Effects of Increased Salinity on Growth, Development and Survival in Early Life Stages of the Green Toad Bufotes variabilis (Anura:Bufonidae)
Soheyla YAGHOBI, Somaye VAISSI, Zeynab Taheri KHAS and Mozafar SHARIFI
Razi University Centre for Environmental Studies, Department of Biology, Baghabrisham 67149, Kermanshah, Iran
1. Introduction
Amphibian populations worldwide are in decline due to a variety of factors including habitat destruction,climate change, diseases, introduction of exotic species,and environmental contamination (Petersonet al., 2002;Stuartet al., 2004). Susceptibility to these factors differs among species and populations (Langhanset al., 2009),and depends, in part, on environmental conditions such as water chemistry (Blaustein and Kiesecker, 2002).Salinity is one of several parameters of water chemistry that influences the survival, development, and fitness of amphibians, and may act as a significant factor in the decline of amphibians (Sparlinget al., 2000). Emphasis on the effects of sodium chloride on amphibians has been placed only recently (Karraker and Ruthig, 2009).However, many amphibian species breed in agricultural run off or storm water ponds, which are located along or close to roads and are expected to come into contact with road de-icing agents that are often applied just before their breeding seasons (Snodgrasset al. 2008).
Road de-icers (dry or liquid chemicals able to lower the freezing point of water) are used in temperate and cold regions worldwide when harsh climatic conditions affect road traffic. In most cases, the active agent used in road de-icers is sodium chloride (Ramakrishna and Viraraghavan, 2005). The salts used in de-icers can run off through overland flow, groundwater infiltration and aerosol sprays to reach various wetlands in the washbasin and causes contamination (Marsalek, 2003; Karraker,2008). De-icing agents, primarily road salt, are applied to roads in 26 states in the United States (Daleyet al., 2009)and in a number of European countries (Karraker, 2007;Wijethungaet al., 2016), however, the scale of impacts of road salt on aquatic organisms remains largely understudied (Corsiet al., 2010; Findlay and Kelly, 2011;Petranka and Francis, 2013; Niyogiet al., 2016). In Iran a mixture of sand and salt is normally used as road de-icer in western and northern part of the country where there is normally a long period of freezing in winter. There are reports of associations between road de-icing, salinity in ground water along highway (Reyahiet al., 2011) and also in surface run off (Aghazadeh etal., 2012) in Iran.
Salinity is one of the important environmental factors affecting survival, growth and distribution of many aquatic organisms (Kumluet al., 2000; Chandet al.,2015). Most amphibians do not adapt well to brackish water because their skin is highly permeable, posing problems for water and ion balance (Duellman and Trueb,1986). Moderate salinity of 2–9 ppt (parts per thousand)can decrease survival, growth, and development(Chinathambyet al., 2006; Sanzo and Hecnar, 2006), and cause morphological abnormalities, such as distortion of tail, abdominal edema and emaciated appearance together with reduction in body water content (Chinathambyet al.,2006; Karraker, 2007). Despite the evidences provided for some populations of amphibian showing that they can locally adapt to saline environments, evidence is emerging that this may not always be possible (Brady, 2013).Several reports of populations of various species such asRana temporaria,Buergeriajaponica,Kaloula pulchra,Microhyla ornata,andBufo bufohave further concluded that salinity causes negative effects onsurvival, growth,development, metamorphosis and behavior of tadpoles(Gordonet al., 1961; Ackrillet al., 1969; Ferreira and Jesus, 1973; Viertel, 1999; Haramura, 2007; Karrakeret al., 2010). In contrast, other species, such asFejervarya cancrivora,F. limnocharis,Bufotes balearicus,Epidalea calamita,Litoria aureaandHoplobatrachus tigerinuscan tolerate higher salinity by maintaining high level of urea in the blood (e. g.,F. cancrivora) or through gradual acclimation to increasing salinity in laboratory experiments (Christy and Dickman, 2002; Gomez-Mestre and Tejedo, 2003; Wu and Kam, 2009).
Most studies on the effects of salt on amphibians have focused on the adult or larval stage, ignoring the eggs,despite some evidence suggesting that embryos and tadpoles may in fact be the most susceptible life-history stage to salt (Karraker and Ruthig, 2009; Nakkrasaeet al., 2016). Adult amphibians rely on integumentary system to retain body fluids through ionic exchange and the ability to hyper synthesize and retain urea to increase body osmolarity (Shoemaker and Nagy, 1977; Balinsky,1981; Katz, 1989). The green toad,Bufotes variabilis(Pallas, 1769), is a common toad of Iran and has a wide distribution in most provinces. This toad is rarely found in central and eastern areas of Iran (Masshaiiet al.,2008). Whether salinity change interferes with hatching,survival, growth and development ofB. variabilistadpoles is unknown. Therefore, main purposes of this study are to examine the influence of salinity on the (1)growth, (2) development and (3) survival of embryos and larvae ofB. variabilis.
2. Materials and Methods
Eggs (embryos within their jelly capsule, Gosner Stage 10–11) from a single cohort ofB. variabiliswere collected on May 2016 in Sarable (34°32' N, 47°01' E),Kermanshah Province, Iran. All eggs of the same trial were at the same stage. Eggs were cultured at various salinities, 0.20, 0.70, 1.70, 2.70, 3.70, 4.70, 5.70, 6.70,7.70, 8.70 and 10 g/l. The salinity we used in this study has been determined based on the expected toleration of a freshwater amphibians reported in the scientific literature ranging from tap water (0.20 g/l) to hyper saline water(10 g/l). Freshwater is generally defined as water in which salinity is less than 3 g/l and sea water as 35 g/l (Nielsenet al., 2003). Each treatment was replicated 3 times for a total of 33 containers (14 cm height and 14 cm diameter),each filled with 2l of dechlorinated tap water with salinity of 0.20 g/l. All containers had 20 eggs, Gosner Stage 14–15. In this study developmental stages are followed as defined by Gosner (1960): embryo <25, hatchling 17–20,larvae >25. The experiment was conducted on a 12h dark:12h light photoperiod at approximately 18°C. Light was supplied by 18 metal halide lamps hanged over laboratory benches to provide a broad spectrum of photosynthetically available irradiant. After hatching larvae were fed with raw spinach: 1 g four times per week for every container.We monitored experimental containers twice a day and removed the bodies of larvae that had died. The larvae were taken out and container were cleaned thoroughly.We did not use any chemical cleaner for this purpose.
We evaluated the impact of salt water on growth of body size by measuring changes in snout to vent length(SVL) during larval period. Regression equations were used to derive the growth rates from the values of length of SVL. Survival was determined as the percent of remaining individuals during embryo and larval period.Photos were taken with a digital camera (SONY, DSCHX9V, 3.6V) on a tripod at a fixed height (30 cm). The larvae were put in a Petri dish which was located over latticed paper. Immediately after photography the larvae were released into their containers. All pictures were analyzed using Digimizer version 4.6.0 (http://digimizer.findmysoft.com/). We measured the snout to vent length(SVL: mm). SVLs were calculated by drawing a line from the tip of the snout to the tip of the vent. Measurements were performed at days 1, 6, 12, 18 and 24. When experiment was completed, the surviving larvae were returned to the pond where they were collected. One-way analysis of variance (ANOVA) was used to examine the effects of salinity on the rate of hatching and on growth,development and survival during embryonic, and larval period. All data are expressed as mean ± SD (standard deviation). The statistical program package SPSS (v. 16)was used for all analyses.
3. Results
In this study, salinity was found to markedly affect the rate of hatching among treatments (ANOVA,P≤0.001).Also, increase in water salinity extended hatching period.Eggs in salinity of 0.20 g/l to 3.70 g/l were hatched after 72 hours while eggs in salinity of 4.70 g/l to 5.70 g/l were hatched after 79.92 hours days. More delay in hatching was found for eggs in salinity 6.70 to 8.70 g/l,were hatched after 96 hours (Table 1). At salinity level of 10 g/1, all eggs shrank and died before hatching.Most unhatched eggs died as indicated by signs of opaqueness and shrinkage, while few eggs were alive but did not hatch. After being exposed to saline water for 24 h post hatching, there was a significant effect of salinity on survival (ANOVA,P≤0.001) of larvae.Approximately, 83.33% of hatched larvae in 0.20 g/l survived for 24 h. Hatched larvae in salinities of 0.70,1.70, 2.70, 3.70, 4.70, 5.70 and 6.70 g/l showed a survival rate of 71.66%, 78.33%, 80.66%, 78.66%, 75.33%, 70%and 66.33%, respectively, 24 h after hatching (Figure 1A). The percentage of survival of larvae to the end of the experiment (Gosner stage 30) was 80.00%, 66.66%,76.66%, 65%, 30% and 0.00% for the 0.20, 0.70, 1.70,2.70, 3.70 and 4.70 to 10 g/l treatments, respectively(Table 1, Figure 1). At salinities over 8.70 g/l unhatched and dead embryos appeared with signs of shrinkage and distorted appearance.
Egg diameter ofB. variabiliswas on average 1.51 mm± 0.01, (Gosner stage 10–11) and jelly capsules diameter was 3.76 mm ± 0.12. Figure 2 and Table 1 demonstrates the average and standard deviation of the (SVL) during larval period ofB. variabilisfrom 6 to 24 days. Growth ofB. variabilisshowed significant difference in the third week (P≤0.05), (Figure 1B).Growth rate for SVL of larvae ofB. variabilisare shown in Table 1.Larvae growth rate of the 0.70 g/l (Linear regression, 0.26 mm/day) was fastest than 0.20 g/l (Linear regression, 0.24 mm/day) and followed by the 1.70 g/l (Linear regression,0.23 mm/day), 2.70 g/l (Linear regression, 0.22 mm/day)and 3.70 g/l (Linear regression, 0.15 mm/day).Various salinity treatments affected developmental rate in live embryos and larvae but this difference was not significant(ANOVA,P≤0.07). The Gosner stages for development of the eggs of the same clutch reared at four treatments of various salinity treatments at 6 to 24 days are shown in Table 1.
4. Discussions
The increase in water salinity in wetland and aquatic ecosystems can result from natural factors such as climate change or sea water intrusion into freshwater wetlands.Various man-made processes such as deforestation,excessive irrigation, salt mining, and road de-icing cause changes in water salinity (Nielsen and Brock, 2009).Increase in salt content in natural aquatic ecosystems under natural or anthropogenic processes is now recognized as a threat to the biological communitiesas a whole and represents an environmental stress for many species (Jinet al., 2011). Amphibians with their permeable skin are at risk in hyposaline and hypersaline water, because they gain or lose water across the skin surface at rates that may rapidly be fatal (Wijethungaet al., 2016). A highly permeable skin makes amphibian osmotically sensitive organisms, because their osmoregulation works at a certain range of water salinity(Gomez-Mestreet al., 2004; Haramura, 2007).

Table 1 Percentage and time of hatching (Gosner stage 20) and survival rate of Bufotes variabilis larvae in various salinity treatments for 24 h (Gosner stage 26) and 24 days (Gosner stage 30) after hatching. Snout to vent length (SVL: Mean ± SD) was measured at 6 and 24 days.Growth rate (mm/day) of body size (SVL) is determined as daily increase of SVL during 6 and 24 days.

Figure 1 Effect of different salinities on survival rate (%) of embryo (E) and larvae (L) of Bufotes variabilis from 1 to 24 days(age). ***: P≤0.001; **: P≤0.01; *: P≤0.05.
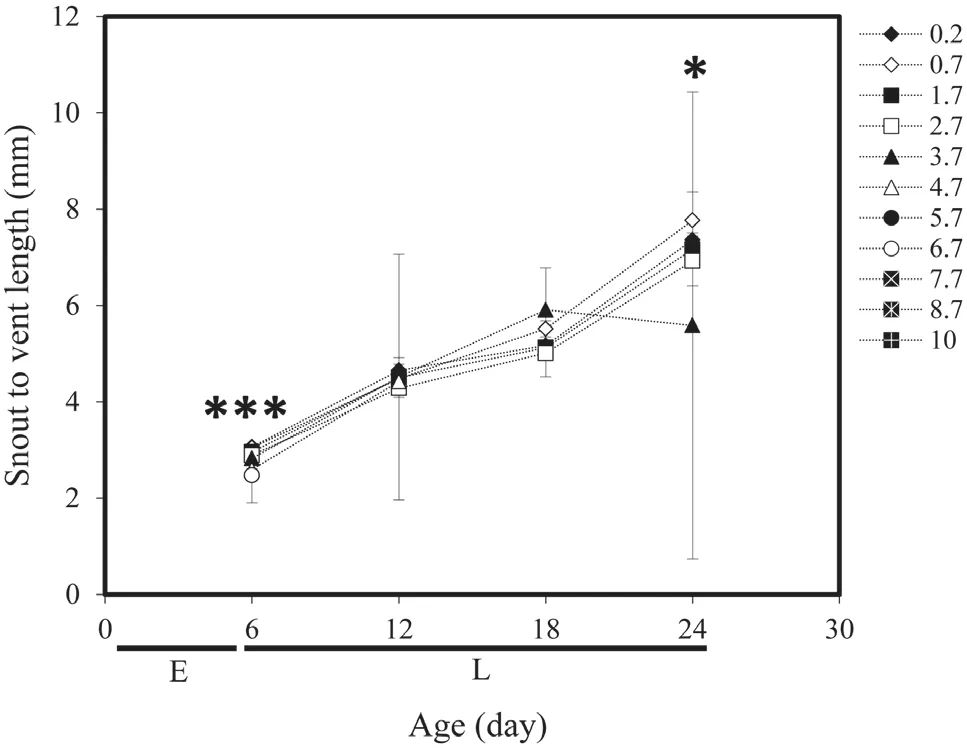
Figure 2 Effect of different salinities on snout to vent length (mm)of larvae (L) of Bufotes variabilis from 1 to 24 days (age). ***:P≤0.001; **: P≤0.01; *: P≤0.05.
Experimental evaluation of the impact of elevated salinity normally showed a considerable reduction in growth, and the rate of metamorphosis. These are also reports of associations between an increase in mortality rate in anuran adults and larvae (Christy and Dickman,2002). There is a general consensus in the literature that amphibian embryos are most sensitive to water salinity,followed by larvae, with adults being most tolerant (e. g.,Chinathambyet al., 2006; Brandet al., 2010; Petranka and Doyle, 2010; Bernabet al., 2013; Hopkinset al.,2014; Thirion, 2014). There are also some opposing data and evidence that show the sensitivity can also change with age within a particular life stage (Alexanderet al.,2012).
Recent investigations indicated that de-icing salts were associated with reduced survival and increased frequency of malformations in some amphibians e.g. the Spotted Salamander,Ambystoma maculatum, and the Wood FrogRana sylvatica(Nakkrasaeet al., 2015). A reduction in survival in embryonicA. maculatumandR. sylvaticawas probably caused by physiological constraints imposed on embryos at higher salinities (Nakkrasaeet al., 2015).In contrast, survival of embryos and larvae of green frogs (Lithobates clamitans), which breed principally in permanent wetlands, was not significantly influenced but frequency of malformations increased with chloride concentration (Karraker, 2007). While a reduction in growth may result from the increased energy expense required for osmoregulation, delayed development may result from a decreased thyroid hormone (TH) level in response to hormonal involvement in osmoregulation(Gomez-Mestreet al., 2004).
Present study covered embryonic, hatching and early larval periods in the course of 20 days after hatching. On this short period of exposure to different water salinity the reaction was slow or limited to retard development of eggs, hatchlings and larvae (Table 1) which may eventually lead to smaller size at metamorphosis. Weather this can result in a reduction in survival rate in adult is not known. There are several studies that show small body size at metamorphosis decreases the ability to tolerate dehydration (Newman and Dunham, 1994) and the likelihood of post-metamorphic survival (Smith,1987; Berven, 1990; Chelgrenet al., 2006). Present study did not cover entire developmental period but previous studies showed that time to metamorphosis of larvae decreased when reared in intermediate salinity treatments compared with freshwater or low salinity treatment.(Chinathambyet al., 2006; Sanzo and Hecnar, 2006;Wu and Kam, 2009).
The Green Toad (B. variabilis)of Europe and the Middle East (Gordon, 1962) have been reported from aquatic environments of salinities as high as 2 g/l, in northern Europe (Gislén and Kauri, 1959). Results obtained from current experiment showed that impact of salinity on embryonic mortality, hatching and survival rate of larvae began at concentration over 3.70 g/l.Although present salinity of freshwater wetland where the eggs have been collected for this experiment is much lower than the water salinity in the laboratory but the interaction of a shortened metamorphosis period and a smaller body size at high salinity with other ecological factors is difficult to anticipate under natural condition.There are now increasing evidences showing that in many regions of the world a net negative impact of the recent climate change is expected to occur in freshwater ecosystems (IPCC 2007). However, the intensity and characteristics of the impact can significantly vary from region to region. In many arid and semi-arid regions such as Iran, trends toward warmer climate and increased precipitation variability are linked to warming streams and rivers (IPCC 2007). Amphibians are well adapted to environmental fluctuations but the novel situation resulting from the combined impact of various environmental factors may cause an irreversible impact on their existence.
AcknowledgementsWe thank the Razi University for the support of this study as an MSc research project.Collection of Green Toad eggs was permitted by the Regional Office of Environment in Kermanshah Province.
Ackrill P., Hornby R., Thomas S. 1969. Responses ofRana temporariaandRana esculentato prolonged exposure to a saline environment. Comp Biochem Physiol, 28(3): 1317–1329
Aghazadeh N., Nojavan M., Mogaddam A. A. 2012. Effects of road-de-icing salt (NaCl) and saline water on water quality in the Urmia area, northwest of Iran.Arab J Geosci, 5(4): 565–570
Alexander L. G., Lailvaux S. P., Pechmann J. H. K., DeVries Philip. J. 2012. Effects of salinity on early life stages of the Gulf Coast toad,Incilius nebulifer(Anura: Bufonidae). Copeia,2012(1): 106–114
Balinsky J. B. 1981. Adaptation of nitrogen metabolism to hyperosmotic environment in Amphibia.J Exp Zool A Ecol Genet Physiol, 215(3): 335–350
Bernab I., Bonacci A., Coscarelli F., Tripepi M., Brunelli E.2013. Effects of salinity stress onBufo balearicusandBufo bufotadpoles: tolerance, morphological gill alterations and Na+/K+-ATPase localization.Aquat Toxicol, 132: 119–133
Berven K. A. 1990. Factors affecting population fluctuations in larval and adult stages of the wood frog (Rana sylvatica).Ecology, 71(4): 1599–1608
Blaustein A. R., Kiesecker J. M. 2002. Complexity in conservation: lessons from the global decline of amphibian populations.Ecol Lett, 5(4): 597–608
Brady S. P. 2013. Microgeographic maladaptive performance and deme depression in response to roads and runoff.Peer J, 1: e163
Brand A. B., Snodgrass J. W., Gallagher M. T., Casey R. E.,Van Meter R. 2010. Lethal and sublethal effects of embryonic and larval exposure ofHyla versicolorto stormwater pond sediments.Arch Environ Contam Toxicol, 58(2): 325–331
Chand B. K., Trivedi R. K., Dubey S. K., Rout S. K., Beg M.M., Das U. K. 2015. Effect of salinity on survival and growth of giant freshwater prawnMacrobrachium rosenbergii(de Man).Aquacul Rep, 2: 26–33
Chelgren N. D., Rosenberg D. K., Heppell S. S., and Gitelman A.I. 2006. Carryover aquatic effects on survival of metamorphic frogs during pond emigration. Ecol Appl, 16(1): 250–261
Chinathamby K., Reina R. D., Bailey P. C. E., Lees B. K. 2006.Effects of salinity on the survival, growth and development of tadpoles of the brown tree frog,Litoria ewingii.Aust J Zool,54(2): 97–105
Christy M. T., Dickman C. R. 2002. Effects of salinity on tadpoles of the green and golden bell frog (Litoria aurea).Amphiba-Reptila, 23(1): 1–11
Corsi S. R., Graczyk, D. J., Geis, S. W., Booth N. L, Richards K. D. 2010. A fresh look at road salt: aquatic toxicity and waterquality impacts on local, regional, and national scales.Environ Sci Technol, 44(19): 7376–7382
Daley, M. L., Potter J. D., McDowell W. H. 2009. Salinization of urbanizing New Hampshire streams and groundwater: effects of road salt and hydrologic variability.J N Am Benthol Soc, 28(4):929–940
Duellman W. E., Trueb L. 1986. Biology of amphibians.New York: McGraw-Hill, 670
Ferreira H. G., Jesus C. H. 1973. Salt adaptation inBufo bufo.J physiol, 228(3): 583–600
Findlay S. E. G., Kelly V. R. 2011. Emerging indirect and longterm road salt effects on ecosystems. Ann N Y Acad Scien,1223(1): 58–68
Gislén T., Kauri H. 1959. Zoogeography of the Swedish amphibians and reptiles: With notes on their growth and ecology. Stockholm,Almqvist and Wiksell. Acta vertebratica, Vol. 1, No. 3
Gomez-Mestre I., Tejedo M. 2003. Local adaptation of an anuran amphibian to osmotically stressful environments.Evolution 57(8): 1889–1899
Gomez-Mestre I., Tejedo M., Ramayo E., Estepa J. 2004.Developmental alterations and osmoregulatory physiology of a larval anuran under osmotic stress.Physiol Biochem Zool, 77(2):267–274
Gordon M. S. 1962. Osmotic regulation in the green toad (Bufo viridis).J Exp Biol, 39(2): 261–270
Gordon M. S., Schmidt-Nielsen K., Kelly H. M. 1961. Osmotic regulation in the crab-eating frog (Rana cancrivora). J Exp Biol,38(3): 659–678
Gosner K. L. 1960. A simplified table for staging anuran embryos and larvae with notes on identification.Herpetologica, 16(3):183–190
Haramura T. 2007. Salinity tolerance of eggs of Buergeria japonica(Amphibia, Anura) inhabiting coastal areas.Zool sci, 24(8):820–823
Haramura T. 2011. Use of oviposition sites by a rhacophorid frog inhabiting a coastal area in Japan.J Herpetol 45(4): 432–437
Hopkins G. R., Brodie Jr. E. D., French S. S. 2014.Developmental and evolutionary history affect survival in stressful environments.PloS One, 9(4): e95174
Jin L., Whitehead P., Siegel D. I., Findlay S. 2011. Salting our landscape: An integrated catchment model using readily accessible data to assess emerging road salt contamination to streams. Environ Pollut, 159(5): 1257–1265
Karraker N. E. 2007. Are embryonic and larval green frogs (Rana clamitans) insensitive to road de-icing salt?.Herpetol Conserv Biol, 2: 35–41
Karraker N. E., Arrigoni J., Dudgeon D. 2010. Effects of increased salinity and an introduced predator on lowland amphibians in Southern China: Species identity matters. Biol Conserva, 143(5): 1079–1086
Karraker N. E., Gibbs J. P., Vonesh J. R. 2008. Impacts of road deicing salt on the demography of vernal pool-breeding amphibians.Ecol Appl, 18(3): 724–734
Karraker N. E., Ruthig G. R. 2009. Effect of road deicing salt on the susceptibility of amphibian embryos to infection by water molds.Environ Res, 109(1): 40–45
Katz U. 1989. Strategies of adaptation to osmotic stress in anuran amphibia under salt and burrowing conditions.Comp Biochem Physiol Part A: Physiology, 93(3): 499–503
Kumlu M., Eroldogan O. T., Aktas M. 2000. Effects of temperature and salinity on larval growth, survival and development ofPenaeus semisulcatus.Aquaculture, 188(1):167–173
Langhans M., Peterson B., Walker A., Smith G. R., Rettig J. E.2009. Effects of salinity on survivorship of wood frog (Rana sylvatica) tadpoles. J Fresh Ecol, 24(2): 335–337
Marsalek J. 2003. Road salts in urban stormwater: An emerging issue in stormwater management in cold climates. Water Sci Technol, 48(9): 61–70
Masshaii N., Balouch M., Mobedi I. 2008. Report about helminth parasites of some Amphibians (Anura: Ranidae, Bufonidae) from the North and Northeast of Iran. J Sci Univ Tehran, 33(4): 9–13
Nakkrasae L. I., Phummisutthigoon S., Charoenphandhu N. 2016. Low salinity increases survival, body weight and development in tadpoles of the Chinese edible frogHoplobatrachus rugulosus. Aquacul Res, 47(10): 3109–3118.
Newman R. A., Dunham A. E. 1994. Size at metamorphosis and water loss in a desert anuran (Scaphiopus couchii).Copeia,372–381
Nielsen D. L., Brock M. A. 2009. Modified water regime and salinity as a consequence of climate change: Prospects for wetlands of Southern Australia. Clim Change, 95(3): 523–533
Nielsen D. L., Brock M. A., Rees G. N., Baldwin D. S. 2003.Effects of increasing salinity on freshwater ecosystems in Australia. Aust J Bot.51(6): 655–665
Niyogi S., Blewett T. A., Gallagher T., Fehsenfeld S., Wood C.M. 2016. Effects of salinity on short-term waterborne zinc uptake, accumulation and sub-lethal toxicity in the green shore crab (Carcinus maenas).Aquat Toxicol, 178: 132–140
Peterson A. T., Ortega-Huerta M. A., Bartley J., Sjnchez-Cordero V., Sobern J., Buddemeier R. H., Stockwell D. R.B. 2002. Future projections for Mexican faunas under global climate change scenarios.Nature, 416(6881): 626–629
Petranka J. W., Doyle E. J. 2010. Effects of road salts on the composition of seasonal pond communities: Can the use of road salts enhance mosquito recruitment? Aquat Ecol, 44(1): 155–166
Petranka J. W., Francis R. A. 2013. Effects of road salts on seasonal wetlands: poor prey performance may compromise growth of predatory salamanders. Wetlands, 33(4): 707–715
Pora A. E., Stoicovici F. 1955. Cercetari asupra rolului sistemului nervos de laBufo viridisin fenomenele de adaptare la salinitate.Bull ttiint Acad romdne, 7: 59–89
Ramakrishna D. M., Viraraghavan T. 2005. Environmental impact of chemical de-icers–a review.Water Air and Soil Pollut,166(1–4): 49–63
Reyahi K., Nafea M. M., Mahjub H., Hashemy M., Parchian M.2011. Effects of road deicing salt on the quality of ground water resources in hamadan province, west of Iran. J res health sci,11(1): 39–44
Sanzo D., Hecnar S. J. 2006. Effects of road de-icing salt (NaCl)on larval wood frogs (Rana sylvatica).Environ Pollut, 140(2):247–256
Shoemaker V., Nagy K. A. 1977. Osmoregulation in amphibians and reptiles.Annu Rev Physiol, 39(1): 449–471
Smith D. C. 1987. Adult recruitment in chorus frogs: Effects of size and date at metamorphosis.Ecology, 68(2): 344–350
Snodgrass J. W., Casey R. E., Joseph D., Simon J. A. 2008.Microcosm investigations of stormwater pond sediment toxicity to embryonic and larval amphibians: Variation in sensitivity among species.Environ Pollut, 154(2): 291–297
Sparling D. W., Bishop C. A., Linder G. 2000. The current status of amphibian and reptile ecotoxicological research. Society of Environmental Toxicology and Chemistry, 13pp
Stuart S. N., Chanson J. S., Cox N. A., Young B. E., Rodrigues A. S. L., Fischman D. L., Waller R. W. 2004. Status and trends of amphibian declines and extinctions worldwide. Science,306(5702): 1783–1786
Thirion J. M. 2014. salinity of the reproduction habitats of the Western spadefoot ToadPelobates cultripes(cuvier, 1829),along the atlantic coast of France. Herpetozoa, 27: 13–20
Viertel B. 1999. Salt tolerance of Rana temporaria: Spawning site selection and survival during embryonic development(Amphibia, Anura).Amphiba-Reptila, 20(2): 161–171
Wijethunga U., Greenlees M., Shine R. 2016. Living up to its name? The effect of salinity on development, growth, and phenotype of the “marine” toad (Rhinella marina). J Comp Physiol B, 186(2): 205–213
Wu C. S., Kam Y. C. 2009. Effects of salinity on the survival,growth, development, and metamorphosis of Fejervarya limnocharis tadpoles living in brackish water. Zool Sci, 26(7):476–482
 Asian Herpetological Research2018年2期
Asian Herpetological Research2018年2期
- Asian Herpetological Research的其它文章
- Sexual Dimorphism, Female Reproductive Characteristics and Egg Incubation in an Oviparous Forest Skink (Sphenomorphus incognitus) from South China
- Amphibian Species Contribute Similarly to Taxonomic, but not Functional and Phylogenetic Diversity: Inferences from Amphibian Biodiversity on Emei Mountain
- A Rapid, Non-invasive Method for Anatomical Observations of Tadpole Vertebrae in Vivo
- Three New Ranidae Mitogenomes and the Evolution of Mitochondrial Gene Rearrangements among Ranidae Species
- A New Species of Gracixalus (Anura: Rhacophoridae) from West Guangxi, China
- A New Species of the Genus Sinomicrurus Slowinski, Boundy and Lawson, 2001 (Squamata: Elapidae) from Hainan Province, China
