Adsorption of Chlortetracycline from Water by Rectories*
L Guocheng (呂國(guó)誠(chéng))**, WU Limei (吳麗梅) WANG Xiaolong (王曉龍), LIAO Libing(廖立兵)** and WANG Xiaoyu (王小雨) School of Materials Sciences and Technology, China University of Geosciences, Beijing 00083, China
School of Engineering and Technology, China University of Geosciences, Beijing 100083, China
1 INTRODUCTION
Antibiotics have become new pollutants in eco-environment due to their abuse of feed and aquaculture. When antibiotics are fed to animals, most of them will be discharged in the stool and only a little stays in the animal body. The discharged antibiotics will maintain active and cause serious pollution to soil and water [1]. To remove hydrophobic organic compounds from water, the adsorbent with higher total organic carbon content will achieve a better effect.However, for ionizable compounds, which are quite hydrophilic, their affinity to solid surface is strongly affected by the solution pH and their hydrophilicity,lowering the adsorption to activated carbon [2]. Antibiotics have different charges on different sites depending on solution pH. For example, for chlortetracycline(CTC), a widely used antibiotics additives, when solution pH is below 3.30, the dimethyl group will be protonated and CTC is present as a cation. At pH between 3.30 and 7.44, phenolic-pentanedione will lose protons and CTC exists as a zwitterion. At pH higher than 7.44,both carbonyl and phenolic-pentanedione will lose protons, so CTC presents a monovalent anion or a divalent anion (Fig. 1) [3].
The study of antibiotics adsorption on soils and clays began in the 1950s [4] but are still limited [5, 6].Due to the hydrophilicity and surface electricity, antibiotic molecules could be adsorbed by minerals and fixed in mineral voids [7]. Recent studies on antibiotic removal used some montmorillonite, such as bentonite,on which the adsorption capacity of diphenhydramine is as high as 192 mg·g-1at pH 5-7 [8-10], while kaolinite,rectories, and other soil components are used less often.Rectory is a regular interstratified mineral consisting of dioctahedral mica-unit and montmorillonite-unit layer in a ratio of 1∶1. It contains exchangeable cations and interlayer water. The special structure determines its expansibility, ion exchange properties and good adsorption performance [11]. It is widely used in removing humic acid, toxic organic compounds or heavy metal cations from urban sewage [12, 13].
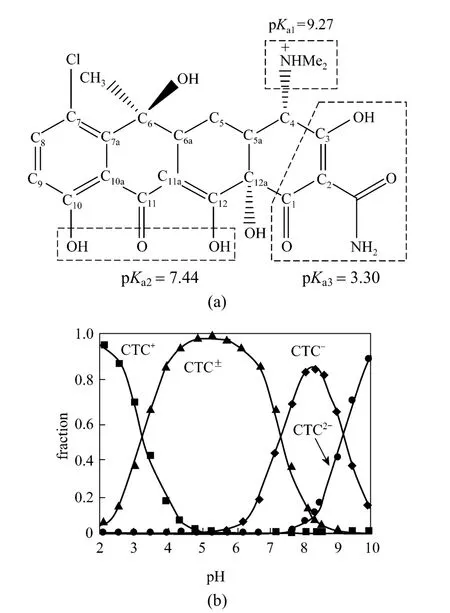
Figure 1 Molecular structure of CTC on a planar view(a) and speciation of CTC under different pH values (b)
In view of the excellent wastewater treatment performance of rectories and the urgency of treating the pollution of antibiotics, we explore the mechanism about adsorption of CTC on rectories in this study.The effects of adsorption time, concentration of CTC solution, temperature and pH on the adsorption rate are investigated. The results are characterized with X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR).
2 EXPERIMENTAL
2.1 Materials
The rectories used is the clay obtained from Zhongxiang City, Hubei Province, China, and was used as received. Its rectories content is 70%-80%,and its chemical constituents are mainly SiO2, Al2O3,Fe2O3, CaO, MgO, K2O, Na2O, P2O5, S, C and loss on ignition (LOI). CTC obtained from Tianjin Dongpeng Co., Ltd., China, is also called “chlortetracycline”,which is a kind of tetracyclines, with formula mass of 515.34 g·mol-1, pKa1, pKa2, and pKa3values of 9.27,7.44, and 3.30, respectively.
2.2 Instruments
CTC was quantified by HPLC method. UV-Vis spectrophotometer (UV-751) at a wavelength of 290 nm was used for CTC detection. The standards were adjusted to the same pH as that in the experiments.Calibration was made with 6 standards between 0 and 100 mg·L-1with the value ofr2no less than 0.99.
Powder XRD analysis was performed at 40 kV and 100 mA. Samples were scanned from 3° to 70° 2θat 8(°)·min-1with a scanning step of 0.02° per step.
The FTIR spectra were obtained by accumulating 256 scans at a resolution 4 cm-1in the range of 4000-400 cm-1.
2.3 Batch experiment
For all batch experiments (each repeated twice),the amount of rectories used was 0.10 g, while the volume of solution was 25 ml. They were mixed in 100 ml conical flasks on the oscillator at 150 r·min-1with constant temperature and separated in 50 ml centrifuge tubes at 2500 r·min-1. The supernatant was taken to a UV-Vis spectrophotometer (UV-751) at a wavelength of 290 nm [14]. The removal rate or adsorption was calculated to evaluate the effects of adsorption time, CTC solution concentration, temperature and pH. For batch kinetic study, the initial CTC concentration was 500 mg·L-1while pH was maintained at 5-6. The mixtures were shaken for 0.1, 0.25,0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7,7.5, 8, 10, and 12 h at room temperature (298 K). For investigating the effects of concentration and temperature on the adsorption, pH was 5-6 and the initial CTC concentration was adjusted to 100, 500, 1000, 2000 and 2500 mg·L-1, while the temperature was maintained at 298, 308, and 318 K. For the effect of pH, the initial CTC concentration was 500 mg·L-1, while the pH value was 1.98, 4, 6, 7.9, 10 and 11.5 at room temperature (298 K).
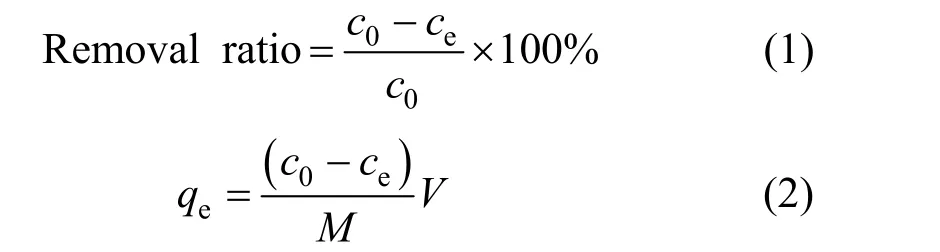
wherec0(mg·L-1) is the initial concentration of solute,ce(mg·L-1) is the equilibrium concentration,qe(mg·g-1)is the amount of CTC adsorbed at equilibrium,V(L)is the volume of solution, andM(g) is the quality of adsorbent.
3 RESULTS AND DISCUSSION
3.1 CTC adsorption kinetics
The results showed that the adsorption reached equilibrium in 8 h (Fig. 2). The maximum removal ratio was about 90% and the equilibrium adsorption was 113.1 mg/g. In subsequent experiments the equilibration time was set for 8 h.
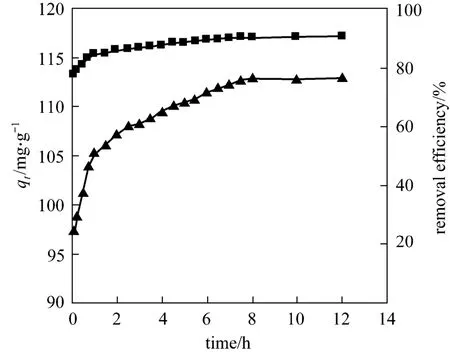
Figure 2 The effect of time on removal efficiency and adsorption▲ qt; ■ removal efficiency
The pseudo-second-order kinetic model fits the experimental data well (Table 1). Its integrated form is [15]
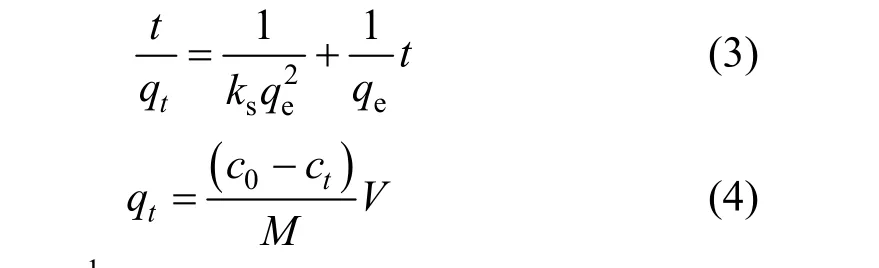
whereqt(mg·g-1) is the amount of CTC adsorbed on the surface of adsorbent at timet(h),ks(g·mg-1·h-1) is the pseudo-second-order kinetic constant, andct(mg·L-1)is the solute concentration at timet. The pseudo-secondorder kinetic model is plotted in Fig. 3. Based on the equation for the straight line (R2=0.999), we obtain that the theoretical equilibrium adsorption of CTC is 113.6 mg·g-1, which is close to the experimental value of 113.1 mg·g-1.
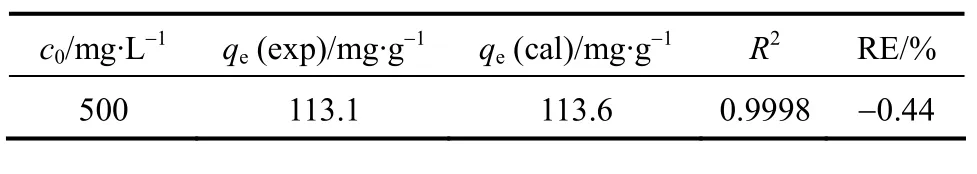
Table 1 Parameters in the pseudo-second-order kinetic model
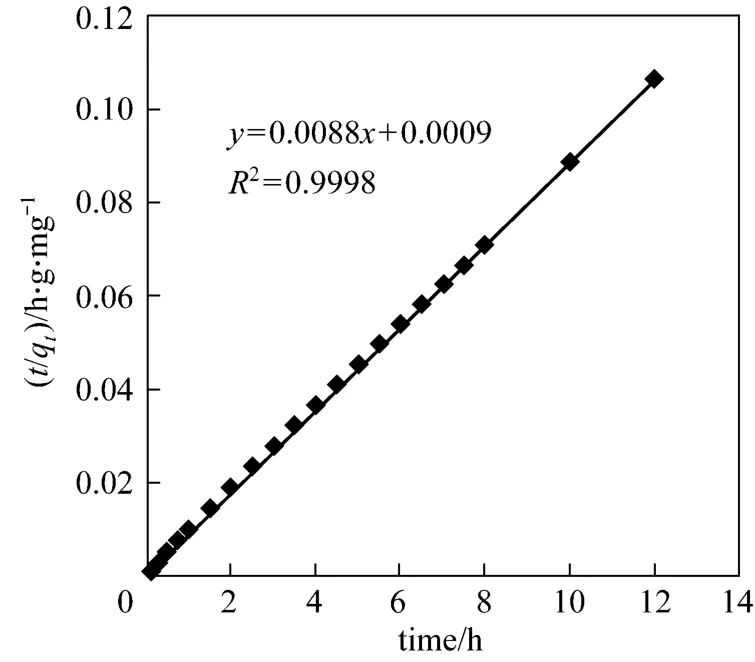
Figure 3 Adsorption kinetics of CTC on rectories
3.2 CTC sorption isotherm
Figure 4 shows the adsorption of CTC on rectories at different temperatures. The adsorption capacity is 177.7, 206.5 and 239.8 mg·g-1at 298, 308 and 318 K,respectively, which is higher than that of ciprofloxacin(CIP) on rectories (135.9 mg·g-1) [16]. The experimental data fit the Langmuir type isotherm [17] well (Table 2)
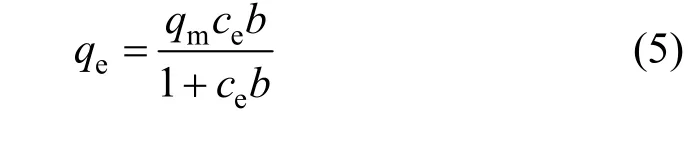
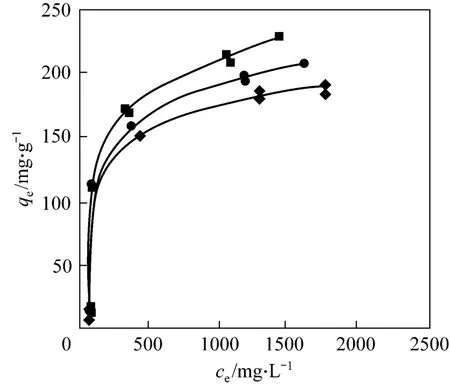
Figure 4 Adsorption isotherm of CTC on rectories■ 318 K; ● 308 K; ◆ 298 K
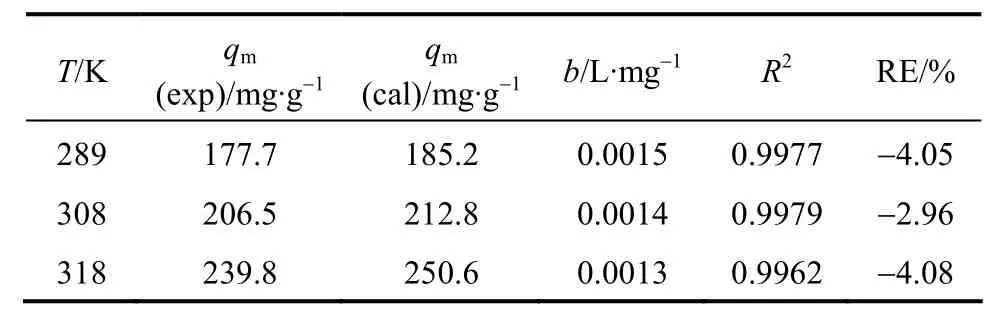
Table 2 Parameters in Langmuir type isotherm model

whereqm(mg·g-1) is the maximum adsorption, andb(L·mg-1) is the Langmuir type isotherm constant. The Langmuir type isotherm is plotted in Fig. 5. Based on the equation of the straight line, we obtain that the theoretical maximum adsorption of CTC on rectories is 181.8 mg·g-1at room temperature (298 K), which is close to the experimental value of 177.7 mg·g-1(298 K).The Langmuir type isotherm constantb=0.015 L·mg-1withR2>0.99. The interlayer surface of rectories is relatively homogeneous and the adsorption obeys the Langmuir monolayer adsorption.
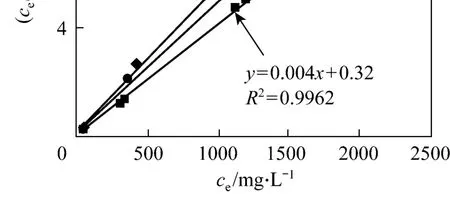
Figure 5 Langmuir type isotherm◆ 298 K; ● 308 K; ■ 318 K
3.3 Effect of temperature on CTC adsorption
Figure 6 shows the adsorption of CTC on rectories at different concentrations and temperatures. The thermodynamic parameters of adsorption are expressed as

whereKd(L·g-1) is the ratio of the amount of CTC adsorbed at the equilibrium CTC concentration, ΔH(kJ·mol-1) is the change in enthalpy, ΔS(kJ·mol-1·K-1)is the change in entropy,R=8.314 J·mol-1·K-1is the gas constant, andT(K) is the reaction temperature.The free energy of adsorption can be determined by

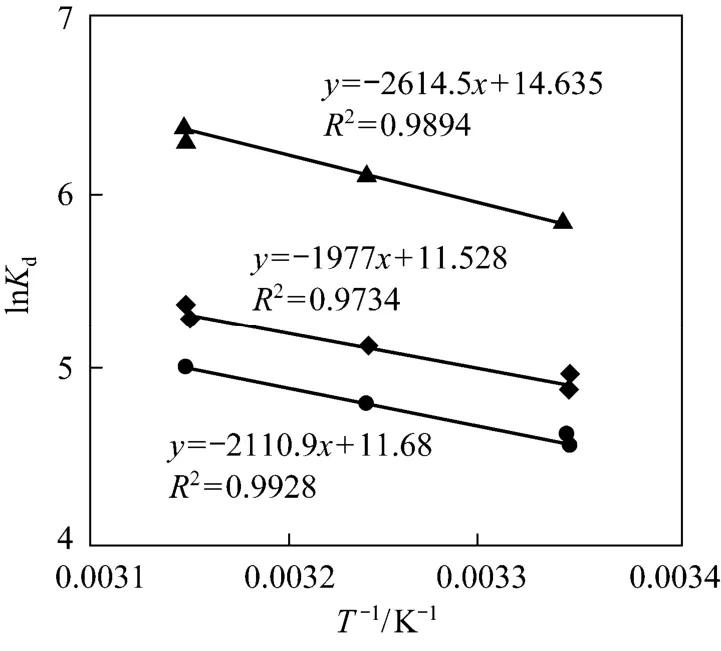
Figure 6 Effect of temperature on CTC adsorption on rectories▲ 1000 mg·L-1; ◆ 2000 mg·L-1; ● 2500 mg·L-1
The calculated theoretical parameters are listed in Table 3. The negative value of ΔGindicates attractive interaction between CTC and rectories, so the adsorption of CTC on rectories is spontaneous. Positive value of ΔHindicates an endothermic reaction and higher temperature is good for the reaction. According to the value ofKdand ΔH, we obtain a small positive value of ΔS, so the adsorption is spontaneous. It suggests that the adsorbed CTC molecules may adopt a randomly oriented manner instead of arranging themselves in an orderly pattern on the external surface of rectories. It is different from the adsorption of ciprofloxacin on rectories [16].
3.4 Effect of pH on CTC adsorption
The amount of CTC adsorbed ranged from 114.2 to 124.8 mg·g-1or 91% to 99% at initial concentration of 500 mg·L-1and reached the maximum at acidity(2-6) at room temperature (298K) (Fig. 7), so the adsorption capacity of rectories depends on the pH environment. This is closely related to the surface electricity of CTC and rectories. When pH <3.30, CTC is in the cationic state (CTC+) and exchanges the interlayer cations at 1∶1. When pH=3.30-7.44, CTC is in the neutral state (CTC0), which reduces the cation exchange capacity, while the molecular adsorption remains similar. When pH>7.44, CTC is in the anion state (CTC-, CTC2-), which hinders the cation exchange capacity. Besides, under alkaline condition, the surface of rectories is electronegative [18] and creates a repulsive energy against CTC-and CTC2-. Thus the adsorption decreases rapidly under alkaline condition.
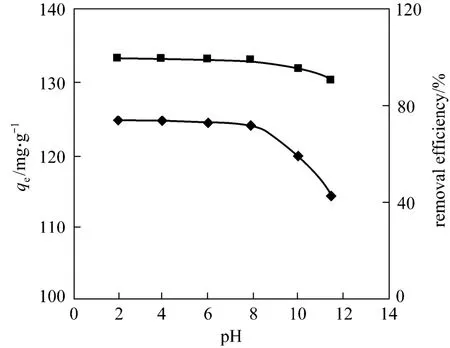
Figure 7 Effect of pH on the adsorption of CTC on rectories◆ qe; ■ removal efficiency
3.5 XRD analysis
Figure 8 shows the XRD patterns of raw rectories and those after adsorbing CTC at different initial concentrations. The XRD patterns are similar, indicating similar structure of rectories with and without the adsorption. However, for 1000 and 2500 mg·L-1concentration of CTC, the important peak located at 5°-10°(2θ) shifts compared with that of raw material.After adsorbed a certain amount of CTC, two weak peaksd002andd003appear instead of one strong peakd002of raw rectories. Thed-spacing of rectories increases. Thed-spacing of raw rectories is 1.12 nm, and that of adsorbed one at the 2500 mg·L-1initial concentration of CTC increases to 1.38 nm. Thus CTC intercalates into the interlayer of rectories and adsorption occurs between the layers.
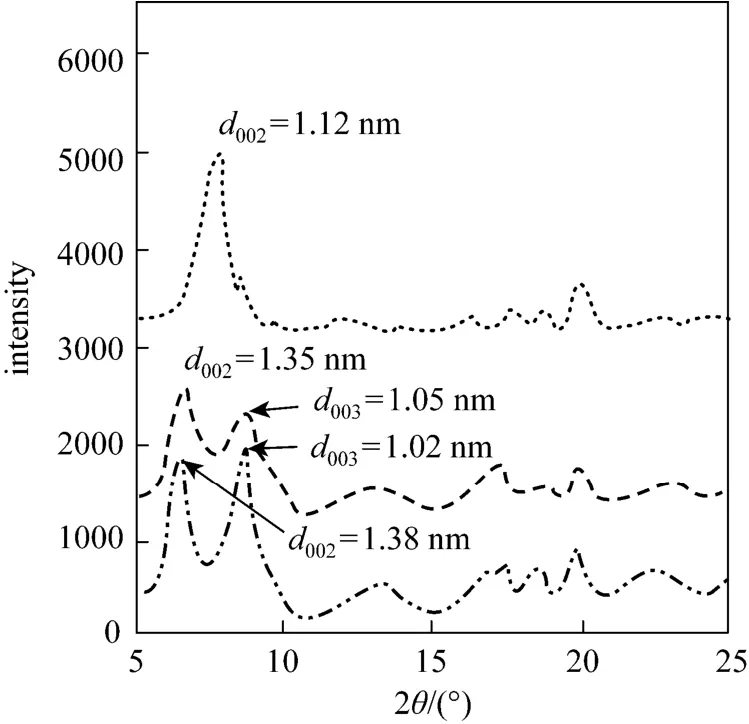
Figure 8 The XRD of rectories after adsorbed CTC at different initial concentrations raw; 1000 mg·L-1; 2500 mg·L-1
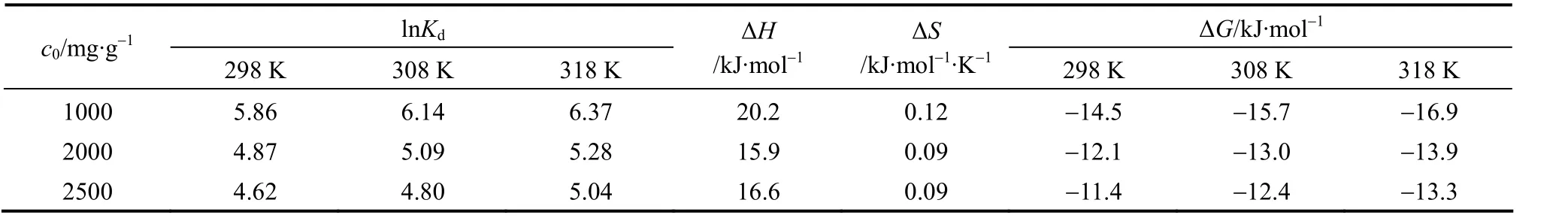
Table 3 Thermodynamic values of CTC adsorption on rectories
3.6 FTIR analyses
Figure 9 shows the FTIR spectra of raw rectories and rectories with absorbed CTC at different initial concentrations. There is no significant change in the vibration of the backbone of the silicate structure of rectories before and after CTC adsorption, which indicates that the adsorbed CTC does not alter the structure, consistent with XRD observation. The FTIR spectrum shows SiO and C O stretching vibration near 1030 cm-1, C O stretching vibration near 1500-1650 cm-1, COOH and OH stretching vibration near 3600 cm-1. The most obvious changes are those in 1500-1650 cm-1and 3300-3600 cm-1. At wave number 1529 cm-1, the rectories after CTC adsorption show a strong peak compared with the raw material,characterizing the reaction between rectories and CTC.Besides, the bands at 3581 cm-1shift to higher frequencies, indicating a strong interaction between rectory surface and the intercalated CTC molecules [18].
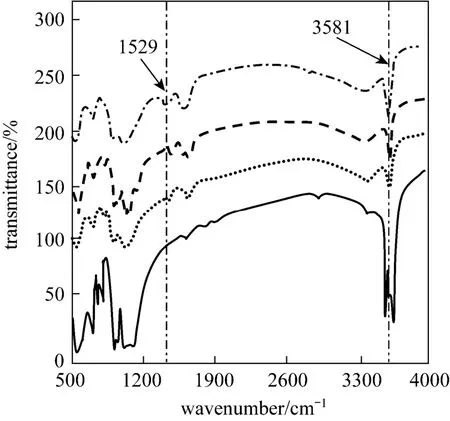
Figure 9 FTIR spectra of rectories in equilibrium with CTC solutionsraw; 50 mg·L-1; 500 mg·L-1; 2500 mg·L-1
4 CONCLUSIONS
The adsorption of CTC on rectories follows the pseudo-second-order kinetics and the Langmuir adsorption isotherm well. The adsorption equilibrium is reached in 8 h and the maximum adsorption is 177.7 mg·g-1at room temperature. The optimal adsorption pH is 2-6 and the adsorption decreases rapidly over pH=7.
The adsorption of CTC on rectories is a spontaneous and endothermic reaction. ΔGis in the range of-10 to -30 kJ·mol-1and ΔHis 10-30 kJ·mol-1. Thus,higher temperature will enhance the uptake of CTC by rectories. The small value of ΔSsuggests that the adsorbed CTC molecules may adopt a randomly oriented manner instead of arranging themselves in order on the external surface of rectories.
XRD analyses show that the intercalated CTC produces an interlayer space with a height of 1.38 nm,which is 1.12 nm in the raw rectories. The FTIR analyses show a stronger peak after adsorption of CTC compared with that without adsorption, characterizing the reaction between rectories and CTC. The mechanism is the interlayer adsorption and the adsorbed CTC does not alter the structure based on the characterization of XRD and FTIR analysis.
1 Chen, Y., Zhang, H., Luo, Y., “Occurrence and dissipation of veterinary antibiotics in two typical swine wastewater treatment systems in east China”,Environment Monitoring and Assessment, 184 (4),2205-2217 (2012).
2 Torres-Pérez, J., Gérente C., Andrès, Y., “Sustainable activated carbons from agricultural residues dedicated to antibiotic removal by adsorption”,Chin.J.Chem.Eng., 20 (3), 524-529 (2012).
3 Parolo, M.E., Avena, M.J., Pettinari, G.R., “Influence of Ca2+on tetracycline adsorption on montmorillonite”,Journal of Colloid and Interface Science, 368 (1), 420-426 (2012).
4 Jefferys, E.G., “The stability of antibiotics in soils”,J.Gen.Microbiol., 7, 295-312 (1952).
5 Sassman, S., Lee, L., “Sorption of three tetracyclines by several soils:assessing the role of pH and cation exchange”,Environ.Sci.Technol., 39, 7452-7459 (2005).
6 Chen, B.F., Wu, M., Zhang, D., “Research advance in sorption mechanisms of antibiotics in soil inorganic minerals”,Chemical Industry and Engineering Progress, 31 (01), 193-200 (2012).
7 Aristilde, L., Marichal, C., “Interactions of oxytetracycline with a smectite clay: A spectroscopic study with molecular simulations”,Environ.Sci.Technol., 44 (20), 7839-7845 (2010).
8 Wu, Z.S., Li, C., Sun, X.F., “Characterization, acid activation and bleaching performance of bentonite from Xinjiang”,Chin.J.Chem.Eng., 14 (2), 253-258 (2006).
9 Kulshrestha, P., Giese Jr, R.F., Aga, D.S., “Investigating the molecular interactions of oxytetracycline in clay and organic matter: insights on factors affecting its mobility in soil”,Environ.Sci.Technol,38, 4097-4105 (2004) .
10 Li, Z.H., Chang, P.H., Jiang, W.T., “Removal of diphenhydramine from water by swelling clay minerals”,Journal of Colloid and Interface Science, 360, 227-232 (2011).
11 Xue, X.H., He, X.S., Zhao, Y.H., “Adsorptive properties of acid-heat activated rectorite for rhodamine B removal: equilibrium, kinetic studies”,Desalination and Water Treatment, 37, 259-267 (2012).
12 Li, J., Hu, M.Z., “Removal of rhodamine B from wastewater by rectorite and sepiolite-kinetic study and equilibrium isotherm analyses”,Advanced Materials Research, 183-185, 362-366 (2011).
13 Yang, S., Zhao, D., Zhang, H., “Impact of environmental conditions on the sorption behavior of Pb(II) in Na-bentonite suspensions”,J.Hazard.Mater., 183, 632-640 (2010).
14 Saminathan, J., Sankar, A.S., Anandakumar, K., Vetrichelvan, T.,“Simple UV spectrophotometric method for the determination of fluvastatin sodium in bulk and pharmaceutical formulations”,E-Journal of Chemistry, 6 (4), 1233-1239 (2009).
15 Blanchard, G., Maunaye, M., Martin, G., “Removal of heavy-metals from waters by means of natural zeolites”,Water.Res., 18, 1501-1507(1984).
16 Wang, C.J., Li, Z.H., Jiang, W.T., “Adsorption of ciprofloxacin on 2∶1 dioctahedral clay minerals”,Appl.Clay Sci., 53 (4), 723-728 (2011).17 Langmuir, I., “The constitution and fundamental properties of solids and liquids”,J.Am.Chem.Soc., 38, 2221-2295 (1916).
18 Turku, I., Sainio, T., Paatero, E., “Thermodynamics of tetracycline adsorption on silica”,Environ.Chem.Lett., 5, 225-228 (2007).
 Chinese Journal of Chemical Engineering2012年5期
Chinese Journal of Chemical Engineering2012年5期
- Chinese Journal of Chemical Engineering的其它文章
- Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin*
- Reactive Distillation for Producing n-Butyl Acetate: Experiment and Simulation
- One Step Bioleaching of Sulphide Ore with Low Concentration of Arsenic by Aspergillus niger and Taguchi Orthogonal Array Optimization*
- Optimization of Fermentation Media for Enhancing Nitrite-oxidizing Activity by Artificial Neural Network Coupling Genetic Algorithm*
- Effect of Propanoic Acid on Ethanol Fermentation by Saccharomyces cerevisiae in an Ethanol-Methane Coupled Fermentation Process*
- Numerical Study on Laminar Burning Velocity and Flame Stability of Premixed Methane/Ethylene/Air Flames*
