Seeding and Cross-Seeding Aggregations of Aβ40 and hIAPP in Solution and on Surface
Miaomiao Liu, Wenjuan Wang, Xiuping Hao, Xiaoyan Dong
Key Laboratory of Systems Bioengineering of the Ministry of Education, Department of Biochemical Engineering, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300354, China.
Abstract:Alzheimer’s disease (AD)and type 2 diabetes mellitus(T2DM), common incurable diseases caused by protein misfolding, have shown extensive correlation with each other via cross-aggregation between their related pathogenic peptide,amyloid β protein (Aβ)and human islet amyloid polypeptide(hIAPP), respectively. However, little is known about how these two peptides affect the cross-amyloid aggregation processin vivo.To better simulate the intracorporal environment, where different forms of amyloid aggregates co-exist and very few aggregates probably attach to the vessel wall as seeds, herein, we study the seeded-aggregation of Aβ and hIAPP in the presence of homogeneous or heterogeneous seeds, both in solution and on the solid surface, with different monomer and seed concentrations. In this study, Thioflavin T (ThT)fluorescence assay, atomic force microscopy (AFM), and far-UV circular dichroism (CD)were performed to investigate the aggregation process in solution. Moreover, the binding of monomers with seeds on solid surface was detected by quartz crystal microbalance with dissipation (QCM-D). The 3-(4,5-dime-thylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)assays with human neuroblastoma cells (SH-SY5Y)were finally used to test the cytotoxicity caused by the aggregates. Series of analyses confirmed that a small amount of Aβ40 or hIAPP seeds (1/50 of the monomers in solution)significantly changed the aggregation pathway, forming heterogeneous aggregates with different morphologies and increased β-sheet structures.MTT result showed that the heterogeneous aggregates obtained with Aβ40 and hIAPP seeding reduced the cell viability to 70.5% and 74.4%, respectively, both causing higher cytotoxicity than homogeneous aggregates (82.9% and 76.5%,respectively). The results in solution and on the solid surface both prove that Aβ40 and hIAPP seeds can not only induce rapid aggregation of their homogeneous monomers but also promote the heterogeneous monomers to aggregate, but monomer-heterogeneous seed binding efficiency is lower than that between homogeneous species. The differences in seeding and cross-seeding ability of Aβ40 and hIAPP indicate the barriers depended on the sequence similarity and structural compatibility between different amyloid aggregates. In the case of heterogeneous aggregation, aggregation features largely depend on the seeds. Furthermore, hIAPP seeds exhibited higher cross-seeding efficiency than Aβ40 seeds on the solid surface, which is different from the result in solution where Aβ40 seeds indicating the influence of interfacial properties on aggregation process. This finding would give a deep understanding of the cross-seeding aggregation process and we hope that this work will stimulate more research to explore all possible fundamental and practical aspects of amyloid cross-seeding.
Key Words:Amyloid β protein;Human islet amyloid polypeptide;Amyloid aggregation;Cross-seeding;Interface
1 Introduction
Misfolding or abnormal conformation of proteins can cause various incurable diseases. To date, more than 20 diseases have been caused by protein misfolding, such as Alzheimer’s disease(AD), Parkinson’s disease, and type 2 diabetes mellitus (T2DM)1-3.The misfolded proteins associated with these diseases are amyloid β protein (Aβ)4, α-nuclear synapse protein3,5, and human islet amyloid polypeptide (hIAPP)2,6. However, for AD,a complex multifactorial disease, its pathogenic mechanism is still controversial. More and more studies have shown a close relationship between AD and T2DM7-9. One-fifteenth to tenth of the world’s dementia may be due to the effects of T2DM10,and T2DM patients often experience a significant decline in cognitive function, with a higher risk of eventually developing to AD9,10. What’s more, there are many common pathological features between AD and T2DM, such as insulin resistance,inflammatory, oxidative damage, and amyloid aggregation and deposition11-13. As for the discovery from genetic and pathological researches, a related study about genome-wide interaction revealed that a hIAPP gene polymorphism is associated with Aβ burden and global cognitive decrements in AD14, and hIAPP species have been found to colocalize with Aβ deposition in AD brain15.
Behind this, the two misfolded proteins associated with the two diseases have many similarities. They both follow similar kinetic processes of nucleation, elongation, and maturation towards cross-β architectures16,17. The finally formed amyloid deposits accumulate on the cell membrane, which leads to membrane rupture and subsequent cell death13,18,19. Misfolding generally occurs between proteins having identical or highly similar amino acid sequences and interactions20. However, it is theoretically possible for one misfolded protein to interact with another as long as they have good conformational complementarity. This process is referred to as heterogeneous nucleation or cross-seeding2,17. As for cross-seeding of Aβ and hIAPP, it happens when hIAPP cross the blood-brain barrier or be expressed in sensory neurons21-23. Therefore, it is of importance to explore the co-aggregation or cross-seeding of Aβ and hIAPP to discover the relationship and mutual influence between the two diseases.
Some studies on the co-aggregation of Aβ and hIAPP showed that the mixture of soluble Aβ and hIAPP monomer solution has a shorter aggregation lag phase than Aβ alone, but slightly longer than hIAPP24. It also has been reported that hIAPP delayed the aggregation of Aβ25. Such differences may be caused by the use of different Aβ. But both studies demonstrated the coaggregation of Aβ and hIAPP monomers. Several studies have investigated the cross-seeding interaction between Aβ and hIAPP, showing that both peptides could nucleate to promote aggregation of another peptide, and the cross-seeding of Aβ and hIAPP was less efficient than the self-seeding20,26.
Previous studies on the aggregation of Aβ40 and hIAPP monomers always performed at a proportion of 1 : 1. Actually,various peptides are not likely to present in equal proportionsin vivo. When hIAPP peptides penetrate the blood-brain barrier and enter the brain, they may exist in the form of aggregates in minuscule amounts. Similarly, only a small quantity of Aβ enters the pancreas through blood circulation in different forms.Considering the reality mentioned here and the fact that once these initial seeds formed, fibrils grow through elongation when monomers are available20,27, we studied the seeded-aggregation of Aβ and hIAPP in the presence of low concentration of homogeneous or heterogeneous seeds.
Besides, the researchers mainly studied the interaction between Aβ40/42 and hIAPP in the solution environment, but the interaction between Aβ40/42 and hIAPP on the fixed surface has been little reported. In fact, some amyloid aggregates may adhere to the cell membrane or blood vessel wallin vivo18,19,while Aβ or hIAPP monomers could further bind to and aggregate on these seeds, leading to changes in membrane stability and permeability and the cell structure damage19,28.Therefore, it is necessary to study the aggregation of Aβ and hIAPP on the surface with the immobilized nuclei. In this study,we first investigated the aggregation kinetics, morphology of the final aggregates, changes in secondary structure in the presence of Aβ40 or hIAPP seeds in solution using thioflavin T (ThT)fluorescence assay, atomic force microscopy (AFM), and far-UV circular dichroism (CD). Then, we focused on the deposition rates and structural changes of Aβ40 and hIAPP monomers on immobilized homogeneous/heterogeneous seeds on solid surface by quartz crystal microbalance with dissipation (QCMD). Connections between the aggregation behavior in solution and on the surface were analyzed. Finally, cell viability assays were conducted with SH-SY5Y cells to provide more insight into the effects of seeding and cross-seeding aggregations.
2 Materials and methods
2.1 Materials
Hexafluoroisopropanol (HFIP), ThT, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)were purchased from Sigma-Aldrich (St. Louis, MO). 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) was purchased from Sangon Biotech (Shanghai, China).N-hydroxysuccinimide (NHS)and 1-ethyl-3-(3-dimethylami nopropyl)carbodiimide hydrochloride (EDC)were purchased from J&K Scientific (Beijing, China). Aβ40 and hIAPP were purchased from GL Biochem (Shanghai, China), Bicinchonininc acid (BCA)kit was purchased from Dingguo Biotech (Beijing,China). Other chemicals purchased from local sources were of the highest grade. Deionized water was used for all solution preparations.
2.2 Preparation of Aβ40 and hIAPP monomers
1.0 mg·mL-1of Aβ40 or hIAPP purchased was dissolved in HFIP solution, placed at rest for 2 h at 4 °C and then sonicated for 30 min with ice bath to remove the pre-existing amyloid aggregates. After that, the solution was frozen at -80 °C overnight, processed by vacuum freeze drier later (Labconco,MO, USA)to remove HFIP and the obtained dry peptide powder was stored at -20 °C. Right before using, the lyophilized Aβ40 or hIAPP was dissolved in 20 mmol·L-1NaOH, sonicated for 20 min and then centrifuged at 16000×gfor 30 min at 4 °C. The 75% of the supernatant was carefully retaken and diluted with HEPES buffer (30 mmol·L-1HEPES plus 160 mmol·L-1NaCl,pH 7.4)to the final concentrations which were determined by BCA kit (see below). For the seeding and cross-seeding experiments, the concentrations of monomer solutions were fixed to 25 μmol·L-1.
2.3 Preparation of Aβ40 and hIAPP seeds
To get the seeds suspension with final concentration of 10 μmol·L-1(equivalent to monomer concentration), Aβ40 or hIAPP monomer solution with 25 μmol·L-1was first incubated for 48 h in air bath shaker set of 37 °C at 100 r·min-1shaking continuously, then centrifuged at 16000×gfor 30 min at 4 °C.The 75% of the supernatant was removed and determined its peptide concentration by the BCA kit. The precipitate’s concentration can be indirectly confirmed by the difference between them and it was resuspended with the HEPES buffer to obtain the needed concentration.
2.4 BCA assay for protein quantification
BCA assay followed the process described in the literature29.Briefly, to obtain the standard concentration curve of bovine serum albumin (BSA), series of concentrations (final at 0, 5, 10,20, 30, 40, 50 μg·mL-1)of standard BSA were obtained by mixing the working reagent with the BSA solution, then measured absorption at 562 nm in a Tecan Infinite M200 PRO(TECAN Austria GmbH, Gr?dig, Austria)plate reader.Similarly, mixtures of the BCA working reagent with the Aβ40,hIAPP monomer solution, the upper supernatant of Aβ40 seeds and hIAPP seeds obtained as mentioned above were added into the 96-well plate to get the absorbance. All samples were first incubated at 60 °C for 30 min, then cooled to room temperature in 15 min. The standard curve was averages of three parallel data. The protein concentrations of the experimental group were determined with it.
2.5 Aggregation kinetics determined by Thioflavin T fluorescence
All samples of fluorescent experiment were fixed to the final volume of 200 μL, incubated in the 96-well plate. The plate was sealed to avoid evaporation of the sample and put into the Tecan Infinite M200 PRO plate reader set at 37 °C with 30 s shaking every 30 min to monitor the ThT fluorescence (excitation at 445 nm and emission at 480 nm). For the cross-seeding aggregation of monomers and seeds, experimental group contained 25 μmol·L-1Aβ40 or hIAPP monomers with 0.5 μmol·L-1Aβ40 or hIAPP seeds and 25 μmol·L-1ThT in the HEPES. The samples containing no seeds were used as controls, and the samples containing only ThT as well as containing ThT and seeds were used as blank controls. Three repeats were performed of every sample and the data was averaged. The aggregation kinetic curves were fitted by the sigmoidal curves using the following equation30,31,

where isythe fluorescence intensity at timet,ymaxandy0are the maximum and minimum fluorescence intensities, respectively,kis the apparent first-order aggregation constant, andt1/2is the time required to reach half the maximum fluorescence intensity.Then the lag phase time (Tlag)can be calculated from the following equation,

the averagekandTlagwere obtained from three parallel samples.
2.6 Atomic force microscopy
The morphologies of 25 μmol·L-1Aβ40 and hIAPP aggregates formed in the absence and presence of 0.5 μmol·L-1seeds were observed after incubation in the plate reader for 72h by AFM (CSPM5500, Benyuan, China)at least three independent regions. The samples prepared as mentioned before were dropped on freshly cleaved mica substrates for 5 min,carefully washed with deionized water, air-dried overnight at room temperature, and then put it in observation29.
2.7 Circular dichroism (CD)spectroscopy
The secondary structures of the cross-seeding experimental group were examined by CD spectroscopy (J-810 circular dichroism spectropolarimeter, JASCO)at room temperature.Each sample prepared as described before was incubated of 300 μL for 72 h and then put into a quartz cell with 0.1 mm path length. The spectra were recorded from 260 to 190 nm at 100 nm·min-1scan rate in the continuous scanning mode with a 1 nm bandwidth. All data were averaged of three repeats and corrected by subtracting the background from it. Experimental ellipticity were converted into molar ellipticity by the following equation32,
[θ] = 1000θ/l·c·Nr(3)whereθis ellipticity (mdeg), [θ] is molar ellipticity(deg·cm2·dmol-1),lis optical path length (0.1 mm),cis concentration of Aβ peptides (25·μmol·L-1)andNris the number of peptide residues (40 for Aβ40, 37 for hIAPP).
2.8 Quartz crystal microbalance with dissipation(QCM-D)monitoring
The process of immobilizing fibrils onto the sensor surface was conducted as described in the literature29,33. Briefly,electrodes made of gold-coated AT-cut quartz crystals were first immersed in piranha solution (98% H2SO4: 30% H2O2at 4 : 1)for 10 min, and then rinsed thoroughly with deionized water.After that it was treated with 5 mmol·L-111-MUA/ultrapure ethanol solution and then activated by 0.4 mol·L-1EDC and 0.1 mol·L-1NHS for 30 min. The activated sites reacted with fibrils by immersing the electrodes in seeds solution overnight. And the remaining sites were blocked with 1 mol·L-1ethanolamine at pH 8.0 for 1 h after carefully rinsing with HEPES buffer and water.All the sensor chips needed for seeding and cross-seeding experiment were performed at the same time.
The QCM-D monitoring of nucleation growth was performed by a Biolin Scientific AB Explorer in the QCM cell at constant 37 ± 0.050 °C, and a maintained flow rate of 50 μL·min-1. The QCM-D system was first stabilized by rinsing the decorated chips with 30 mmol·L-1HEPES buffer until getting a stable baseline and then pumped 25 μmol·L-1Aβ40 or hIAPP monomer solution over the chips for 30 min. The resonance frequency and dissipation were recorded at the third, fifth, seventh, ninth and eleventh overtone corresponding to 15, 25, 35, 45 and 55 MHz,respectively. Once the analyte is attached to the chip surface, the mass increases and the oscillation frequency decreases, as expressed by the Sauerbrey equation34,

where Δfand Δmare frequency and mass change, respectively,andais a scale factor. To better match the hydrophilic surface with sticky boundary condition, a chip surface with amyloid fibrils, avoid errors caused by the Sauerbrey models that are only applicable to the thin rigid adhesive layer in contact with air or a vacuum35, Q-Tools software was used to fit frequency change ΔFand dissipation change ΔDat the third, fifth, and seventh overtone and to estimate the mass change Δmon the chip surface through the Kelvin-Voigt model36.
2.9 Cell viability assay
The cytotoxicity of seeding or cross-seeding aggregates was assessed with SH-SY5Y cells by MTT assay. A total of 8 × 103SH-SY5Y cells/well were cultured in DMEM/F12 supplemented with 10% FBS, 100 U·mL-1penicillin, and 100 U·mL-1streptomycin at 37 °C under 5% CO2for 24 h in 96-well plates.Then, the cells were treated with 20 μL pre-aged samples (25 μmol·L-1Aβ40 or hIAPP fibrils incubated for 72 h with or without 0.5 μmol·L-1Aβ40 or hIAPP seeds)and incubated for another 24 h. After that, 10 μL MTT solution (5.5 mg·mL-1in HEPES)was added into each well and incubated for 4 h. The medium was centrifuged at 1500 r·min-1for 10 min to remove the supernatant and replaced with 100 μL dimethyl sulfoxide to dissolve the formazan crystals. Finally, the plate was shaken at 150 r·min-1for 10 min, and the absorbance at 570 nm was measured in a microplate reader (TECAN Austria GmbH,Gr?dig, Austria). The average cell viability of each group was obtained from six parallel samples, and the standard deviation was reported as an error bar in the figures. The Tukey test was used to analyze the significance of data for each group.
3 Results and discussion
3.1 Seeding and cross-seeding aggregation kinetics of Aβ40 and hIAPP in solution
To study the seeding and cross-seeding aggregation kinetics of Aβ40 and hIAPP, the growth kinetic curves, as shown in Fig.1, were monitored by ThT fluorescence method through fitting Eq. (1)to the experimental data. The lag times and the apparent first-order aggregation constants obtained from Eq. (2)are listed in Table 1. From Fig. 1 and Table 1, Aβ40 alone showed a longer lag time (38.2 h)than hIAPP (10.2 h). And the addition of both Aβ40 seeds and hIAPP seeds can significantly shorten the lag phase of Aβ40. Specifically, Aβ40 seeds had a more remarkable effect on accelerating nucleation, reducing the lag time of Aβ40 from 38.2 h to 4.86 h, while hIAPP seeds reduced it to 21.1 h.From Fig. 1b and Table 1, Aβ40 seeds slightly extended the lag time of hIAPP from 10.2 h to 12.8 h, accelerated the elongation rate from 0.152 to 0.471 and enhanced the maximum ThT intensities from 4000 to 7500. Meanwhile, hIAPP seeds reduced the lag time of hIAPP to 8.79 h and enhanced the ThT intensities on the plateau to 6000 (Fig. 1b).
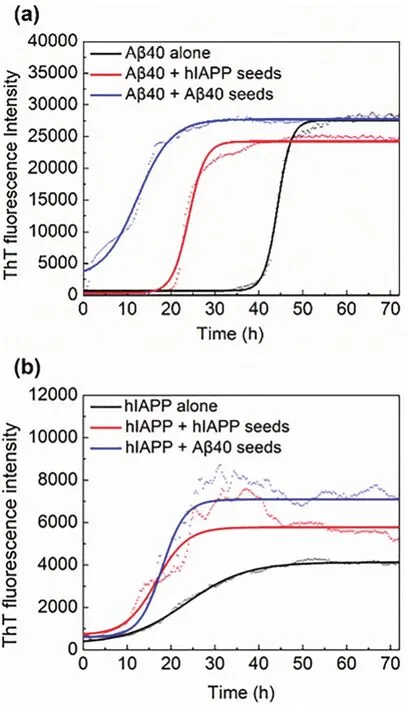
Fig. 1 Aggregation kinetics of hIAPP and Aβ40 monomers incubated with or without seeds by ThT assays. (a)Kinetic traces for Aβ40 in the absence (black)or presence of 5% hIAPP seeds (red), Aβ40 seeds (blue).(b)Kinetic traces for hIAPP in the absence (black)or presence of 5% hIAPP seeds (red), Aβ40 seeds (blue).

Table 1 Lag phase time (Tlag)and apparent first-order aggregation constant (k)of Aβ or hIAPP aggregation kinetics in different conditions.
These results indicate that Aβ40 and hIAPP seeds can not only induce rapid aggregation of their homogeneous monomers but also promote the aggregation of the heterogeneous monomers,and the ability of cross-seeding is relatively weaker than selfseeding, which is consistent with literature20. Furthermore, it can be seen that Aβ40 seeds resulted in a slightly prolonged lag phase time for hIAPP compared with hIAPP alone, whereas hIAPP seeds accelerated the nucleation of Aβ40. This result implies that with the addition of heterogeneous seeds, the lag phase time trended to be similar with the aggregation characteristics of the peptide that acted as the seeds. The above differences in seeding and cross-seeding ability indicate the barriers depended on the sequence similarity and structural compatibility between different amyloid aggregates.
3.2 Morphological observation and secondary structure of seeding-induced Aβ40 and hIAPP aggregates
To more intuitively observe the products of cross-seeding, we got the morphology of Aβ40 and hIAPP aggregates induced by homogeneous and heterogeneous seeds by AFM. As shown in Fig. 2a, the pure Aβ40 aggregated to form longer mature fibers37,38, while the pure hIAPP mainly formed shorter fibers and interconnected to form a net structure (Fig. 2d). The morphology of amyloid aggregates induced by homogeneous seeds is similar to that without seeds. For the Aβ40 aggregates induced by Aβ40 seeds and the pure Aβ40 aggregates, they were both elongated and fibrous (Fig. 2b). As for hIAPP, the aggregates formed with or without the addition of the homogeneous seeds were reticular (Fig. 2f,d). On the contrary,the addition of heterogenous seeds altered the morphology of aggregates evidently. The Aβ40 aggregates induced by hIAPP seeds retained some slender fibers, but more spherical granules and some net structures were also observed in Fig. 2c, which resembles the pure hIAPP. In Fig. 2e, the hIAPP aggregates induced by Aβ40 seeds dispersed in short fibers, with a length of 200-400 nm, and some long fibers (about 2 μm)which are the typical characteristics of the pure Aβ40 were locally observed.The results showed that the morphology of aggregates has the trend to be similar to the form of aggregates used as seeds. Aβ40 seeds altered the morphology of aggregates more obvious than hIAPP seeds, no matter in terms of the number, shape or length of aggregates.

Fig. 2 AFM images of Aβ or hIAPPin situ incubated for 72 h in the absence and presence of seeds. (a)Aβ40 alone, (b)Aβ40 with Aβ40 seeds,(c)Aβ40 with hIAPP seeds, (d)hIAPP alone, (e)hIAPP with Aβ40 seeds, (f)hIAPP with hIAPP seeds.
Comparing Fig. 2 with Fig. 1, some experimental groups had similar ThT fluorescence intensity but showed different morphologies, suggesting they had different aggregation paths.
To further investigate the effect of seeding and cross-seeding on the conformational transformation of Aβ and hIAPP aggregation, the secondary structures of Aβ40 or hIAPP aggregates induced by Aβ40 or hIAPP seeds were monitored using far-UV CD spectroscopy (Fig. 3). In Fig. 3, the spectra of different samples have a negative peak at 218 nm and a positive peak at 197 nm, which represents the β-sheet structure39,indicating that both Aβ40 and hIAPP formed into β-sheet-rich aggregates.

Fig. 3 Far-UV circular dichroism spectra of Aβ and hIAPP aggregates formed in the absence or presence of 0.5 μmol·L?1 seeds.
To quantify the secondary structure of different aggregates and clearly compare the effect of homogeneous/heterogeneous seeds on the structure, the BeStSeL algorithm(http://bestsel.elte.hu/)was used to analyze the CD spectra(Table 2). The introduction of Aβ40 seeds and hIAPP seeds increased the content of the β-sheet structure in Aβ40 aggregates from 46% to 60% and 54%, respectively. hIAPP aggregates induced by Aβ40 seeds (79%)contained more β-sheet structure than pure hIAPP aggregates (52%)and the one induced by homogeneous seeds (50%). It was consistent with AFM results(Fig. 2e). These results indicate that the introduction of heterogeneous seeds increased the β-sheet structure of Aβ40/hIAPP aggregates, and the Aβ40 seeds exerted greater influence than hIAPP. In addition, no significant relation was observed between the fibrils length and the secondary structure content in the cross-seeding aggregation, which may be due to the different morphology and structure properties of the two kinds of amyloid peptides29,40.

Table 2 The contents of secondary structure in Aβ and hIAPP aggregates.
Overall, the results of cross-seeding fibrillation of Aβ and hIAPP in solution monitored by ThT fluorescence (Fig. 1 and Table 1), AFM (Fig. 2)and far-UV CD spectroscopy (Fig. 3 and Table 2)reveal that the addition of seeds, regardless of homogeneous or heterogeneous seeds, can change the lag phase and make the final aggregates form more β-sheet secondary structures compared with the control group without seeds addition. These indicate that Aβ and hIAPP could efficiently cross-seed each other and accelerate the formation of β-rich structures. The cross-seeding is supposed to initiateviathe association of two highly similar U-shaped β-sheet structures of Aβ and hIAPP9,24. Moreover, according to the results from heterogeneous seeding of Aβ40 and hIAPP elongation, the morphology and conformations of hybrid amyloid aggregates depend largely on the seeds that take up a small proportion.
3.3 Seeding and cross-seeding aggregation of Aβ40 and hIAPP on surface
To study the interaction of Aβ40 and hIAPP monomers with homogeneous/heterogeneous seeds immobilized to the solid sensor, QCM-D was used to detect the change of oscillation frequency and energy dissipation caused by monomer solution flowing through the fixed Aβ40 and hIAPP seeds (Fig. 4), and the experimental data were analyzed by Q-tools software. The obtained frequency variation (ΔF)and dissipation variation (ΔD)were further converted into mass variation (Fig. 5a). As shown in Fig. 4, the injection of monomers onto the chips without immobilized seeds caused very slight frequency variation. While after flowing monomers solution through the solid sensor coupled seeds, significantly frequency decreases were detected.It demonstrated that there wasn’t nonspecific adsorption between the flowing monomers and the chips and the detected signal changes came from the interaction among monomers and seeds.

Fig. 4 Frequency change (ΔF)of the quartz crystal oscillator during the absorption of (a)Aβ40 monomers (b)hIAPP monomers to the chips without seeds fibrils (control, black line)and chips immobilized with Aβ40 seeds (red line)or hIAPP seeds (blue line).
When Aβ40 or hIAPP monomers were injected onto the immobilized Aβ40 seeds, the former one brought a continuous increase in mass (Fig. 5a, black line)but the later one brought no changes of mass signal (Fig. 5a, red line), indicating that the Aβ40 monomers continuously bond to the immobilized Aβ40 seeds but the hIAPP monomers hardly not within 30 min. When Aβ40 or hIAPP monomers were injected onto the immobilized hIAPP seeds, a continuous increase in mass was detected (Fig.5a, pink and blue lines), indicating that hIAPP and Aβ40 monomers could both bind to the immobilized hIAPP seeds.

Fig. 5 Growth of Aβ40 and hIAPP monomers on immobilized seeds.(Aβ stands for Aβ, hI stands for hIAPP)(a)Change of molar density during the adsorption of Aβ40 monomers on immobilized Aβ40 seeds(black)and hIAPP seeds (pink), hIAPP monomers (25 μmol·L?1)on immobilized Aβ40 seeds (red)and hIAPP seeds (blue). (b)The average adsorption rate for adsorption of Aβ40 and hIAPP monomers on seeds.
To further get the average binding rates of Aβ/hIAPP monomers to the seeds, we calculated the deposition mass per unit area per unit time within 30 min (Fig. 5b). It shows that the average binding rate of Aβ40 and hIAPP to the homogeneous seeds was significantly higher than that of the heterogeneous seeds. This result is consistent with the results of the ThT fluorescence assay (Fig. 1). However, comparing the four groups in Fig. 5a, b, the hIAPP seeds fixed on the solid surface had high efficiency in cross-seeding Aβ40 monomers and the interaction between Aβ40 seeds fixed on solid and hIAPP monomers had low efficiency, which is opposite to the phenomenon of the cross-seeding aggregation in solution (Fig. 1a red line and Fig.1b blue line), the lag phase time of heterogeneous hIAPP seededaggregation is 1.65 times longer than heterogeneous Aβ40 seeded-aggregation. The difference confirmed the influence of interfacial properties on aggregation process, implying that the seeding or cross-seeding of hIAPP is easier occur when the solid interface is absent. And the significant difference between average binding rate of hIAPP monomers on fixed Aβ40 seeds and hIAPP monomers on fixed hIAPP seeds is consistent with the results in the previous study that the aggregation is more efficient in promoting the kinetics of amyloidogenesis for the hIAPP group when short fibrillar species (the morphology of hIAPP is short fibers)are available41.
Moreover, the dissipation variation (ΔD)simultaneously obtained from the adsorption process was used to analyze the structural change of the biomacromolecules on the chip surface.The high ΔD/ΔFvalue means the formed structure is soft and loose. On the contrary, a lower ΔD/ΔFvalue means the molecular layer is more compact42,43. Fig. 6 shows that the ΔDΔFcurves of Aβ40 monomers binding on Aβ40 seeds and hIAPP seeds have the same slope, indicating that Aβ40 undergoes similar structural change during the binding process with Aβ40 seeds and hIAPP seeds. Similarly, the ΔD-ΔFcurves of Aβ40 monomer and hIAPP monomer binding on hIAPP seeds have the same slope indicating that Aβ40 and hIAPP undergo similar structural change during the binding process with hIAPP seeds.It can be inferred from the same ΔD/ΔFthat the monomers and seeds above-mentioned could form structures with a similar degree of tightness, which may be contributed by the high degrees of sequence identity (25%)and similarity (50%)of hIAPP and Aβ17.
3.4 Cytotoxicity of seeding-induced Aβ and hIAPP aggregates
To further investigate the effect of the addition of heterogeneous seeds on the aggregation pathway and test the toxicity of different aggregates to the SH-SY5Y cell line, the MTT assays were conducted (Fig. 7). As shown in Fig. 7a, Aβ seeds or the hIAPP seeds of 0.1 μmol·L-1did not cause significant cytotoxicity. As for pure Aβ40 aggregates and pure hIAPP aggregates of 5 μmol·L-1formed without seeds addition,the cell viability decreased to 83.8% and 78.1%, respectively(Fig. 7b). For seeding and cross-seeding, the homogeneous aggregates obtained with Aβ40 seeding or hIAPP seeding presented (82.9% and 76.5%, respectively)similar cytotoxicity with the pure Aβ40 aggregates or the pure hIAPP aggregates.However, the heterogeneous Aβ40 aggregates obtained with hIAPP seeding reduced the cell viability to 74.4%, and the heterogeneous hIAPP aggregates obtained with Aβ40 seeding reduced the cell viability to 70.5%, both presenting higher toxic than homogeneous aggregates.

Fig. 6 Change of dissipation (ΔD)versus frequency change (ΔF)during the adsorption of Aβ40 monomers on immobilized Aβ40 seeds (black)and hIAPP seeds (pink), hIAPP monomers on immobilized Aβ40 seeds (red)and hIAPP seeds (blue).

Fig. 7 Cell viability assays (a)with preformed Aβ40 seeds and hIAPP seeds and (b)Aβ40 aggregates and hIAPP aggregates incubated with Aβ40 seeds and hIAPP seeds.
Combined with the results of ThT fluorescence (Fig. 1), AFM(Fig. 2)and CD (Table 2), hIAPP seeds changed the aggregation pathway of Aβ40 to form numerous small non-fibrous aggregates containing β-sheet-rich structures, thereby finally causing the higher toxicity. Similarly, Aβ40 seeds had the same effect on the cross-seeding with hIAPP monomers27,44,45. Thus,the presence of heterogeneous seeds not only changed the aggregation process, forming aggregates with different morphologies and more β-sheet secondary structures, but also caused more cell cytotoxicity.
4 Conclusions
In this study, the self-seeding and cross-seeding of Aβ and hIAPP in bulk solution and on solid surface were investigated.Our results show that Aβ40 and hIAPP seeds, even if took up 1/50 of monomers, not only induce rapid aggregation of their homogeneous monomers but also have the ability to crossseeding, promoting the aggregation of the heterogeneous monomers. However, the efficiency of cross-seeding is lower than self-seeding, no matter in solution or on surface. In solution,the addition of heterogeneous seeds changed the aggregation path, increased the amount of β-sheet in Aβ40 and hIAPP aggregates, ultimately altered their morphology and increased their cytotoxicity. Similarly, homo- and hetero-monomers binding on the chip surface with immobilized seeds showed that the binding rate between heterogeneous species was slower than between homogeneous, proving the existence of cross-species barriers. More importantly, hIAPP seeds exhibited higher efficiency than Aβ40 seeds on the solid surface in the crossseeding, which is different from the result in solution,confirming the important influence of the interface on crossseeding. Although the mechanism and driving force of heterogeneous amyloid formation are not clear, our results suggest that the feature of the heterogeneous aggregation process is likely to be determined by the fibrils that act as seeds. This finding would benefit in the deeper understanding of the process of cross-seeding aggregation.
References(1)Westermark, G. T.; F?ndrich, M.; Lundmark, K.; Westermark, P.Csh. Perspect. Med.2018,8(1), a024323.doi: 10.1101/cshperspect.a024323(2)Ren, B.; Zhang, Y.; Zhang, M.; Liu, Y.; Zhang, D.; Gong, X.; Feng,Z.; Tang, J.; Chang, Y.; Zheng, J.J.Mater. Chem. B2019,7(46),7267. doi: 10.1039/c9tb01871a(3)Lim, K. H.Front. Mol. Neurosci.2019,12,158.doi: 10.3389/fnmol.2019.00158(4)Hardy, J.; Selkoe, D. J.Science2002,297(5580), 353.doi: 10.1126/science.1072994(5)Ono, K.; Takahashi, R.; Ikeda, T.; Yamada, M.J. Neurochem.2012,122(5), 883. doi: 10.1111/j.1471-4159.2012.07847.x(6)Palotay, J. L.; Howard, C. F.Vet. Pathol.1982,19(Suppl. 7), 181.doi: 10.1177/030098588201907s14(7)Despa, F.; Goldstein, L. B.; Biessels, G. J.Ann. Neurol.2019,87(3),486. doi: 10.1002/ana.25668(8)Baram, M.; Atsmon-Raz, Y.; Ma, B.; Nussinov, R.; Miller, Y.Phys.Chem. Chem. Phys.2016,18(4), 2330. doi: 10.1039/c5cp03338a(9)Zhu, H.; Tao, Q.; Ang, T. F. A.; Massaro, J.; Gan, Q.; Salim, S.; Zhu,R.-Y.; Kolachalama, V. B.; Zhang, X.; Devine, S.;et al.JAMA Netw.Open2019,2(8), e199826. doi: 10.1001/jamanetworkopen.2019.9826
(10)Biessels, G. J.; Strachan, M. W. J.; Visseren, F. L. J.; Kappelle, L. J.;Whitmer, R. A.Lancet Diabetes Endo.2014,2(3), 246.doi: 10.1016/S2213-8587(13)70088-3
(11)Verdile, G.; Keane, K. N.; Cruzat, V. F.; Medic, S.; Sabale, M.;Rowles, J.; Wijesekara, N.; Martins, R. N.; Fraser, P. E.; Newsholme,P.Mediat. Inflamm.2015,2015, 105828. doi: 10.1155/2015/105828
(12)Schultz, N.; Byman, E.; Wennstr?m, M.Neurobiol. Aging2018,69,94. doi: 10.1016/j.neurobiolaging.2018.05.003
(13)Eisenberg, D.; Nelson, R.; Sawaya, M. R.; Balbirnie, M.;Sambashivan, S.; Ivanova, M. I.; Madsen, A. O.; Riekel, C.Acc.Chem. Res.2006,39(9), 568. doi: 10.1021/ar0500618
(14)Roostaei, T.; Nazeri, A.; Felsky, D.; De Jager, P. L.; Schneider, J. A.;Pollock, B. G.; Bennett, D. A. Voineskos, A. N.Mol. Psychiatr.2017,22(2), 287.doi: 10.1038/mp.2016.35
(15)Jackson, K.; Barisone, G. A.; Diaz, E.; Jin, L. W.; DeCarli, C.;Despa, F.Ann. Neurol.2013,74(4), 517. doi: 10.1002/ana.23956
(16)Soto, C.; Pritzkow, S.Nat. Neurosci.2018,21(10), 1332.doi: 10.1038/s41593-018-0235-9
(17)Armiento, V.; Spanopoulou, A.; Kapurniotu, A.Angew. Chem. Int.Edit.2020,59(9), 3372. doi: 10.1002/anie.201906908
(18)Jucker, M.; Walker, L. C.Ann. Neurol.2011,70(4), 532.doi: 10.1002/ana.22615
(19)Kiriyama, Y.; Nochi, H.Cells2018,7(8), 95.doi: 10.3390/cells7080095
(20)O'Nuallain, B.; Williams, A. D.; Westermark, P.; Wetzel, R.J. Biol.Chem.2004,279(17), 17490. doi: 10.1074/jbc.M311300200
(21)Mulder, H.; Leckstrom, A.; Uddman, R.; Ekblad, E.; Westermark, P.;Sundler, F.J. Neurosci. 1995,15(11), 7625.
(22)Fawver, J. N.; Ghiwot, Y.; Koola, C.; Carrera, W.; Rodriguez-Rivera,J.; Hernandez, C.; Dineley, K. T.; Kong, Y.; Li, J. R.; Jhamandas, J.;et al.Curr. Alzheimer Res.2014,11(10), 928.doi: 10.2174/1567205011666141107124538
(23)Banks, W. A.; Kastin, A. J.Peptides1998,19(5), 883.doi: 10.1016/S0196-9781 (98)00018-7
(24)Hu, R. D.; Zhang, M. Z.; Chen, H.; Jiang, B. B.; Zheng, J.ACS Chem. Neurosci.2015,6(10), 1759.doi: 10.1021/acschemneuro.5b00192
(25)Yan, L. -M.; Velkova, A.; Tatarek-Nossol, M.; Andreetto, E.;Kapurniotu, A.Angew. Chem. Int. Edit. 2007,46(8), 1246.doi: 10.1002/anie.200604056
(26)Moreno-Gonzalez, I.; Edwards, G.; Salvadores, N.; Shahnawaz, M.;Diaz-Espinoza, R.; Soto, C.Mol. Psychiatr.2017,22(9), 1327.doi: 10.1038/mp.2016.230
(27)Kakinen, A.; Sun, Y. X.; Javed, I.; Faridi, A.; Pilkington, E. H.;Faridi, P.; Purcell, A. W.; Zhou, R. H.; Ding, F.; Lin, S. J.;et al.Sci.Bull.2019,64(1), 26. doi: 10.1016/j.scib.2018.11.012
(28)Seeliger, J.; Weise, K.; Opitz, N.; Winter, R.J. Mol. Biol.2012,421(2-3), 348. doi: 10.1016/j.jmb.2012.01.048
(29)Hao, X. P.; Zheng, J.; Sun, Y.; Dong, X. Y.Langmuir2019,35(7),2821. doi: 10.1021/acs.langmuir.8b03599
(30)Naiki, H.; Nakakuki, K.Lab. Invest.1996,74(2), 374.
(31)Nielsen, L.; Khurana, R.; Coats, A.; Frokjaer, S.; Brange, J.; Vyas, S.;Uversky, V. N.; Fink, A. L.Biochemistry2001,40(20), 6036.doi: 10.1021/bi002555c
(32)Syed, S. B.; Khan, F. I.; Khan, S. H.; Srivastava, S.; Hasan, G. M.;Lobb, K. A.; Islam, A.; Hassan, M. I.; Ahmad, F.Int. J. Biol.Macromol.2018,117, 1252. doi: 10.1016/j.ijbiomac.2018.06.025
(33)Wang, C. G.; Xu, L.; Cheng, F.; Wang, H. Q.; Jia, L. Y.RSC Adv.2015,5(38), 30197. doi: 10.1039/c5ra02314a
(34)Sauerbrey, G.Z. Phys.1959,155(2), 206.
(35)Michaels, T. C. T.; Buell, A. K.; Terentjev, E. M.; Knowles, T. P. J.J. Phys. Chem. Lett.2014,5(4), 695. doi: 10.1021/jz4024833
(36)Voinova, M. V.; Rodahl, M.; Jonson, M.; Kasemo, B.Phys. Scr.1999,59(5), 391. doi: 10.1238/Physica.Regular.059a00391
(37)Li, S.; Liu, F. F.; Yu, L. L.; Zhao, Y. J.; Dong, X. Y.Acta Phys. -Chim.Sin. 2016,32(6), 1391. [李松, 劉夫鋒, 余林玲, 趙彥嬌, 董曉燕.物理化學(xué)學(xué)報(bào), 2016,32(6), 1391.]doi: 10.3866/PKU.WHXB201603221
(38)Deng, J.; Ma, T.; Chang, Z. W.; Zhao, W. Z.; Yang, J.Acta Phys. -Chim. Sin., 2020,36(4), 1905019. [鄧靜, 馬濤, 常自偉,趙偉靜, 楊俊. 物理化學(xué)學(xué)報(bào), 2020,36(4), 1905019.]doi: 10.3866/PKU.WHXB201905019
(39)Mao, X. B.; Wang, C. X.; Liu, L.; Ma, X. J.; Niu, L.; Yang, Y. L.;Wang, C.Acta Phys. -Chim. Sin. 2010,26(4), 850. [毛曉波, 王晨軒, 劉磊, 馬曉晶, 牛琳, 楊延蓮, 王琛. 物理化學(xué)學(xué)報(bào), 2010,26(4), 850.] doi: 10.3866/PKU.WHXB20100440
(40)Qahwash, I. M.; Boire, A.; Lanning, J.; Pytel, T. K. P.; Meredith, S.C.J. Biol. Chem.2007,282(51), 36987.doi: 10.1074/jbc.M702146200
(41)Trigg, B. J.; Lee, C. F.; Vaux, D. J.; Jean, L.Biochem. J.2013,456(1), 67. doi: 10.1042/BJ20130605
(42)He, C. X.; Yuan, A. P.; Zhang, Q. L.; Ren, X. Z.; Li, C. H.; Liu, J. H.Acta Phys. -Chim. Sin.2012,28(11), 2721. [何傳新, 袁安朋, 張黔玲, 任祥忠, 李翠華, 劉劍洪. 物理化學(xué)學(xué)報(bào), 2012,28(11), 2721.]doi: 10.3866/PKU.WHXB201207191
(43)Saraiva, A. M.; Pereira, M. C.; Brezesinski, G.Langmuir2010,26(14), 12060. doi: 10.1021/la101203h
(44)Haataja, L.; Gurlo, T.; Huang, C. J.; Butler, P. C.Endocr. Rev.2008,29(3), 303. doi: 10.1210/er.2007-0037
(45)Zhang, Y. C.; Lu, L.; Jia, J. P.; Jia, L. F.; Geula, C.; Pei, J. J.; Xu, Z.Q.; Qin, W.; Liu, R. Q.; Li, D.;et al.PLoS One2014,9(1), e85885.doi: 10.1371/journal.pone.0085885
- 物理化學(xué)學(xué)報(bào)的其它文章
- High-Performance Palladium-Based Catalyst Boosted by Thin-layered Carbon Nitride for Hydrogen Generation from Formic Acid
- Eu(III)在蒙脫石上的吸附及碳酸根和磷酸根對(duì)其吸附的影響
- 鋯合金初始氧化行為的原位近常壓XPS研究
- Methanol Synthesis by COx Hydrogenation over Cu/ZnO/Al2O3 Catalyst via Hydrotalcite-Like Precursors: the Role of CO in the Reactant Mixture
- 自交聯(lián)共軛亞油酸囊泡基熒光納米點(diǎn)的構(gòu)筑及其熒光特性
- Controllable Formation of PbI2 and PbI2(DMSO)Nano Domains in Perovskite Films through Precursor Solvent Engineering

