Effect of Propanoic Acid on Ethanol Fermentation by Saccharomyces cerevisiae in an Ethanol-Methane Coupled Fermentation Process*
1 INTRODUCTION
Cassava is an attractive ethanol fuel feedstock compared to other fuel feedstocks, because it is not a staple food of the Chinese people and can grow in marginal lands where other crops, such as corn, wheat,rice, and sugarcane, do not thrive [1]. Thus, using cassava for ethanol production would not raise major ethical and moral questions as would corn [2]. Currently, >1 million tons of cassava ethanol is being produced annually in China, but in ethanol production from cassava starch, large amounts of stillage, the remainder after ethanol distillation, is produced, which causes serious water pollution. A reputed “full stillage” recycling for ethanol fermentation has been widely studied regarding water pollution reduction [3-5]. The main processes of full stillage recycling is a solid-liquid separation, direct recycling of 15%-30% of the thin stillage, and the balance is then treated successively by anaerobic and aerobic wastewater treatments. There are several drawbacks in full stillage recycling. First,solids separation equipment is severely corroded during solid-liquid separation because the separation is performed under high temperature and acidic conditions [6], and the sand in stillage further exacerbates corrosion. Second,S.cerevisiaemetabolic coproducts in thin stillage can accumulate during recycling and thus degrade ethanol fermentation [7]. Finally, after aerobic treatment, the chemical oxygen demand of the wastewater is usually >300 mg·L-1, which can severely threaten the environment.
Therefore, an alternative process called ethanolmethane coupled fermentation has been proposed to resolve the stillage pollution problems [8, 9]. In this process, the stillage generated from ethanol fermentation was first treated by anaerobic digestion (methane fermentation) and then the anaerobic effluent utilized for the next ethanol fermentation batch after reclamation treatment. As a result, wastewater was not discharged to the environment (Fig. 1) and in addition,separation equipment corrosion and metabolic coproduct inhibition were avoided. This coupled process has been confirmed by laboratory scale operation through >20 successive batches and could arrive at a water and mass balance [9, 10]. The main drawback to this system is that organic acids in the anaerobic effluent, mainly acetic and propanoic acid, are potential inhibitors of ethanol fermentation.
The inhibitory effects of organic acids, mainly acetic and lactic acid, on ethanol fermentation have been widely investigated [11-15]. These acids are mainly produced by contaminating bacteria, such as acetogenic and lactogenic bacteria. The inhibitory effects of propanoic acid on yeast growth have also been reported [16, 17], but these reports are mostly related to food preservation. Usually, no propanoic acid is introduced from cassava, and negligible propanoic acid is produced by yeast and/or contaminating bacteria,and thus, reports of propanoic acid effects on ethanol fermentation byS.cerevisiaehave been scarce.
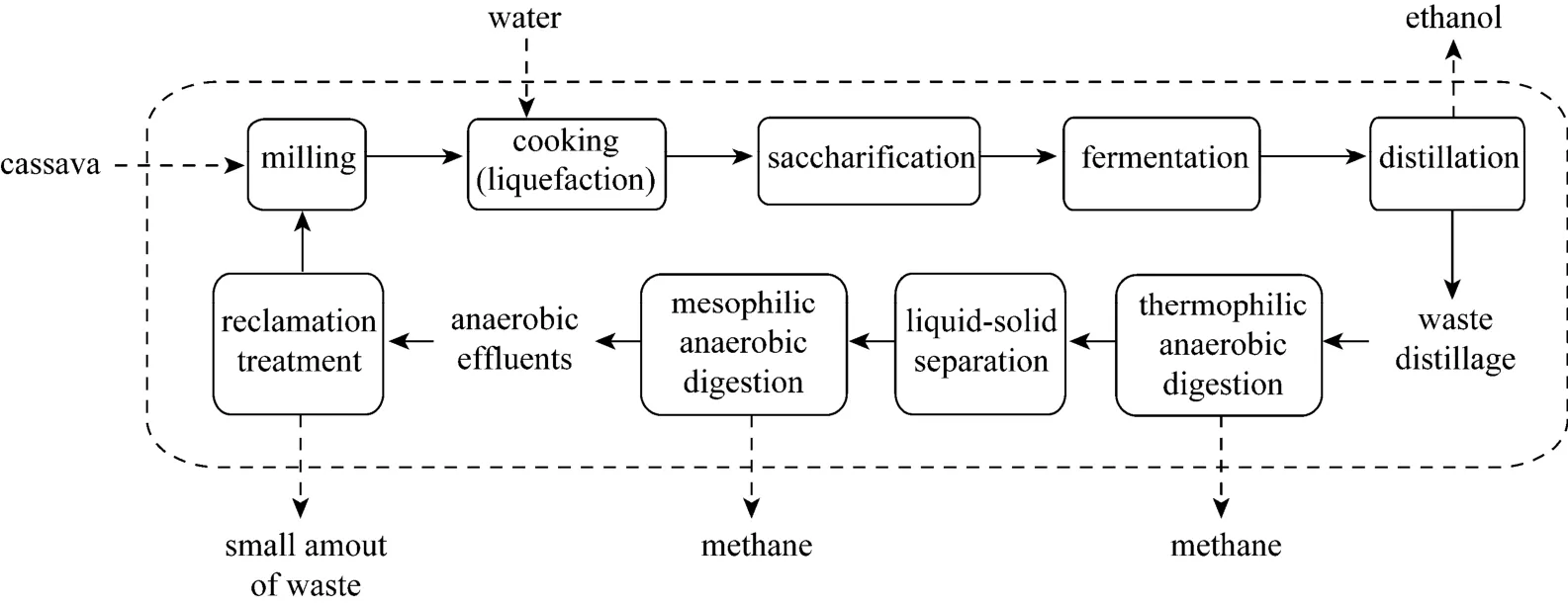
Figure 1 Flowchart of the coupled ethanol-methane fermentation process
In the ethanol-methane coupled process here,stillage generated from ethanol fermentation was first treated by anaerobic digestion (methane fermentation),which involved a spectrum of bacteria consisting of three major groups, which perform hydrolysis, acidification,or methane conversion. Propanoic acid is produced by propanoic producing groups of acidogenic bacteria during acidification [8], and in some cases its concentration can reach 2.96 g·L-1[8]. Moreover, the rate of propanoic acid conversion to methane is much lower than that of acetic acid under the same conditions [18, 19].Consequently, as production proceeds, propanoic acid more easily and gradually accumulates, which can potentially cause collapse of the coupled process. Thus,propanoic acid is one of the most important factors for the successful operation of ethanol-methane coupled fermentation. Furthermore, concentration of propanoic acid can be always detected in the anaerobic effluent.As a result, in preparation for the application of an ethanol-methane coupled fermentation at the industrial scale, it was necessary to evaluate the influence of propanoic acid on ethanol fermentation. Moreover, description of a functional method for avoiding potential ethanol inhibition originating from high propanoic acid concentrations in the anaerobic effluent would be valuable.
In this study, the effects of propanoic acid concentration onS.cerevisiaegrowth, ethanol fermentation,and glycerol production in cassava mash were investigated, focusing on the influence of pH on these parameters.
2 MATERIALS AND METHODS
2.1 Microorganism and growth conditions
Two strains ofS.cerevisiaewere employed: AG strain (“Angel”, a commercial strain ofS.cerevisiaefor ethanol production) obtained from Hubei Angel Yeast Co., China and TG 1308 strain, an industrial strain, kindly provided by Henan Tianguan Fuel Ethanol Co., China. Yeast was cultivated in an orbital shaker at 100 r·min-1and 30 °C for 19 h and the seed medium contained (in g·L-1) glucose, 20; yeast extract,8.5; ammonium sulfate, 1.3; magnesium sulfate heptahydrate, 0.1; and calcium chloride dihydrate, 0.06.
2.2 Mashing of cassava
Liquefied mash prepared from 100 g of cassava powder (starch content 65%-70%, ~0.45 mm diameter,kindly provided by the Henan Tianguan Fuel Ethanol Co., China) was added to 300 ml of water. The pH of the resulting slurry was adjusted to 6.2-6.4 with 30%H2SO4. Liquefaction was accomplished by adding 10 IU of thermostable α-amylase (20000 IU·ml-1, optimal temperature range 95-105 °C, Genencor China Co.)per gram of powder, heating at 100 °C for 60 min, and then autoclaving at 121 °C for 20 min—water lost during autoclaving was replaced with sterile water. No propanoic acid was detected in the mash.
2.3 Ethanol fermentation
Triplicate fermentations were carried out in 250 ml flasks containing 150 ml of cassava mash, and experiments involved propanoic acid at 0, 15, 30, 45, 60, and 75 mmol·L-1. After acid addition, slurry pH was adjusted to pH 4.0, 5.0, or 6.0 using 30% H2SO4or 10%NaOH. A bolus of 130 IU of glucoamylase (130000 IU·ml-1, Genencor China Co.) per gram of cassava powder was added to promote saccharification, with 0.3% of urea added as nitrogen source. Seed broth at 10% of the flask volume (15/150 ml) was inoculated into the mash to produce 10 million cells per miniliter mash, and the temperature maintained at 30°C in an incubator. Fermentations were terminated when residual total sugar concentration decreased to <10 g·L-1.
2.4 Analytical methods
Fehling’s reagent was utilized to measure reducing sugar concentration as well as total sugars after samples were acid hydrolyzed (in 2%, hydrochloric acid, at 100 °C for 120 min) [20].
Concentration of ethanol, propanoic acid, and glycerol was determined by high performance liquid chromatography (UltiMate 3000 HPLC, Dionex Corp.,California, USA), with samples pretreated as described by Graveset al[14]. A 20-μl aliquot from a suitably diluted sample was analyzed using an Aminex HPX-87H ion exclusion column (Bio-Rad Laboratories, California, USA) coupled to a refractive index detector (Shodex RI-101, Showa Denko K.K., Oita,Japan). The column was operated at 65 °C, the mobile phase 0.005 mol·L-1sulfuric acid at 0.6 ml·min-1, and the data processed using the Chromeleon Software(Dionex, USA).

Figure 2 Final ethanol concentrations produced by two strains of S. cerevisiae in cassava mash at 30 °C, containing increasing propanoic acid concentrations at different pH values (Error bars too small to show; standard deviation, <2.5%)pH: ■ 4.0; ◆ 5.0; ▲ 6.0
All final ethanol, glycerol, and biomass data were analyzed using software SPSS version 13.0 (SPSS Inc.,Illinois, USA), which was widely used in statistic analysis. Differences among means were analyzed at a significance level of 0.05 by one-way ANOVA. Thep-values were calculated to estimate significance:p<0.01 meant highly significant, 0.01<p<0.05 significant, andp>0.05 not significant.
3 RESULTS AND DISCUSSION
3.1 Effect of propanoic acid on ethanol production by S. cerevisiae
Propanoic acid addition significantly (p<0.01)affected the ethanol production from both yeast strains at pH 4.0 and 5.0 (Fig. 2), but the effect was not significant (p>0.05) at pH 6.0. For strain TG 1308, lower pH conditions increased the acid’s inhibitory activity at each acid concentration (30 mmol·L-1,p<0.05; 45,60, and 75 mmol·L-1,p<0.01, Fig. 2). For example, at pH 6.0 and 5.0, 75 mmol·L-1propanoic acid did not decrease final ethanol concentrations but, at pH 4.0,ethanol fermentation was completely inhibited. It has been reported that the inhibitory effect of weak organic acids on microorganisms is due to their undissociated forms which are determined by the medium’s pH [11]; lower pH increases undissociated acid concentration and, in turn, increases propanoic acid inhibitory activity (Table 1). Statistical analysis showed that medium pH without propanoic acid addition did not significantly (p>0.05) affect the ethanol fermentation by strain TG 1308, whereas strain AG was more sensitive to the pH alone (p<0.05, without propanoicacid). This difference is derived perhaps from the different strain characteristics.
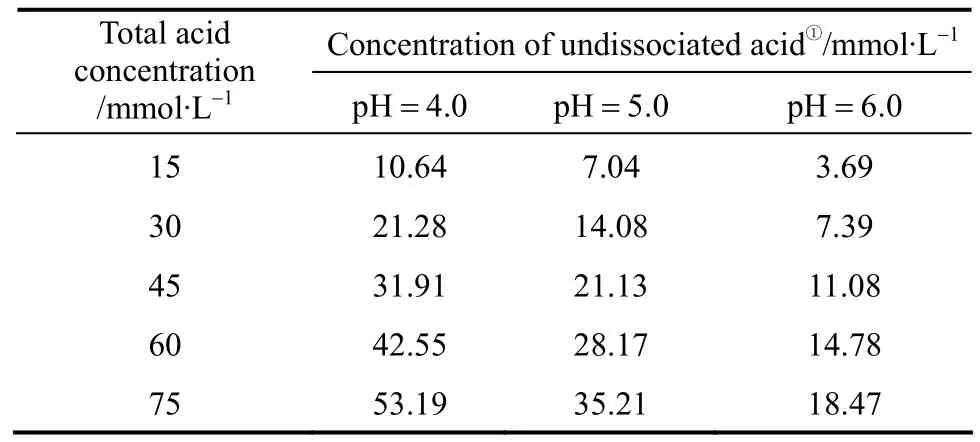
Table 1 Percentages of undissociated and anions of propanoic acid in cassava mash at different pH values corresponding to various total propanoic acid concentrations
Propanoic acid addition increased (p<0.01) the final ethanol concentration at pH 4.0 and 5.0, with total propanoic acid concentration <30.0 mmol·L-1(Fig. 3). Similar stimulating effects by low concentration acetic acid have been reported [11]; acetic acid could stimulate ethanol production in some cases by as much as 20% [12], but this phenomenon has not been not consistently observed. In the present study,the final ethanol concentration was increased by 5.5%and 7.6% withS.cerevisiaeTG 1308 and AG strains,respectively, consistent with results by Zhaoet al. [11].The stimulatory effect of propanoic acid on ethanol fermentation observed here could be explained in two ways. First, propanoic acid conversion to propenyl-CoA,and its subsequent utilization in the 2-methylcitrate pathway [21], spared some pyruvic acid from metabolic reactions associated with anaerobic cell growth,thus allowing increased fermentative ethanol production [22]. And second, in the presence of propanoic acid, a greater proportion of glucose was diverted for energy production (as ATP) to maintain cytosolic pH homeostasis [12]. Overall, propanoic acid stimulated the energy producing glycolytic pathway, thus increasing ethanol production. Other researchers have also reported that the presence of acid may modify glycolytic controls, and enolase and/or phosphorylating enzymes(hexokinase and phosphofructokinase) are presumably involved in the process [23].
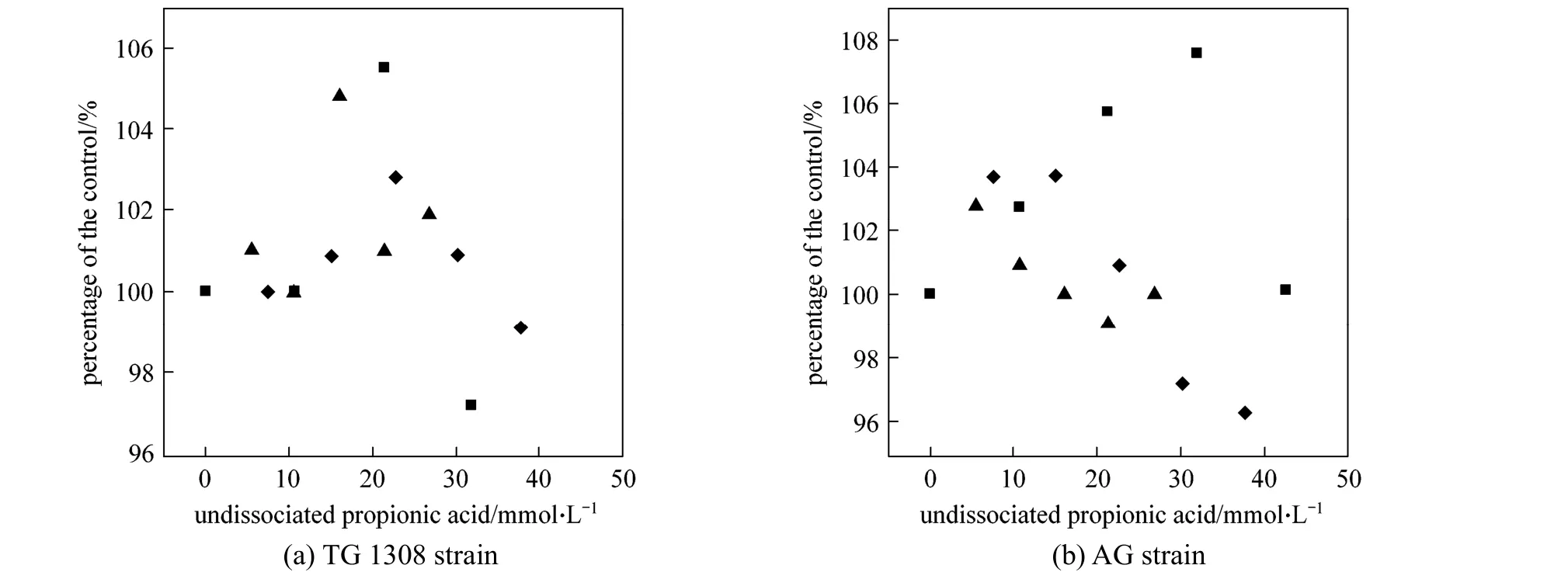
Figure 3 Final ethanol concentrations, as percentage of controls, produced by two strains of S. cerevisiae in cassava mash at 30 °C and containing different concentrations of undissociated propanoic acid (Error bars too small to show; standard deviation<2.5%)pH: ■ 4.0; ◆ 5.0; ▲ 6.0
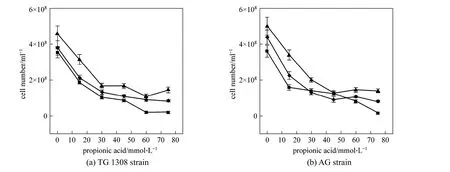
Figure 4 Effect of propanoic acid on cell numbers of two S. cerevisiae strains at termination of fermentation in cassava mash at 30 °C at different pH valuespH: ■ 4.0; ◆ 5.0; ▲ 6.0
3.2 Inhibition of S. cerevisiae growth by propanoic acid
Yeast growth was also determined, as it is critical to the ethanol fermentation rate. As the yeast dry weight in these incubations was difficult to measure due to solids in the media, yeast growth was evaluated by yeast cell counts by hemocytometer. Addition of propanoic acid significantly (p<0.01) decreased cell numbers for both yeast strains at all pH conditions(Fig. 4), which meant that total acid concentration determined the growth of yeast strain. When the propanoic acid concentration was 60 or 75 mmol·L-1at pH 4.0, strain TG 1308 growth was totally inhibited.Cell numbers also declined with decreased medium pH without propanoic acid addition but to a smaller extent (p<0.05); similar results were obtained with strain AG. The different characteristics of these strains resulted most likely in the growth differences observed here in the presence of propanoic acid [16, 17].
The decreased biomass production observed here could be explained by the classical weak acid theory,in which undissociated propanoic acid, which diffuses into yeast cells, dissociates due to the higher intracellular pH, thus acidifying the cytoplasm. To maintain intracellular pH in an optimal metabolic range, cells pump protons to the exterior at the expense of metabolic energy,in a ratio of 1 mol ATP per 1 mol transported H+[24].Increased diversion of energy to export protons thus results in decreased biomass yield with respect to glucose.
Narendranathet al. have reported that 100 mmol·L-1acetic acid inhibits completely yeast growth in minimal medium [13], while here 75 mmol·L-1propanoic acid totally inhibited yeast growth. The present results revealed that propanoic acid was more lethal toS.cerevisiaethan acetic acid, probably due to propanoic acid has a longer aliphatic group, making it more lipophilic than acetic acid. Besides causing cytoplasm acidification, propanoic acid can also cause disordering of membrane structure [25], which may also have contributed in part to its greater inhibitory effect on yeast ethanol fermentation.
3.3 Effect of propanoic acid on glycerol production by S. cerevisiae
As an important ethanol fermentation by-product,glycerol production was measured in the present experiments and, compared to the control, glycerol production was found to decease (p<0.01) with propanoic acid addition (Fig. 5). At pH 4.0, the glycerol produced by strain TG 1308 decreased (p<0.01) from(7.57±0.31) g·L-1to (4.56±0.24) g·L-1when propanoic acid increased from 0 to 45 mmol·L-1; similar results were obtained at pH 5.0 and 6.0. Under anaerobic conditions, glycerol is formed during reoxidation of NADH, in a great extent, from cellular material synthesis [26]. Consequently, decreased biomass should have resulted in decreased glycerol production. It is noted that, in both strains, pH without propanoic acid addition also affected (p<0.01, strain TG 1308;p<0.05,strain TG) glycerol production.
3.4 Effect of propanoic acid on ethanol fermentation by S. cerevisiae
When ethanol fermentation was complete (residual sugar at <10 g·L-1), propanoic acid addition to the medium improved ethanol fermentation performance in terms of product yield; the ethanol yield increased and the coproduct yield (glycerol and biomass yield)decreased (Table 2). However, ethanol productivity must be considered in light of industrial scale production. Although the final ethanol concentration and ethanol yield increased when propanoic acid was present, ethanol productivity decreased in some cases because the fermentation time was prolonged (Table 3).For example, with 45 mmol·L-1propanoic acid, the final ethanol concentration, ethanol yield, and ethanol productivity by strain AG at pH 4.0 was (88.4±1.5)g·L-1, (0.468±0.008) g ethanol per gram sugar, and(1.84±0.01) g·L-1·h-1, respectively, while the control showed (82.1±1.3) g·L-1, (0.434±0.007) g ethanol per gram sugar, and (2.74±0.03) g·L-1·h-1, respectively(Table 3). The prolonged fermentation time reflects the time required for the yeast to pump out excess protons to produce the required intracellular pH for growth and active fermentation [13]. Here, accelerated ethanol formation in the presence of propanoic acid was found (data not shown), which was similar to other observations regarding the presence of low acetic acid concentrations [11, 12]. Consequently, ethanol productivity did not decrease with low propanoic acid concentrations but, with high propanoic acid, ethanol productivity decreased (Table 3). Compared to the gains obtained in the ethanol yield, the losses caused by significantly decreased ethanol productivity was greater, and would be thus unacceptable in industrial scale ethanol production. The results here suggested that, to avoid decreased ethanol productivity (increased fermentation time), the propanoic acid concentration should be at <30.0 mml·L-1(Table 3).However, the potential inhibitory effect by propanoic acid could be avoided by elevating the medium pH as an ameliorating condition because, as observed here,at pH 6.0 the effect of propanoic acid on ethanol fermentation by yeast became insignificant (p>0.05). In this case, the risk of contamination in ethanol fermentation must be considered because the growth of contaminating bacteria, such as acetogenic and lactogenic bacteria, during ethanol fermentation is usually inhibited by lowering the fermentation medium’s initial pH[14]. As a result, when the medium pH is increased,the growth of contaminating bacteria would no longer be strongly inhibited.
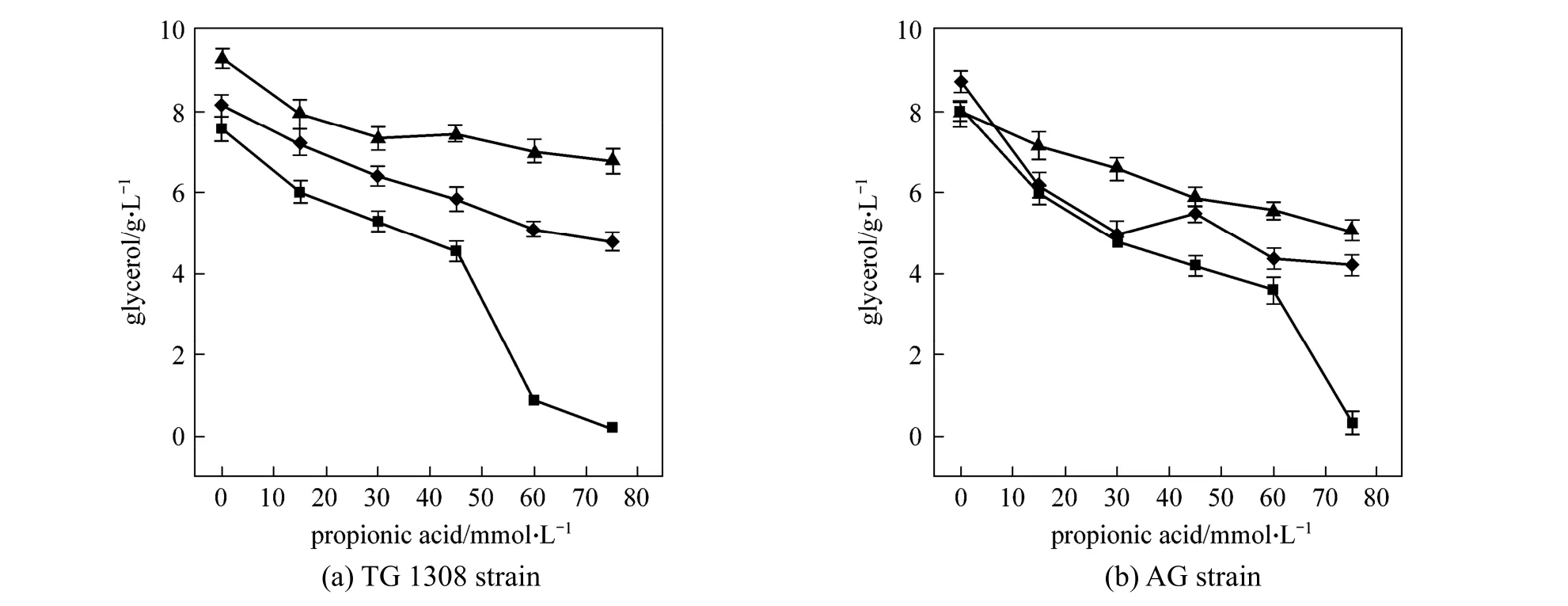
Figure 5 Glycerol concentrations produced by two strains of S. cerevisiae at termination of fermentation in cassava mash at 30 °C, containing increasing propanoic acid concentrations at different pH valuespH: ■ 4.0; ◆ 5.0; ▲ 6.0

Table 2 (a) Ethanol and coproduct fermentation performance by strain TG 1308 in cassava mash at 30°C,containing increasing propanoic acid concentrations and adjusted to different pH values
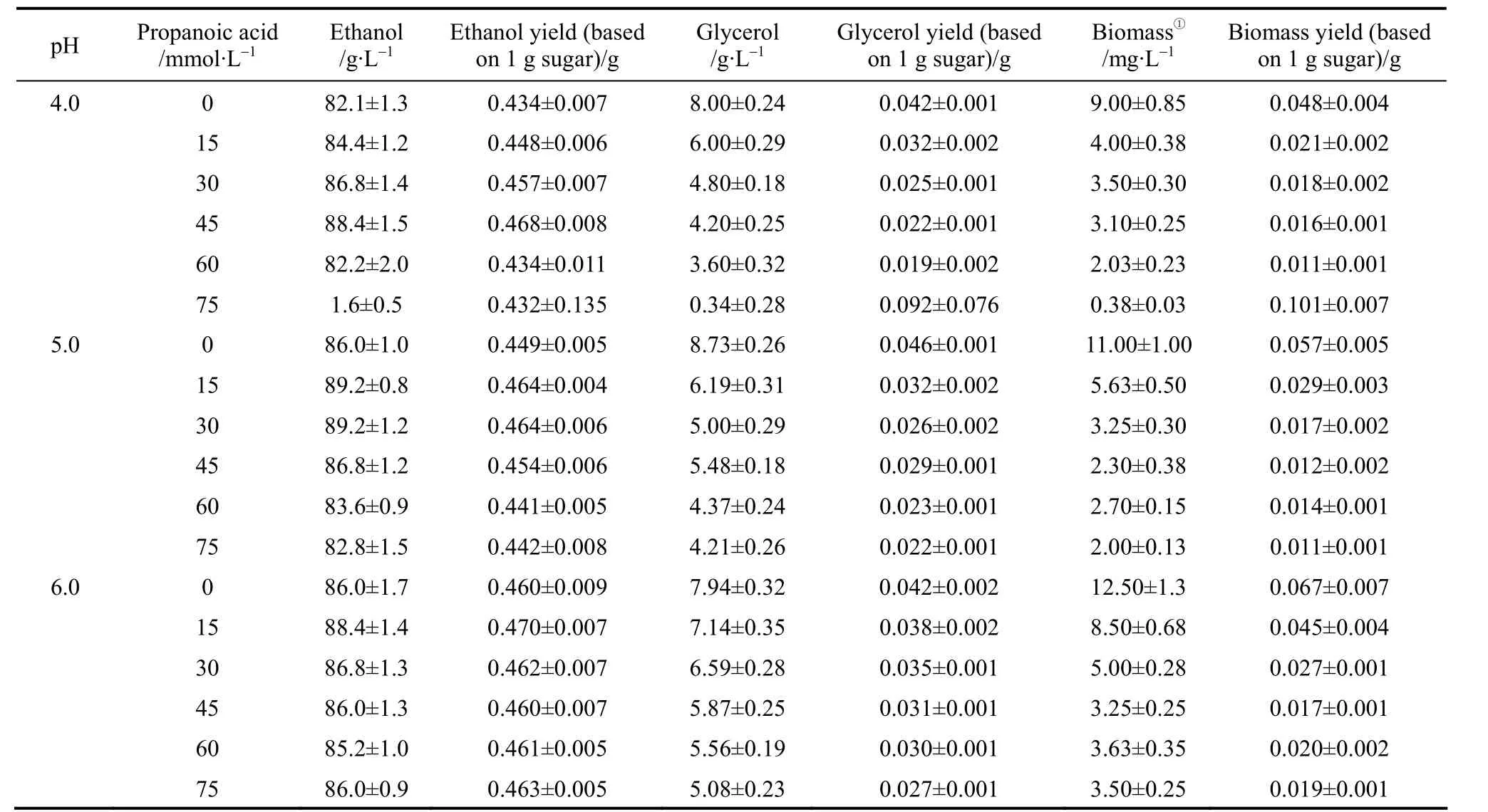
Table 2 (b) Ethanol and coproduct fermentation performance by strain AG in cassava mash at 30 °C,containing increasing propanoic acid concentrations and adjusted to different pH values
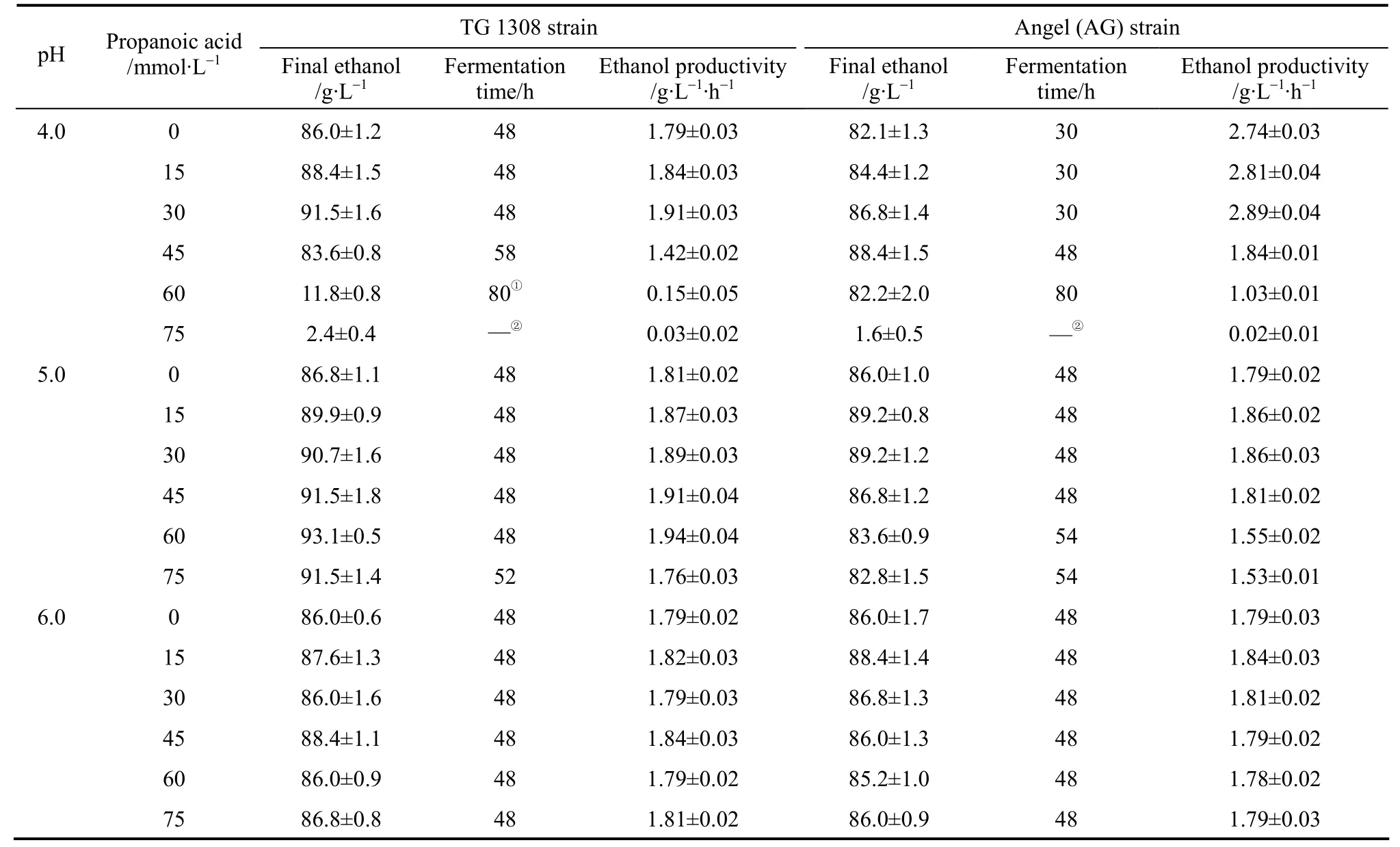
Table 3 Ethanol fermentation time and ethanol productivityby both yeast strains in cassava mash at 30°C,containing increasing propanoic acid concentrations at different pH values
4 CONCLUSIONS
In the ethanol-methane coupled fermentation process, abnormal methane fermentation usually results in increased concentrations of organic acid, particularly propanoic acid, in the anaerobic effluent. If this anaerobic effluent is used for subsequent ethanol fermentation, the fermentation performance could deteriorate. The present results suggested that in a traditional ethanol fermentation with an initial pH of 4.0, the propanoic acid concentration should be <30 mmol·L-1;otherwise, the ethanol fermentation would be prolonged (45 and 60 mmol·L-1) or totally inhibited (75 mmol·L-1). However, the potential inhibitory effect by propanoic acid could be avoided by the ameliorating effect of elevating the medium pH.
Interestingly, although propanoic acid exhibited an inhibitory effect on ethanol fermentation byS.cerevisiae, low concentration propanoic acid stimulated ethanol production as much as 7.6% under the proper conditions. This improved ethanol fermentation could partly be explained by the observed decreased biomass and decreased glycerol production resulting from propanoic acid addition. In the ethanol-methane coupled process, it was not necessary to decrease the acid content in the anaerobic effluent to zero. Actually,this acid-free requirement would be almost impossible to be meet by controlling the anaerobic digestion(methane fermentation) when considering industrial production efficiency.
As the effect of propanoic acid on ethanol fermentation byS.cerevisiaewas also influenced by other environmental conditions, such as temperature and substrate concentration, related research into other important parameters are under way.
1 Dai, D., Hu, Z., Pu, G., Li, H., Wang, C., “Energy efficiency and potentials of cassava fuel ethanol in Guangxi region of China”,Energy Convers.Manage., 47, 1686-1699 (2006).
2 Pimentel, D., “Ethanol fuels: energy balance, economics, and environmental impacts are negative”,Nat.Resour.Res., 12 (2), 127-134(2003).
3 Bialas, W., Szymanowska, D., Grajek, W., “Fuel ethanol production from granular corn starch usingSaccharomyces cerevisiaein a long term repeated SSF process with full stillage recycling”,Bioresour.Technol., 101 (9), 3126-3131 (2010).
4 Miao, J., Li, L.L., Chen, G.H., Gao, C.J., Dong, S.X., “Preparation ofN,O-carboxymethyl chitosan composite nanofiltration membrane and its rejection performance for the fermentation effluent from a wine factory”,Chin.J.Chem.Eng., 16 (2), 209-213 (2008).
5 Grossmann, I.E., Martín, M., “Energy and water optimization in biofuel plants”,Chin.J.Chem.Eng., 18 (6), 914-922 (2010).
6 Mao, Z.G., Zhang, J.H., “Trend of ‘zero energy consumption and wastewater’ in fuel ethanol production”,Chin.J.Biotechnol., 24 (6),946-949 (2008).
7 Castro, G.A., Caicedo, L.A., Almeciga-Diaz, C.J., Sanchez, O.F.,“Solvent extraction of organic acids from stillage for its re-use in ethanol production process”,Waste Manage.Res., 28 (6), 533-538(2010).
8 Zhang, C.M., Mao, Z.G., Wang, X., Zhang, J.H., Sun, F.B., Tang, L.,Zhang, H.J., “Effective ethanol production by reutilizing waste distillage anaerobic digestion effluent in an integrated fermentation process coupled with both ethanol and methane fermentations”,Bioprocess Biosyst.Eng., 33 (9), 1067-1075 (2010).
9 Sun, F.B., Mao, Z.G., Zhang, J.H., Zhang, H.J., Tang, L., Zhang,C.M., Zhang, J., Zhai, F.F., “Water-recycled cassava bioethanol production integrated with two-stage UASB treatment”,Chin.J.Chem.Eng., 18 (5), 837-842 (2010).
10 Zhang, Q.H., Lu, X., Tang, L., Mao, Z.G., Zhang, J.H., Zhang, H.J.,“A novel full recycling process through two-stage anaerobic treatment of distillery wastewater for bioethanol production from cassava”,J.Hazard.Mater., 179, 635-641 (2010).
11 Zhao, R., Bean, S.R., Crozier-Dodson, B.A., Fung, D.Y.C., Wang,D.H.,“Application of acetate buffer in pH adjustment of sorghum mash and its influence on fuel ethanol fermentation”,J.Ind.Microbiol.Biotechnol., 36 (1), 75-85 (2009).
12 Taherzadeh, M., Niklasson, C., Lidén, G., “Acetic acid-friend or foe in anaerobic batch conversion of glucose to ethanol bySaccharomyces cerevisiae?”,Chem.Eng.Sci., 52 (15), 2653-2659 (1997).
13 Narendranath, N.V., Thomas, K.C., Ingledew, W.M., “Effects of acetic acid and lactic acid on the growth ofSaccharomyces cerevisiaein a minimal medium”,J.Ind.Microbiol.Biotechnol., 26 (3), 171-177(2001).
14 Graves, T., Narendranath, N., Dawson, K., Power, R., “Effect of pH and lactic or acetic acid on ethanol productivity bySaccharomyces cerevisiaein corn mash”,J.Ind.Microbiol.Biotechnol., 33 (6),469-474 (2006).
15 Graves, T., Narendranath, N.V., Dawson, K., Power, R., “Interaction effects of lactic acid and acetic acid at different temperatures on ethanol production bySaccharomyces cerevisiaein corn mash”,Appl.Microbiol.Biotechnol., 73 (5), 1190-1196 (2007).
16 Lind, H., Jonsson, H., Schnurer, J., “Antifungal effect of dairy propionibacteria—contribution of organic acids”,Int.J.FoodMicrobiol., 98 (2), 157-165 (2005).
17 Mollapour, M., Shepherd, A., Piper, P.W., “Novel stress responses facilitateSaccharomyces cerevisiaegrowth in the presence of the monocarboxylate preservatives”,Yeast, 25 (3), 169-177 (2008).
18 Wang, Y., Zhang, Y., Wang, J., Meng, L., “Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria”,Biomass Bioenergy, 33 (5), 848-853 (2009).
19 Ren, N.Q., Liu, M., Wang, A., Ding, J., Li, H., “Organic acids conversion in methanogenic-phase reactor of the two-phase anaerobic process”,Chin.J.Environ.Sci., 24 (4), 89-93 (2003).
20 Schneider, F., Sugar Analysis: Official and Tentative Methods Recommended by the International Commission for Uniform Methods of Sugar Analysis (ICUMSA), ICUMSA, Englang (1979).
21 Pronk, J.T., van der Linden-Beuman, A., Verduyn, C., Scheffers,W.A., van Dijken, J.P., “Propionate metabolism inSaccharomyces cerevisiae: implications for the metabolon hypothesis”,Microbiology, 140, 717-722 (1994).
22 Abbott, D.A., Ingledew, W.M., “Buffering capacity of whole corn mash alters concentrations of organic acids required to inhibit growth ofSaccharomyces cerevisiaeand ethanol production”,Biotechnol.Lett., 26 (16), 1313-1316 (2004).
23 Pampula, M.E., Loureiro-Dias, M.C., “Activity of glycolytic enzymes ofSaccharomyces cerevisiaein the presence of acetic acid”,Appl.Microbiol.Biotechnol., 34 (3), 375-380 (1990).
24 Pampulha, M.E., Loureiro-Dias, M.C., “Energetics of the effect of acetic acid on growth ofSaccharomyces cerevisiae”,FEMS Microbiol.Lett., 184 (1), 69-72 (2000).
25 Piper, P., Calderon, C.O., Hatzixanthis, K., Mollapour, M., “Weak acid adaptation: the stress response that confers yeasts with resistance to organic acid food preservatives”,Microbiology, 147,2635-2642 (2001).
26 Zhang, A.L., Chen, X., “Improve ethanol yield through minimizing glycerol yield in ethanol fermentation ofSaccharomyces cerevisiae”,Chin.J.Chem.Eng., 16 (4), 620-625 (2008).
27 Devantier, R., Pedersen, S., Olsson, L., “Characterization of very high gravity ethanol fermentation of corn mash. Effect of glucoamylase dosage, pre-saccharification and yeast strain”,Appl.Microbiol.Biotechnol., 68 (5), 622-629 (2005).
 Chinese Journal of Chemical Engineering2012年5期
Chinese Journal of Chemical Engineering2012年5期
- Chinese Journal of Chemical Engineering的其它文章
- Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin*
- Reactive Distillation for Producing n-Butyl Acetate: Experiment and Simulation
- One Step Bioleaching of Sulphide Ore with Low Concentration of Arsenic by Aspergillus niger and Taguchi Orthogonal Array Optimization*
- Adsorption of Chlortetracycline from Water by Rectories*
- Optimization of Fermentation Media for Enhancing Nitrite-oxidizing Activity by Artificial Neural Network Coupling Genetic Algorithm*
- Numerical Study on Laminar Burning Velocity and Flame Stability of Premixed Methane/Ethylene/Air Flames*
