, Y., “Cultivation and characteristics analysis of nitrifying granules in sequencing batch reactor”,J.Civ.Environ.Architect.Eng., 32, 119-123 (2010).
16 Guo, J., Peng, Y.Z., Wang, S.Y., Zheng, Y.A., Huang, H.J., Wang,Z.W., “Long-term effect of dissolved oxygen on partial nitrification performance and microbial community structure”,Bioresour.Technol., 100, 2796-2802 (2009).
17 APHA, Standard Methods for the Examination of Water and Wastewater, Greenburg, A.E., Clesceri, L.S., Eation, A.D., ed., 18th American Public Health Association, American Water Works Association and Water Environment Federation, Washington DC (1992).
18 Amann, R.I., “In situidentification of micro-organisms by whole cell hybridization with rRNA-targeted nucleic acid probes”, Molecular Microbial Ecology Manual, Kluwer Academic Publishers,Netherland (1995).
19 Wang, J.L.,Yang, N., “Partial nitrification under limited dissolved oxygen conditions”,Process.Biochem., 39, 1223-1229 (2004).
20 Bae, W., Baek, S., Chung, J., Lee, Y., “Optimal operational factors for nitrite accumulation in batch reactors”,Biodegradation., 12,359-366 (2001).
21 Charley, R.C., Hooper, D.G., McLee, A.G., “Nitrification kinetics in activated-sludge at various temperatures and dissolved-oxygen concentrations”,Water Res., 14, 1387-1396 (1980).
22 Randall, C.W., Benefield, L.D., Buth, D., “The effects of temperature on the biochemical reaction-rate of the activated-sludge process”,Water Sci.Technol., 14, 413-430 (1982).
23 Painter, H.A., Loveless, J.E., “Effect of temperature and pH value on the growth-rate constants of nitrifying bacteria in the activated-suldge process”,Water Res., 17, 237-248 (1983).
24 Wijffels, R.H., Englund, G., Hunik, J.H., Leenen, E., Bakketun, A.,Gunther, A., Decastro, J.M.O., Tramper, J., “Effects of diffusion limitation on immobilized nitrifying microorganisms at low-temperatures”,Biotechnol.Bioeng., 45, 1-9 (1995).
25 Weon, S.Y., Lee, S.I., Koopman, B., “Effect of temperature and dissolved oxygen on biological nitrification at high ammonia concentrations”,Environ.Technol. 25, 1211-1219 (2004).
26 Simm, R.A., Mavinic, D.S., Ramey, W.D., “A targeted study on possible free ammonia inhibition ofNitrospira”,J.Environ.Eng.Sci., 5,365-376 (2006).
27 Yang, S.F., Tay, J.H., Liu, Y., “Inhibition of free ammonia to the formation of aerobic granules”,Biochem.Eng.J., 17, 41-48 (2004).
28 Carrera, J., Jubany, I., Carvallo, L., Chamy, R., Lafuente, J., “Kinetic models for nitrification inhibition by ammonium and nitrite in a suspended and an immobilised biomass systems”,Process.Biochem.,39, 1159-1165 (2004).
29 Mahne, I., Princic, A., Megusar, F., “Nitrification/denitrification in nitrogen high-strength liquid wastes”,Water Res., 30, 2107-2111(1996).
30 Hunik, J.H., Meijer, H.J.G., Tramper, J., “Kinetica of nitrosomonas-europaea at extreme substrate, product and salt concentrations”,Appl.Microbiol.Biotechnol., 37, 802-807 (1992).
31 Martins, A.M.P., Pagilla, K., Heijnen, J.J., van Loosdrecht, M.C.M.,“Filamentous bulking sludge—A critical review”,Water Res., 38,793-817 (2004).
32 Tay, J.H., Ivanov, V., Pan, S., Tay, S.T.L., “Specific layers in aerobically grown microbial granules”,Lett.Appl.Microbiol., 34, 254-257(2002).
 Chinese Journal of Chemical Engineering2012年5期
Chinese Journal of Chemical Engineering2012年5期
- Chinese Journal of Chemical Engineering的其它文章
- Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin*
- Reactive Distillation for Producing n-Butyl Acetate: Experiment and Simulation
- One Step Bioleaching of Sulphide Ore with Low Concentration of Arsenic by Aspergillus niger and Taguchi Orthogonal Array Optimization*
- Adsorption of Chlortetracycline from Water by Rectories*
- Optimization of Fermentation Media for Enhancing Nitrite-oxidizing Activity by Artificial Neural Network Coupling Genetic Algorithm*
- Effect of Propanoic Acid on Ethanol Fermentation by Saccharomyces cerevisiae in an Ethanol-Methane Coupled Fermentation Process*
