Numerical Study on Laminar Burning Velocity and Flame Stability of Premixed Methane/Ethylene/Air Flames*
CHEN Shanshan (陳珊珊), JIANG Yong (蔣勇)**, QIU Rong (邱榕) and AN Jiangtao (安江濤)
State Key Laboratory of Fire Science, University of Science and Technology, Hefei 230026, China
1 INTRODUCTION
Nowadays, as the global economy is developing so fast and energy is subjected to such a massive consumption, energy crisis has attracted more and more attention. As a result, it has been put on the agenda by many researchers to improve energy efficiency and achieve economic energy usage. And lean combustion is proved to be a valid and practicable technology to meet the emerging needs among all kinds of solution.It has been pointed out by many scholars that lean combustion can greatly reduce fuel consumption and emission of pollutants [1-3]. There still remain some problems to be solved, and one of them is that combustion stability can be affected due to the weakly burning flame, and the processes of stretching and heat loss can locally cause extinction [4].
Natural gas, with methane being its major component, has been widely used in both fundamental and practical circumstances [5]. As a fuel, it has some special prospects and is considered to be one of the most promising clean alternative fuels. However, it encounters a number of problems as well, such as low thermal efficiency and poor lean-burn capability [6]. Lean methane combustion has great potential to reduce NOxemission, but there are two distinct shortcomings in using it: a substantial decrease of laminar burning velocity and a large increase in misfire [3]. Some attempts have been made to solve these two problems and an effective approach is to adopt fuel enrichment,i.e., mixing nature gas with another fuel that possesses high burning velocity and good flame stability. And reformate gas is turning out to be a good choice.
Since reformate gas is mainly composed of hydrogen, carbon monoxide and nitrogen, the effects of reformate gas as well as hydrogen and carbon monoxide have been investigated extensively by researchers. Yuet al. measured experimentally the laminar flame speeds of hydrocarbon/air mixtures with and without hydrogen addition and the results show that the flame speed is substantially increased with hydrogen addition [7]. Coppenset al. researched the effects of composition on burning velocity and nitric oxide formation in laminar premixed CH4/H2/O2/N2flames,and proved that NO concentration decreases significantly with enrichment by hydrogen [8]. Guoet al.investigated the effects of reformate gas enrichment on extinction limit and NOxformation in counterflow CH4/air premixed flames and found out the significant advantage of the reformate gas enriched lean premixed combustion, that is, it greatly reduces the NO formation by allowing a combustor to operate at leaner condition without any effect on flammable range [9].Chenet al. studied the effects of CO addition on the lean premixed CH4/air flame and revealed that both the laminar burning velocity and the NOxemission decrease when CO is added to the fuel [10].
In the present study, we take ethylene (C2H4) as fuel additive because of its massive production as well as lower ignition temperature than that of methane [11].Ethylene is the most produced organic compound in the world and its global production exceeded 100 million metric tonnes each year. On the other hand, as a key intermediate in the oxidation of higher alkanes and alkenes, ethylene plays an important role in the combustion mechanisms of most practical fuels[12-14]. Thus, the research of simple hydrocarbon fuels here might help to shed light on that of more complex hydrocarbon fuels.
The objective of this paper focuses on the combustion characteristics of premixed CH4/C2H4/air flames. Flames with various ethylene fractions under the condition of lean burning have been predicted at room temperature and atmospheric pressure. The unstretched laminar burning velocity is first investigated.Then the analysis of flame structure is given, with species concentrations and flame temperature included,both of which have important influence on laminar burning velocity. Finally the flame stability is discussed,in terms of Markstein length and Markstein number.
2 SIMULATION DETAILS
Numerical simulations were performed using CHEMKIN 4.0 and two flame models were adopted accordingly. One is the freely-propagating, premixed,planar flame based on PREMIX code [15] to obtain the laminar burning velocity and some parameters to analyze the flame structure, and the other is the opposed-flow,symmetric flame with OPPDIF (opposed diffusion)code [16], through which we acquire Markstein length and Markstein number to expound the flame stability.Both of the two models were developed by Sandia National Laboratory, and have been widely used in fundamental and practical combustion fields [17-19].PREMIX accounts for finite-rate chemical kinetics and multicomponent molecular transport. It uses a hybrid time integration/Newton iteration technique to solve the steady-state mass, species and energy conservation equations. For the opposed-jet flame calculation, the flow is reduced mathematically to one dimension by assuming that the radial velocity varies linearly with the radial distance, and this leads to a simplified form in which the flow field properties are functions of the axial distance only. Equations are solved by using the TWOPNT, a two-point boundary value problem solver in the CHEMKIN package, with the gas-phase kinetics and transport packages which process the chemical reaction mechanism and calculate the species transport properties.
The chemical kinetic model of USC Mech II composed of 111 species and 784 reactions was employed. It is a recently proposed, high-temperature combustion reaction model developed by Wanget al.[20], and has been validated comprehensively on laminar fame speed, ignition delay time, and species profiles in flow reactors and burner stabilized flames for fuels, including hydrogen, carbon monoxide, methane, acetylene, ethylene, ethane, propyne, propene,propane, 1,3-butadience, 1-betene andn-butane [21].Liuet al. have investigated the laminar burning velocity of C2H4/CH4/air flames, with two kinetic models adopted in their study, namely USC Mech II and Mech-118. They calculated the laminar burning velocity at both atmospheric and elevated pressures, compared the simulation with experimental results and concluded that USC Mech II agrees well with the experimental data at atmospheric pressure while Mech-118 yields better agreement with measurements as the system pressure increases [11].
It should be noted that some parameters are used as follows:
(1) the global equivalence ratioφis defined as

whereYfueliis the mass fraction of speciesiin the fuel blends,Yairis the mass fraction of air, and the subscript “actual” and “stoich” refer to the actual and stoichiometric situations respectively.
(2) the mixing ratioαis presented by the fraction of oxygen-consumption weighted mole fractions of the two fuels:

where24CHXand4CHXare mole fractions of ethylene and methane in the fuel blends respectively. The extreme values of 0α= and 1α= correspond to pure methane and ethylene reacting with air respectively.The composition of air is 21% of oxygen and 79% of nitrogen in volume.
3 RESULTS AND DISCUSSION
3.1 Laminar burning velocity
Laminar burning velocity is an important parameter in combustion as it contains fundamental information regarding reactivity, diffusivity and exothermicity. It can be used to validate chemical reaction mechanisms and design internal combustion engines.The burning velocity is defined as the linear velocity that the flame front travels relative to the unburned gas, and usually expressed as the volume of unburned gas consumed per unit time divided by the area of the flame front in a one-dimensional domain. In the freely-propagating, laminar one-dimensional flame,the mixing of fuel and oxidizer proceeds in the form of molecular diffusion, and it is expected that the flow rate of the mixture does not influence the flame structure. As a result, the unstretched laminar burning velocity can be acquired.
The calculation of CH4/C2H4/air flames with different ethylene fractions was performed at room temperature and atmospheric pressure. The fixed temperature and the mass flow rate are set as 400 K and 0.04 g·cm-2·s-1respectively. Adaptive mesh parameters are GRAD=0.4 and GURV=0.6 (GRAD: adaptive grid control based on solution gradient; GURV:adaptive grid control based on solution curvature).Absolute and relative error criteria are ATOL=1×10-9and RTOL=1×10-4(ATOL: absolute tolerance; RTOL:relative tolerance). The ending axial position is 0.3 cm.
To provide validation of our calculation with USC Mech II mechanism, Fig. 1 shows the comparison between the computed laminar burning velocities by using USC Mech II and experimental results from Ref. [11]. Good agreement is found over a wide range of ethylene fractions, in spite of a slight overestimation in numerical results. The results show that USC Mech II can well produce the laminar burning velocity of CH4/C2H4/air flames at fuel-lean condition.

Figure 1 Comparison between the computed laminar burning velocities by using USC Mech II and experimental results for CH4/C2H4/air flamesφ: ■ 0.8; ● 1.0; ▲ 1.2
The laminar burning velocity is plotted as a function of equivalence ratio for different mixing ratios in Fig. 2. It can be observed that (1) the laminar burning velocity reaches its maximum value at near the stoichiometric condition, and the peak velocity appears at the equivalence ratios of 1.1 and 1 respectively in flames with and without ethylene addition; (2) the laminar burning velocity increases with the increase of ethylene fraction, especially when the equivalence ratio is higher than 0.8.

Figure 2 Laminar burning velocity versus equivalence ratio for different mixing ratiosα: ■ 0; ● 0.2; ▲ 0.4; ▼ 0.6; ★ 0.8; ◆ 1.0
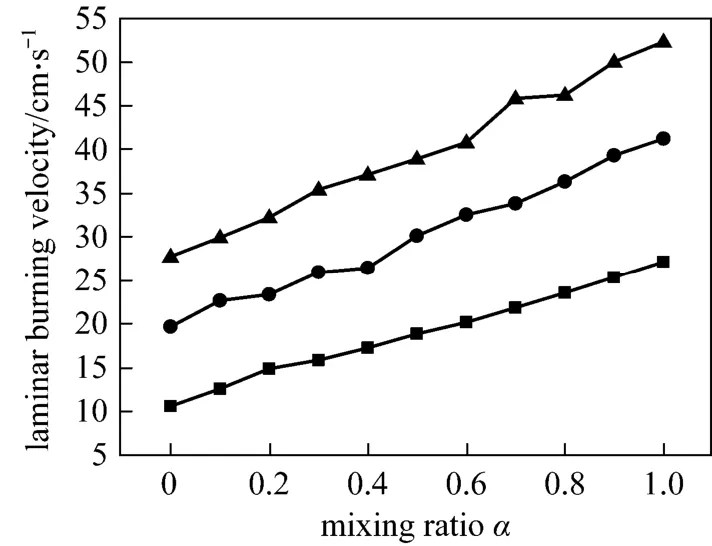
Figure 3 Laminar burning velocity versus mixing ratio at different equivalence ratiosφ: ■ 0.6; ● 0.7; ▲ 0.8
We obtain the laminar burning velocity as a function of mixing ratio at the equivalence ratios of 0.6,0.7 and 0.8 in Fig. 3. It is clear that the increase of ethylene fraction results in the increase of laminar burning velocity in lean combustion, and the slope of increase becomes slightly steeper with a larger equivalence ratio. That is to say, ethylene addition in the methane/air mixture leads to faster flame propagation and the effect improves as fuel is enriched. This positive effect of ethylene addition on laminar burning velocity of CH4/air flame is similar to that of hydrogen. Huet al. have conducted experimental and numerical study on laminar burning characteristics of the premixed CH4-H2-air flames at room temperature and atmospheric pressure, and found that the laminar burning velocity is increased with the increase of hydrogen fraction [22].
3.2 Flame structure
Predicted structures of methane/air flames with and without ethylene addition are presented in Fig. 4.For each pair of graphs, the left plot shows the concentrations of reactants (CH4, C2H4, O2) and products(CO2, H2O, CO) as well as the temperature distribution, whereas the right one presents the concentration profiles of radical species (H, O, OH, CH3), as a function of distance through the flame. Besides, the maximum mole fractions of H, O and OH radicals are also revealed.
From the curves in Fig. 4, it is observed that the fuel is fully consumed by 0.1 cm more or less; the O2concentration drops sharply as fuel is consumed and keeps on declining slightly after the fuel is used up,before settling down to its final value. CO gives its peak concentration almost at the same position where the fuel concentration falls to zero, while the increase of CO2concentration delays owing to CO formation and will continue as CO is oxidized later. The main temperature rise (80% approximately) is accomplished also at the same position where the fuel is completely depleted, and then the temperature reaches gradually the equilibrium value after this position.

Figure 4 Flame structures in CH4/C2H4/air flames at the equivalence ratio of 0.7
The major radicals H, O and OH rise to maximum concentrations and then decrease slowly in the post-flame zone [23]. The OH radical has the largest peak concentration in the flame, while H gives the lowest, and O is in between. The concentrations of H,O and OH radicals are increased as ethylene is added in the fuel, which will promote the combustion. Huetal. studied the typical predicted structures of plane unstretched CH4/H2/air flames at stoichiometric condition and noted that the radical OH has the largest peak concentration, with H having roughly 10%-30%lower than that of OH, and the concentration of O is less than half of H concentration [24]. Our results are somewhat different from theirs, which can be partly attributed to the lean combustion in our study in contrast with theirs at stoichiometric condition, and the excessive amount of oxygen results in larger O concentration. Another reason may be the hydrogen addition in their fuel blends which brings about higher H concentration.
3.3 H and OH radical behaviors
Huet al. investigated the relationship between the laminar burning velocity and the peak concentration of H + OH in the reaction zone of premixed CH4/air flames at stoichiometric condition and pointed out that there exists a strong correlation between them[24, 25]. That is, the laminar burning velocity is increased almost linearly with the increase of peak H + OH mole fraction. Similar conclusions are acquired for CH4/air flame at the equivalence ratio of 0.7 as shown in Figs. 5 and 6. The best-fitting correlation is as follows:
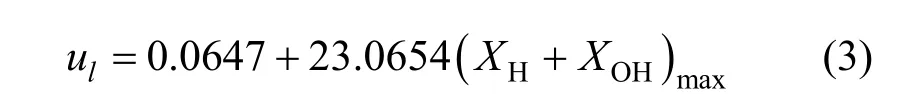
whereulis the laminar burning velocity (m·s-1), andXHandXOHare mole fractions of H and OH in the reaction zone respectively.
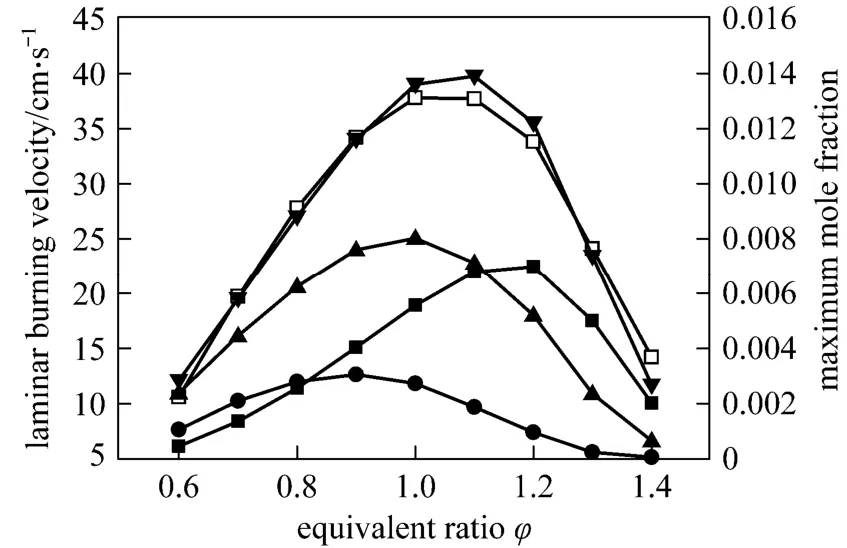
Figure 5 Laminar burning velocity and maximum H, O,OH and H + OH mole fractions versus equivalence ratio in the CH4/air flame■ H; ● O; ▲ OH; ▼ H + OH; □ ul

Figure 6 Laminar burning velocity versus maximum H + OH mole fraction in the CH4/air flame

Figure 7 Laminar burning velocity and maximum H + OH mole fraction versus mixing ratioφ: ■, □ 0.6; ●, ○ 0.7; ▲, △ 0.8
Figure 7 shows the laminar burning velocity and maximum H + OH mole fraction with different mixing ratios at the equivalence ratios of 0.6, 0.7 and 0.8. It is concluded that: (1) both the laminar burning velocity and the maximum H + OH mole fraction increase as more ethylene is added in the CH4/air flame; (2) the variation trends of laminar burning velocity are in keeping with that of maximum H + OH mole fraction and a linear correlation can be acquired, which is observed in Fig. 8 as well. And the linear correlation can be expressed by the following formulas:

Figure 9 H and OH production rate integrals of reaction steps at the equivalence ratio of 0.7 α:0;0.2


Figure 8 Laminar burning velocity versus maximum H+OH mole fractionφ: ■ 0.6; ● 0.7; ▲ 0.8
In order to get a better understanding of the production and consumption of H and OH radicals, the main reaction steps related in the USC Mech II mechanism are analyzed. Fig. 9 presents the H and OH production rate integrals of different reaction steps, and steps with integral less than 10-6have been neglected. Chemical reactions of all the steps are illustrated in Table 1. The results of CH4/air flame and the CH4/C2H4/air flame with mixing ratio of 0.2 were compared. As can be seen, H is predominantly produced by the reaction steps of R31 (CO + OH=CO2+ H),R89 (CH3+ O=CH2O + H) and R3 (OH + H2=H +H2O), and is mainly consumed by R1 (H + O2=O +OH) and R12 [H + O2(+M)=HO2(+M)]. On the other hand, R1 (H + O2=O + OH), R4 (O + H2O=OH + OH)and R16 (HO2+ H=OH + OH) play a positive role in the OH formation, whereas R31 (CO + OH=CO2+ H),R125 (CH4+ OH=CH3+ H2O) and R84 (CH2O + OH=HCO + H2O) do the opposite. As ethylene is added,the intensities of these reaction steps are increased except for R125, whose intensity goes near to steadiness.

Table 1 Main reactions related to the H and OH production and consumption
3.4 Flame temperature
As remarked above, ethylene addition will increase the laminar burning velocity of CH4/air flame. One reason is that more ethylene addition leads to the increase of H and OH radical concentrations, which promotes chemical reactions and consequently enhances the laminar burning velocity, and another reason is the increase of flame temperature as ethylene is added,which exerts a significant influence on the laminar burning velocity [26].
The flame temperature profiles of CH4/C2H4/air flames with different mixing ratios at the equivalence ratio of 0.7 are given in Fig. 10. It shows that as ethylene fraction increases, the flame temperature rise more rapidly and the adiabatic flame temperature rises.From CH4/air flame to CH4/C2H4/air flame with mixing ratio of 0.2, the adiabatic flame temperature increases by 41 K, and another 73 K increase is achieved as the mixing ratio increases to 0.6. When it comes to C2H4/air flame, 178 K increase is gained totally in comparison with the original.

Figure 10 Temperature profiles for different mixing ratios at the equivalence ratio of 0.7a=0, 1761; a=0.2, 1802; a=0.4, 1820;a=0.6, 1875; a=0.8, 1903; a=1.0, 1939
Peters and Williams [27] introduced the inner layer temperatureT0in fuel consumption, which characterizes the balance between chain-branching and chain-termination reactions and is interpreted as the critical temperature at and above which reactions take place. Within the temperature profile of a premixed flame,T0demonstrates a transition position from inert preheat zone to reaction zone, and this is also the position where the first derivative gives the maximum value and the second derivative vanishes.Fig. 11 presents the temperature profiles, the first and second derivatives of temperature in CH4/C2H4/air flames at the equivalence ratio of 0.7, withT0also displayed. The results show that the value ofT0becomes lower with the increase of ethylene fraction(except for C2H4/air flame), which consequently reduces the overall activation energy and makes the occurrence of combustion reaction easier [28].
3.5 Flame stability

Figure 11 Temperature profiles and the first and second derivatives of temperature for different mixing ratios at the equivalence ratio of 0.7a: 0; 0.2; 0.4; 0.6; 0.8; 1.0
Understanding and quantifying the effect of stretch is important for both the practical laminar flames and the modeling of turbulent flames based on the laminar flamelet concept, and flame stability is usually predicated by use of response of burning velocity to the variation of stretch [29]. Hence, Markstein length is introduced to demonstrate the linear relationship between the local burning velocity and the local stretch rate, as a function of the physicochemical properties of the mixture.
Since there are different definitions of Markstein length in the combustion community, Markstein length can be determined by the burning velocity and the stretch rate relative to either the unburned gas or the burned gas. The latter one is adopted here to express as the relationship of stretched flame speedSbwith the local stretch rateKb:whereis the one-dimensional unstretched flame speed, andLbis the Markstein length. The subscript b designates relativeness to the burned gas. Therefore,Lbcan be evaluated ifSbandKbavailable from the equations below:


wherexis the flame distance,m(x) is the local mass flux defined asm(x) =ρu(x),ρis the local gas density anduthe axial velocity.
To be specific, the procedure applied is as follows:The reaction zone is represented by the heat release rate, and the location to determine the burning velocity and the stretch rate is where the heat release rate reaches its peak value. Then, the burning velocity and the stretch rate are extrapolated to the reference surface accordingly, and the required parameters are obtained finally (as shown in Fig. 12). For more details of the determination ofSbandKbone can refer to Ref. [30].

Figure 12 Determination of flame speed and stretch rate relative to the burned gas
In the opposed-flow flame, the injection mixtures from the two opposed nozzle are identical, thus the calculation region is symmetric about the centerline.The temperature at the exit is set as 298 K. Adaptive mesh parameters are GRAD=0.5 and GURV=0.5.Absolute and relative error criteria are ATOL=1×10-6and RTOL=1×10-3. The ending axial position is 2 cm.
Figure 13 shows the flame speed against the stretch rate relative to the burned gas at the equivalence ratio of 0.7. Obviously the flame speedbSdeclines almost linearly as the stretch ratebKincreases for all the ethylene fraction conditions, whereas the slope is slightly larger with a large ethylene fraction.As mentioned above, Markstein lengthbLcan be acquired from the slope of the regression line.

Figure 13 Flame speed versus stretch rate for different mixing ratio at the equivalence ratio of 0.7α: ■ 0; ● 0.2; ▲ 0.4; ▼ 0.6
On the other hand, the non-dimensional Markstein numberbMis also used, which is defined as:

whereδis the unstretched flame thickness and can be obtained from the temperature profile of one-dimensional unstretched premixed flame:

whereTadis the adiabatic flame temperature,Tuis the unburned gas temperature, and ( dT/ dx)maxis the maximum temperature gradient.
The results of Markstein length and Markstein number relative to the burned gas against mixing ratio is shown in Fig. 14. Both Markstein length and Markstein number increase monotonically with the increase of ethylene fraction, indicating that ethylene addition will improve the flame stability.

Figure 14 Markstein length and Markstein number versus mixing ratio at the equivalence ratio of 0.7■ Lb; ● Mb
4 CONCLUSIONS
A numerical study on premixed CH4/C2H4/air flames with various ethylene fractions and equivalence ratios was conducted at room temperature and atmospheric pressure. The effects of ethylene addition on laminar burning velocity, flame structure and flame stability at lean condition (the equivalence ratioφ=0.6,0.7,0.8) have been investigated. The main conclusions are summarized as follows:
(1) The unstretched laminar burning velocity increases with ethylene fraction, and this effect of ethylene is more remarkable at a large equivalence ratio.
(2) Promotion of chemical reactions with ethylene addition is primarily due to higher concentrations of the H, O and OH radicals in the flame as ethylene is added, and a linear correction exists between the laminar burning velocity and the maximum H + OH concentration in the reaction zone.
(3) With the increase of ethylene fraction, the adiabatic flame temperature is raised, while the inner layer temperature becomes lower. Both of these factors contribute to the enhancement of combustion.
(4) Both Markstein length and Markstein number increase as more ethylene is added, indicating the tendency of flame stability to improve with ethylene addition.
1 Cho, E.S., Chung, S.H., “Improvement of flame stability and NOxreduction in hydrogen-added ultra lean premixed combustion”,J.Mech.Sci.Technol., 23, 650-658 (2009).
2 Ren, J.Y., Egolfopoulosa, F.N., Tsotsis, T.T., “NOxemission control of lean methane-air combustion with addition of methane reforming products”,Combust.Sci.Technol., 174 (4), 181-205 (2002).
3 Shy, S.S., Chen, Y.C., Yang, C.H., Liu, C.C., Huang, C.M., “Effects of H2or CO2addition, equivalence ratio, and turbulent straining on turbulent burning velocities for lean premixed methane combustion”,Combust.Flame, 153 (4), 510-524 (2008).
4 Ren, J.Y., Egolfopoulos, F.N., Tsotsis, T.T., “Strain-rate effects on hydrogen-enhanced lean premixed combustion”,Combust.Flame,124 (4), 717-720 (2001).
5 Zhang, X., Wang, B.W., Liu, Y.W., Xu, G.H., “Conversion of methane by steam reforming using dielectric-barrier discharge”,Chin.J.Chem.Eng., 17 (4), 625-629 (2009).
6 Zhang, Y.Y., Jianghong Wu, J.H., Ishizuka, S., “Hydrogen addition effect on laminar burning velocity, flame temperature and flame stability of a planar and a curved CH4-H2-air premixed flame”,Int.J.Hydrogen Energy, 34 (1), 519-527 (2009).
7 Yu, G., Law, C. K., Wu, C. K., “Laminar flame speeds of hydrocarbon + air mixtures with hydrogen addition”,Combustion and Flame,63 (3), 339-347 (1987).
8 Coppens, F.H.V., Ruyck, D.J., Konnov, A.A., “The effects of composition on burning velocity and nitric oxide formation in laminar premixed flames of CH4+ H2+ O2+ N2”,Combust.Flame, 149 (4),409-417 (2007).
9 Guo, H.S., Smallwood, G.J., Gulder, O.L., “The effect of reformate gas enrichment on extinction limits and NOxformation in counterflow CH4/air premixed flames”,Proc.Combust.Inst., 31 (1),1197-1204 (2007).
10 Chen, W.T., Jiang, Y., Qiu, R., “Effects of CO addition on the lean premixed CH4/Air flame”,ActaPhysico-Chimica Sinica, 26 (6),1481-1487 (2010).
11 Liu, W., Kelley, A.P., Law, C.K., “Flame propagation and counterflow nonpremixed ignition of mixtures of methane and ethylene”,Combustion andFlame, 157 (5), 1027-1036 (2010).
12 Delfau, J.L., Biet, J., Idir, M., Pillier, L., Vovelle, C., “Experimental and numerical study of premixed, lean ethylene flames”,Proc.Combust.Inst., 31 (1), 357-365 (2007).
13 Leroy, V., Leoni, E., Santoni, P.A., “Reduced mechanism for the combustion of evolved gases in forest fires”,Combust.Flame, 154(3), 410-433 (2008).
14 Zhu, X.H., Guo, Z.F., Cen, W., Mao, B.Q., “Ethylene polymerization using improved polyethylene catalyst”,Chin.J.Chem.Eng., 19 (1),52-56 (2011).
15 Kee, R.J., Grcar, J.F., Smooke, M.D., Miler, J.A., Meeks, E., “PREMIX: A program for modeling steady, laminar, one-dimensional premixed flames”, Sandia National Laboratories, USA, SAND85-8240(1985).
16 Lutz, A.E., Kee, R.J., Grcar, J.F., Rupley, F.M., “OPPDIF: A program for computing opposed-flow diffusion flames”, Sandia National Laboratories, USA, SAND96-8243 (1997).
17 Andrae, J., Bjornbom, P., Edsberg, L., “Numerical studies of wall effects with laminar methane flames”,Combust.Flame, 128 (1-2),165-180 (2002).
18 Henshaw, P.F., Andrea, T.D., Mann, K.R.C., Ting, D.S.K., “Premixed ammonia-methane-air combustion”,Combust.Sci.Technol.,177 (11), 2151-2170 (2005).
19 Wu, C.Y., Chao, Y.C., Cheng, T.S., Chen, C.P., Ho, C.T., “Effects of CO addition on the characteristics of laminar premixed CH4/air opposed-jet flames”,Combust.Flame, 156 (2), 362-373 (2009).
20 Wang, H., You, X.Q., Joshi, A.V., Davis, S.G., Laskin, A., Egolfopoulos,F., Law, C.K., “USC Mech Version (II) High-temperature combustion reaction model of H2/CO/C1-C4compounds”, Combustion Kinetics Laboratory, University of Southern California, 2007[2011-06-21], http://ignis.usc.edu/ USC_Mech_II.htm.
21 Smallbone, A.J., Liu, W., Law, C.K., You, X.Q., Wang, H., “Experimental and modeling study of laminar flame speed and non-premixed counterflow ignition ofn-heptane”,Proc.Combust.Inst., 32 (1), 1245-1252 (2009).
22 Hu, E., Huang, Z.H., He, J.J., Jin, C., Zheng, J.J., “Experimental and numerical study on laminar characteristics of premixed methane-hydrogen-air flames”,Int.J.Hydrogen Energy, 34 (11),4876-4888 (2009).
23 Bhargava, A., Westmoreland, P.R., “MBMS analysis of a fuel-lean ethylene flame”,Combust.Flame, 115 (4), 456-467 (1998).
24 Hu, E., Huang, Z.H., Zheng, J.J., Li, Q.Q., He, J.J., “Numerical study on laminar burning velocity and NO formation of premixed methane-hydrogen-air flames”,Int.J.HydrogenEnergy, 34 (15),6545-6557 (2009).
25 Hu, E., Huang, Z.H., He, J.J., Miao, H.Y., “Experimental and numerical study on lean premixed methane-hydrogen-air flames at elevated pressures and temperatures”,Int.J.Hydrogen Energy, 34(16), 6951-6960 (2009).
26 Konnov, A.A., “The effect of temperature on the adiabatic laminar burning velocities of CH4-air and H2-air flames”,Fuel, 89 (9),2211-2216 (2010).
27 Peters, N., Williams, F.A., “The asymptotic structure of stoichiometric methane-air flames”,Combustion and Flame, 68 (2), 185-207(1987).
28 Warnatz, J., Maas, U., Dibble, R.W., Combustion: Physical and Chemical Fundamentals, Modeling and Simulation, Experiments,Pollutant formation, 2nd edition, Springer-Verlag, Berlin (1998).
29 Davis, S.G., Quinard, J., Searby, G., “Markstein numbers in counterflow, methane- and propane- air flames: A computational study”,Combust.Flame, 130 (1-2), 123-136 (2002).
30 Davis, S.G., Quinard, J., Searby, G., “Determination of Markstein numbers in counterflow premixed flames”,Combust.Flame, 130(1-2), 112-122 (2002).
 Chinese Journal of Chemical Engineering2012年5期
Chinese Journal of Chemical Engineering2012年5期
- Chinese Journal of Chemical Engineering的其它文章
- Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin*
- Reactive Distillation for Producing n-Butyl Acetate: Experiment and Simulation
- One Step Bioleaching of Sulphide Ore with Low Concentration of Arsenic by Aspergillus niger and Taguchi Orthogonal Array Optimization*
- Adsorption of Chlortetracycline from Water by Rectories*
- Optimization of Fermentation Media for Enhancing Nitrite-oxidizing Activity by Artificial Neural Network Coupling Genetic Algorithm*
- Effect of Propanoic Acid on Ethanol Fermentation by Saccharomyces cerevisiae in an Ethanol-Methane Coupled Fermentation Process*
