Sexual Dimorphism, Female Reproductive Characteristics and Egg Incubation in an Oviparous Forest Skink (Sphenomorphus incognitus) from South China
Li MA, Jianchi PEI, Cuntong ZHOU,3, Yu DU,4, Xiang JI and Wen SHEN
1 School of Sports and Health, Hangzhou Normal University, Hangzhou 310036, Zhejiang, China
2 Jiangsu Key Laboratory for Biodiversity and Biotechnology, College of Life Sciences, Nanjing Normal University,Nanjing 210023, Jiangsu, China
3 College of Ecology, Lishui University, Lishui 323000, Zhejiang, China
4 Hainan Key Lab for Herpetology, College of Tropical Biology and Agronomy, Hainan Tropical Ocean University,Sanya 572022, China
1. Introduction
Forest skinks of the reproductively bimodal genusSphenomorphusFitzinger, 1843 occur in South-East Asia,Asia, Indochina and Central America (Linkemet al.,2011). Of some 145 currently recognizedSphenomorphusspecies (Linkemet al., 2011), six (S. courcyanus,S.incognitus,S. indicus,S. maculatus,S. taiwanensisandS.tonkinensis) can be found in China, withS. taiwanensisendemic to Taiwan Province of the country (Huang,1999; Nguyenet al., 2011, 2012). Despite its wide geographic distribution, high species diversity and the fact that it is morphologically, zoogeographically and taxonomically well known, the ecology and biology of the genusSphenomorphusremain poorly studied. Several investigators have studied sexual dimorphism and female reproduction but, to the best of our knowledge, they only reported descriptive data for five species (S. incognitus:Huang, 2010;S. indicus: Huang 1996; Ji and Du, 2000;Jiet al., 2006;S. jagori: Auffenberg and Auffenberg,1989;S. maculates: Huang, 1999;S. taiwanensis: Huang,1997, 1998). Detailed data on female reproductive traits do not exist for all these species except forS. indicus(Ji and Du, 2000; Jiet al., 2006). For example, ten femaleS. incognitus(Huang, 2010), nine femaleS. taiwanensis(Huang, 1997) and a unknown number of femaleS.jagori(Auffenberg and Auffenberg, 1989) were measured for fecundity (clutch size), but in none of these species were egg mass and reproductive output (clutch mass)documented.
Sphenomorphus incognitusstudied here ranges from Southern-Central China (Anhui, Fujian, Guangdong,Guangxi, Hainan, Hubei, Taiwan, Yunnan and Zhejiang)to North Vietnam (Huang, 1999; Lau, 2005; Nguyenet al., 2012; Tang and Huang, 2014; Chenet al., 2017).This medium sized (up to 107 mm snout-vent length,SVL), oviparous terrestrial skink shows a preference for stream habitats, forest edges and riverbeds (Huang,1999; Nguyenet al., 2012). The skink is morphologically similar toS. indicus, its viviparous congener, and this similarity contributes to the confusion about taxonomic identity, habitat use and geographic distribution of these two species (Chenet al., 2017). Previous studies presented very limited descriptive data forS. incognitusfrom mainland China (Huang, 1999), and a bit more detailed data for a population on Lanyu Island, Taiwan,China (Huang, 2010). From Huang’s (2010) study onS.incognitusfrom Lanyu Island we know the following.First, males are larger in terms of linear body size (SVL)and thusS. incognitusis among species that show malebiased sexual size dimorphism (SSD). Second, females exhibit spring and summer vitellogenesis and lay eggs from March to July. Third, females lay 3–6 eggs per clutch, with clutch size being independent of female SVL.Here, we presented data forS. incognitusfrom South China. Based on morphological measurements taken for adults in the field and clutches laid in the laboratory,we studied sexual dimorphism in body size and shape,female reproduction and egg incubation. Our aims were:(1) to show sexual dimorphism in several morphological characters (body size, head size, head width, abdomen length, and fore- and hind-limb lengths) likely to be associated with reproductive success and performance;(2) to investigate the relationships among egg size (and thus hatchling size), clutch size and female size; and(3) to examine the effects of constant versus fluctuating temperatures on incubation length and hatchling morphology.
2. Materials and Methods
We collected 263 adult skinks (92 females and 171 males) larger than 80 mm SVL in three consecutive years between 2013 and 2015 from Guangzhou, Wuzhishan and Zhaoqing in South China. Most of these skinks (65 females and all males) were released at their point of capture following the collection of morphological data.Measurements taken for each skink with Mitutoyo digital calipers included SVL, abdomen length (AL, between the insertion points of the fore- and hind-limbs), head length(HL, from the snout to the anterior edge of tympanum)and head width (HW, the posterior end of the mandible)(Sunet al., 2012). Of the 263 adults, 123 (42 females and 81 males) were also measured for fore-limb length (FLL,humerus plus ulna) and hind-limb length (HLL, femur plus tibia) (Jiet al., 2007).
We palpated all adult females in the field and transported 27 females with enlarged follicles to our laboratory in Nanjing, where they were individually housed in 540 × 400 × 320 mm3plastic cages placed in a room inside which temperatures varied from 20 °C to 28 °C. All cages had a substrate consisting of moist soil (~150 mm depth) covered with cobblestones, grass and fallen leaves, and females were able to regulate body temperature using natural sunlight. Mealworms(Tenebrio molitor), house crickets (Achetus domesticus),cockroaches (Blaptica dubia) and water enriched with vitamin and minerals were provided or refreshed daily.
Females laid a single clutch of eggs between early May and mid-August. We checked the cages at least thrice daily for freshly laid eggs after the first female laid eggs,thereby collecting, weighing and measuring (for length and width) eggs always less than 6 h post-laying. Postoviposition females were weighed and measured for SVL.Of the 27 females, two were excluded from analyses because they laid unfertilized eggs or abnormal eggs with condensed yolk. We calculated relative clutch mass(RCM) by dividing clutch mass by the post-oviposition female mass (Shine, 1992). To account for the influence of variation in female size on fecundity, we calculated relative fecundity by using the residuals derived from the regression of clutch size on female SVL (Olsson and Shine, 1997).
We collected 142 fertilized egg, of which eight,each from one of eight clutches, were used to identify the Dufaure and Hubert’s (1961) stage of embryonic development at laying. The remaining eggs were individually placed into covered plastic jars (50 ml) with moist vermiculite at –12 kPa (Ji and Bra?a, 1999). All incubating egg were 2/3 buried in the substrate, with the surface near the embryo exposed to air inside the jar. Eggs from the same clutch were assigned as equally as possible among five incubators (Binder, Germany): three set at 22,25 and 28 °C, respectively; the other two set at 25 ± 3 °C and 25 ± 5 °C, respectively. Thermal fluctuations were maintained at 12 h (+) and 12 h (–) and were confirmed with Tinytalk temperature loggers (Gemini Pty, Australia)placed inside jars. We rotated jars at 4-d intervals to minimize the influence of thermal gradients. Substrate water potential was adjusted at 4-d intervals by weighing jars. Incubation length was defined as the time between laying and pipping. Upon emergence, hatchlings were collected, weighed and measured for SVL, AL, HL and HW.
We used linear regression analysis to examine if the relationship between a selected pair of dependent and independent variables was significant. We calculated regression residuals of an examined morphological variable (AL, HL, HW, FLL, or HLL) against SVL, and then used one-way ANOVA to see if the variable differed between male and female adults. Data on egg size,incubation length and hatchling morphology from the same clutch were pooled to avoid pseudo-replication. We usedG-test and one-way ANOVA to see if eggs incubated under different thermal regimes differed in hatching success, mean mass at laying and mean incubation length.We used one-way ANCOVA to test for slope homogeneity of regressions lines and to see if hatchlings from eggs assigned to different treatments differed morphologically after accounting for egg mass at laying. Prior to parametric analyses, all data were tested for normality using the Kolmogorov-Smirnov test, and for homogeneity of variances using Bartlett’s test. All statistical procedures were performed in Statistica 8.0 (StatSoft; Tulsa, OK,USA), and statistical significance was assumed atP<0.05. Values are presented as mean ± standard error (SE)and range.
3. Results and Discussion
3.1. Sexual dimorphismThe largest male and female were 110 mm and 108 mm SVL, respectively. Both values are greater than the maximal sizes ever reported forS.incognitusfrom mainland China (107 mm SVL; Huang,1999) and Taiwan, China (94 mm SVL; Huang, 2010).The mean SVL did not differ between male (97 ± 0.5 mm)and female (96 ± 0.7 mm) adults (ANOVA;F1,261= 0.45,P= 0.50; Figure 1), suggesting thatS. incognitusfrom South China is sexually monomorphic in terms of adult body size (SVL). This pattern of SSD differs from malebiased SSD reported forS. incognitusfrom Taiwan, China(Huang, 2010), and it also does not support the hypothesis that lizards on islands are more likely to exhibit malebiased SSD (Hernández-Salinaset al., 2014). Much more adults were measured in this study (92 females and 171 males) than in the earlier one (43 females and 45 males;Huang, 2010), thus allowing more accurate determination of SSD.
The evolution and maintenance of a given pattern of SSD often result from sexual differences in reproductive success relating to adult body size (Cooper and Vitt, 1989;Hews, 1990; Mouton and Van Wyk, 1993; Reeve and Fairbairn, 2001; Coxet al., 2003). Within scincid lizards,selection through male contest competition is the key factor for male-biased SSD inPlestiodonchinensis(Lin and Ji, 2000),Plestiodon elegans(Du and Ji, 2001; Zhang and Ji, 2004) andEutropismultifasciata(Jiet al., 2006),whereas selection on fecundity or reproductive output is the main cause for increased female size inS. indicus(Ji and Du, 2000),Scincella modestaandScincella reevesii(Yanget al., 2012). Sexual size monomorphism(SSM) often occurs in species where these two selective forces cancel each other out and has been documented in a wide range of lizard taxa. In lizard species so far studied in China, SSM has been documented inCalotes versicolor(Jiet al., 2002),Eremias argus(Chenet al.,2015),Eremias brenchleyi(Xu and Ji, 2003),Eremias multiocellata(Liet al., 2006),Japalura splendida(Lin, 2004),Phrynocephalus frontalis(Quet al., 2011),Phrynocephalus grumgrzimailoi(Liu and Shi, 2009),Phrynocephalus guinanensis(Jiet al., 2009),Shinisaurus crocodilurus(Heet al., 2011),Takydromus septentrionalis(Jiet al., 1998; Zhang and Ji, 2000) andTakydromus sexlineatus(Xuet al., 2014).
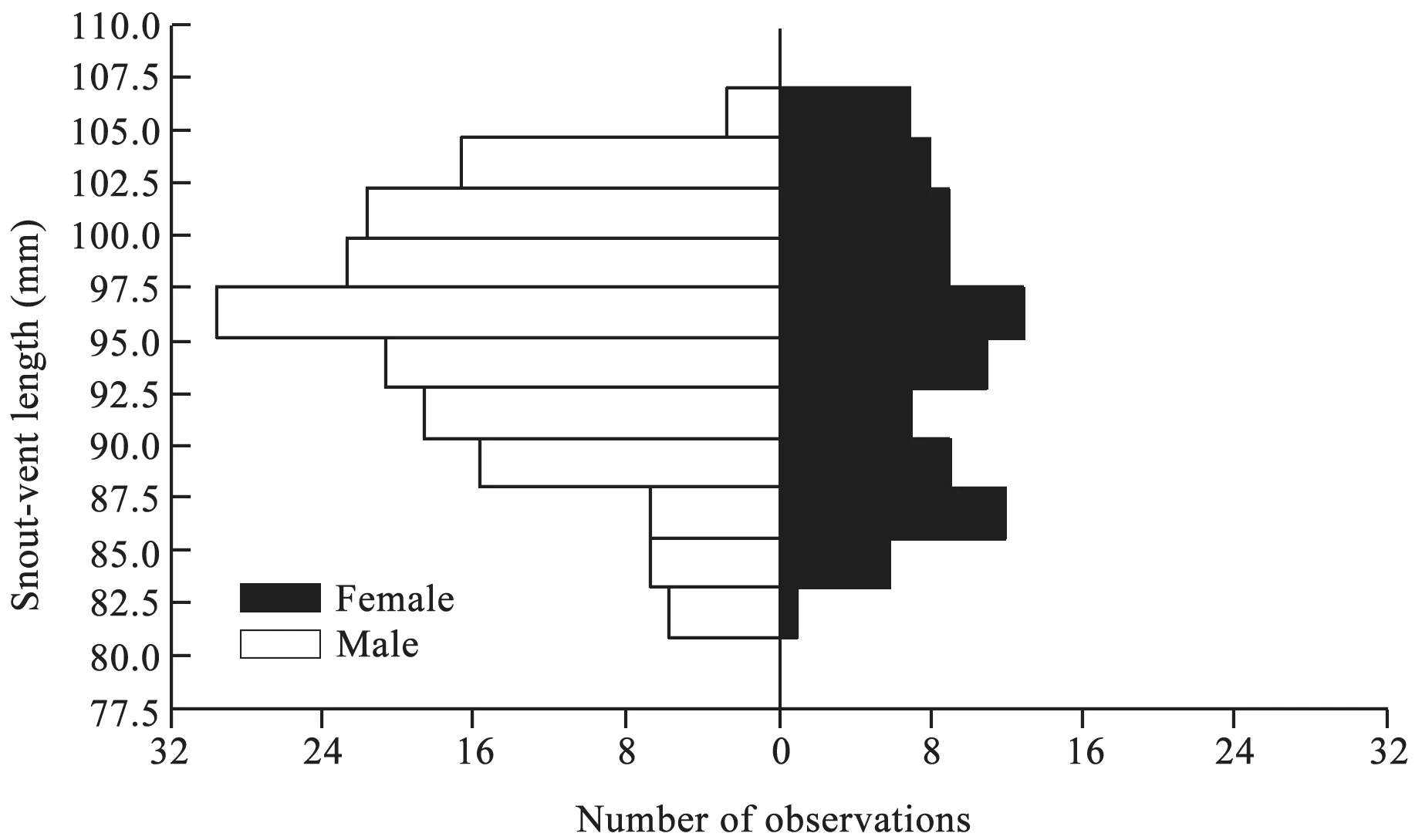
Figure 1 Frequency distributions of SVL of adult Sphenomorphus incognitus (92 females and 171 males), showing sexual size monomorphism.
The rates at which HL (Figure 2a) and HW (Figure 2b) increased with SVL were greater in adult males(ANCOVA for slope homogeneity, bothP< 0.001),and the rates at which AL (Figure 2c), FLL (Figure 2d)and HLL (Figure 2e) increased with SVL did not differ significantly between the sexes (ANCOVA for slope homogeneity; allP> 0.09). The mean values of residuals from the regressions of HL, HW, FLL and HLL on SVL were greater in adult males (ANOVA; allP< 0.0001),whereas the mean value of residuals from the regressions of AL (ANOVA;F1,261= 64.20,P< 0.0001) on SVL was greater in adult females. The greater relative head size in males and the greater relative abdomen size in females are the rule in nearly all lizard lineages (Olssonet al., 2002; Coxet al., 2003; Kratochvílet al., 2003;Pincheira-Donoso and Tregenza, 2011; Sunet al., 2012;see also Huang, 1996). It is therefore not surprising thatS. incognitusshares these features. Head size (both length and width) and abdomen length are sexually dimorphic largely because these traits are directly linked to the reproductive role of each sex (Bultéet al., 2008),although in some species the greater relative head size in males may also have a secondary role in reducing intersexual resource competition by amplifying food niche divergence between the sexes (Bra?a, 1996; Lin and Ji, 2000; Zhang and Ji, 2000, 2004). Sexual dimorphism in appendage (limb) length has been poorly known. LikePhrynocephalus przewalskii(Zhao and Liu, 2014) andS. incognitusfrom Taiwan, China (Huang, 2010),S.incognitusfrom South China shows male-biased sexual dimorphism in appendage length.
3.2. Female reproductive characteristicsTable 1 shows female reproductive traits ofS. incognitusfrom South China. Females laid a single clutch of 3–10 eggs per breeding season from early May to mid-August, with the egg-laying season being about three months longer than that (from March to July) reported forS. incognitusfrom Taiwan, China (Huang, 2010). Clutch size was positively related to female SVL (r2= 0.18,F1,23= 5.09,P= 0.034), suggesting that, as in most other lizard species (Ramírez-Bautistaet al., 2017), female size is an important determinant of fecundity inS. incognitus. Such a relationship between clutch size and female SVL was nonetheless not statistically significant inS. incognitusfrom Taiwan, China (Huang, 2010). The mean clutch size was greater in South China (5.2; Table 1) than in Taiwan,China (4.0; Huang, 2010). This difference could be in part due to the fact that females of this study (81–108 mm SVL; Table 1) were larger than those studied in Taiwan,China (73–87 mm SVL; Huang, 2010), asS. incognitusis among species where larger females are more fecund than smaller ones. Egg mass and clutch mass had never been examined inS. incognitus. In this study, we found that neither clutch mass (r2= 0.12,F1,23= 3.23,P=0.085) nor egg mass (r2= 0.04,F1,23= 0.99,P= 0.33)was significantly related to female SVL. These findings suggest that female size is not an important determinant of reproductive output or investment per offspring inS. incognitus. Egg mass was independent of relative fecundity (r2= 0.03,F1,23= 0.64,P= 0.43), suggesting that, as inEutropis longicaudata(Sunet al., 2012) andS.modesta(Yanget al., 2012), the egg size-number tradeoff does not exist inS. incognitus.
Among oviparous skinks so far studied in mainland China, the mean RCM was smaller inS. incognitus(0.25;Table 1) than inS. modesta(0.72; Yanget al., 2012),E.longicaudata(0.34; Sunet al., 2012),P. chinensis(0.33;Lin and Ji, 2000) andP. elegans(0.31; Du and Ji, 2001),the proportion of variation in clutch mass explained by female SVL was lower inS. incognitus(12%) than inP.chinensis(51%; Lin and Ji, 2000),P. elegans(46%; Du and Ji, 2001),E. longicaudata(42%; Sunet al., 2012) andS. modesta(37%; Yanget al., 2012), and the proportion of variation in clutch size explained by female SVL is lower inS. incognitus(18%) than inP. chinensis(52%;Lin and Ji, 2000),S. modesta(40%; Yanget al., 2012),P. elegans(37%; Du and Ji, 2001) andE. longicaudata(35%; Sunet al., 2012). These comparisons provide an inference that selection on increased maternal body size and thus increased body volume available to hold eggs is comparatively weak inS. incognitus.

Figure 2 Linear regressions of head length (a), head width (b), abdomen length (c), fore-limb length (d) and hind-limb length (e) on SVL in adult Sphenomorphus incognitus. Filled circles: females; open circles: males.
3.3. Egg incubation and hatchling phenotypeEmbryonic stages at laying ranged from Dufaure and Hubert’s (1961) stage 31 to 32, with a mean stage of 31.3.Embryonic stage at laying is a causal factor of inter- and intra-specific variation in incubation length in oviparous lizards (Wanget al., 2013). However, incubation length at any given temperature may vary considerably among species that differ in phylogeny, egg size and/or distribution (Linet al., 2010; Liet al., 2012, 2013; Sunet al., 2013). Within sincid lizards, for example, the mean incubation length at 28 °C is much longer inS. incognitus(~40 d; Table 2) than inS. modesta(~20 d; Luet al.,2006) andP. chinensis(~24 d; Luet al., 2012, 2014; Shenet al., 2017), although the mean DH stage at laying does not differ betweenS. incognitusandS. modesta(31.1; Luet al., 2006) and is about one stage earlier inS. incognitusthan inP. chinensis(~32.5; Luet al., 2012, 2014; Shenet al., 2017). InPhrynocephaluslizards the changeover from the DH stage 30 to 31 shortens the mean incubation length at 28 °C by 3 d (Wanget al., 2013; Zenget al.,2013).
Eggs assigned to the five temperature treatments did not differ significantly in mean mass (F4,42= 2.44,P=0.06) or hatching success (G= 2.62,df= 4,P> 0.50).Hatching successes varied from 64% (16/25) in the 25 ± 5°C treatment to 82% (9/11) in the 28 °C treatment, with a mean of 74% (Table 2). Within each treatment incubation length was independent of egg mass (linear regression analysis: allP> 0.20). Mean values for incubation length differed among the five treatments (F4,42= 45.62,P<0.0001). For eggs incubated at constant temperatures,the mean incubation length was shortened by 22.0 and 13.2 d for every 3 °C increase from 22–28 °C (Table 2).This pattern of thermal sensitivity of incubation length is consistent with earlier studies on turtles (Jiet al., 2003,Duet al., 2007, 2010), lizards (Ji and Bra?a, 1999; Linet al., 2007; Wanget al., 2013; Shenet al., 2017), snakes(Ji and Du, 2001; Linet al., 2005; Linet al., 2010) and crocodiles (Pi?aet al., 2003; Charruau, 2012) where incubation length decreases at an ever decreasing rate as temperature increases across the range where successful embryonic development can take place, explaining why eggs take a longer time to hatch at fluctuating temperatures than at constant temperatures with the same mean in some species (Shine, 2004a; Haoet al., 2006;Bra?a and Ji, 2007; Leset al., 2007; Luet al., 2009; Liet al., 2012). However, contrast to what was expected the fluctuating temperature treatments result in shorter incubation lengths relative to constant temperatures inS.incognitus. This suggests that, as inBassiana duperreyi(Shine, 2004b),Lycaena tityrus(Fischeret al., 2011),Naja atra(Linet al., 2008) andXenochrophis piscator(Luet al., 2009), incubation at stable temperatures may lead to delayed hatchinginS. incognitus.
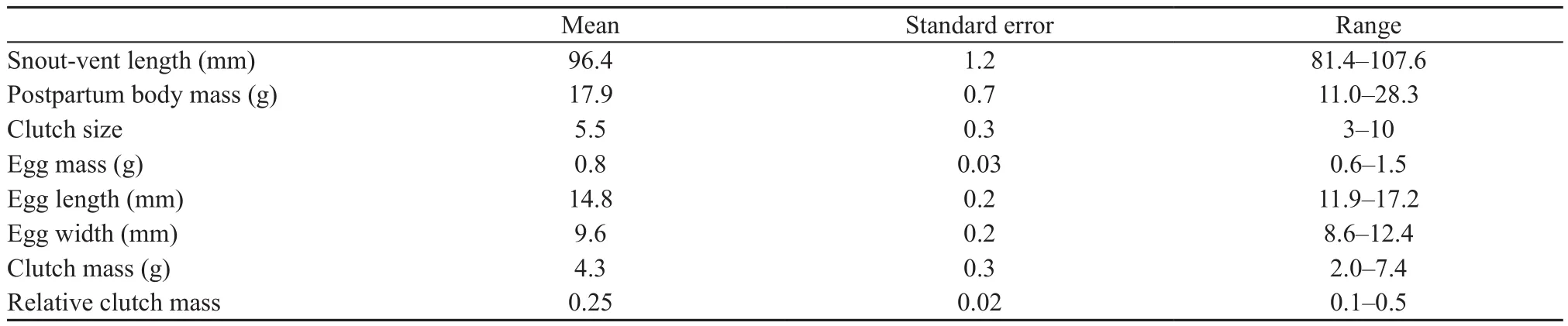
Table 1 Reproductive traits of female Sphenomorphus incognitus (N = 25).
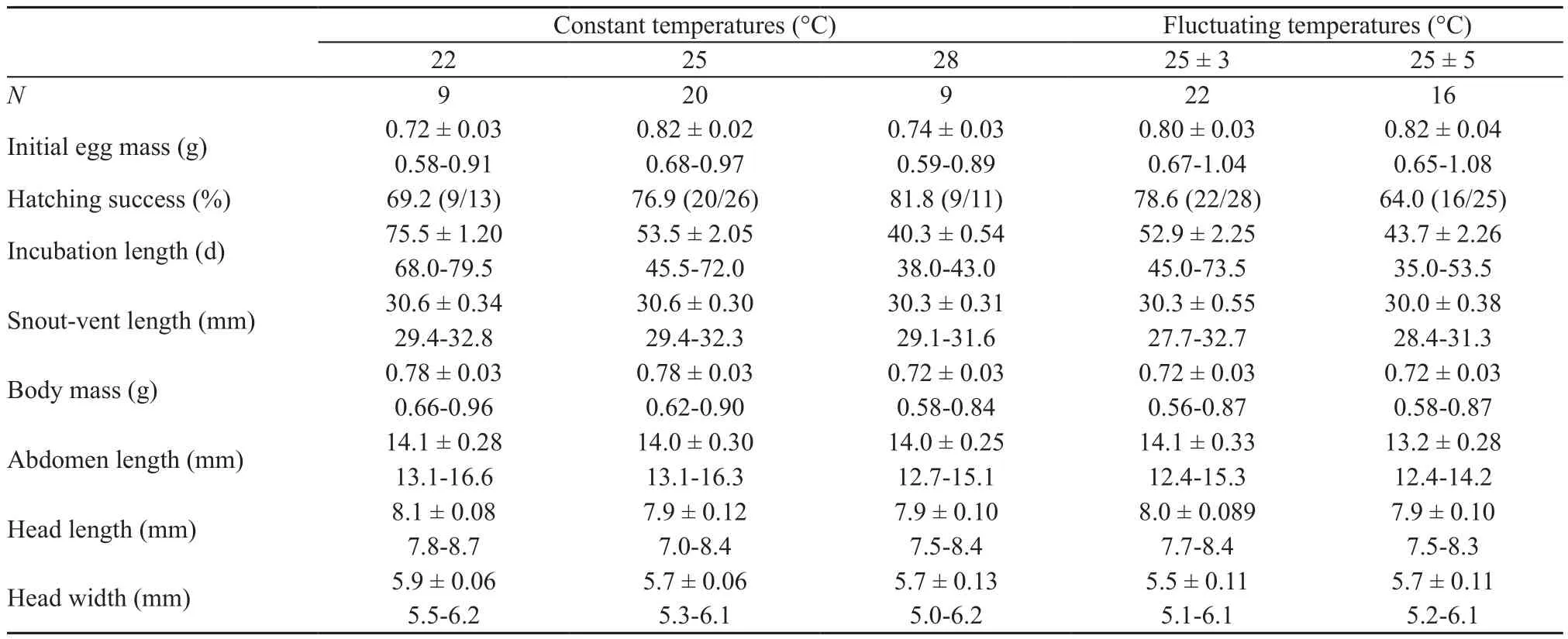
Table 2 Hatching success and descriptive statistics (expressed as mean ± SE and range) for egg mass at laying (initial egg mass), incubation length and wet body mass and morphology of hatchling Sphenomorphus incognitus from eggs incubated under fi ve thermal regimes.
Incubation temperatures higher than 28 °C substantially reduce hatching success and adversely affect hatchling phenotypes in forest skinks (Luet al., 2006; Liet al.,2012). Here we found that hatchlings from eggs incubated at 25 ± 5 °C did not differ from those from eggs incubated under other four thermal regimes in any examined trait after accounting for egg mass at laying (ANCOVA; allP> 0.19; Table 2). This finding is overall consistent with that reported for a wide range of reptile taxa, including turtles (Pelodiscus sinensis: Du and Ji, 2003; Jiet al.,2003), lizards (E. argus: Haoet al., 2006;Heteronotia binoei: Andrewarthaet al., 2010;Lacerta agilis: Liet al., 2013;P. chinensis: Chenet al., 2003) and snakes(Rhabdophis tigrinus lateralis: Chen and Ji, 2002;Ptyas mucosus: Lin and Ji, 2004;N. atra: Linet al., 2008;X.piscator: (Luet al., 2009). In all these species, incubation temperature has no role in modifying hatchling traits as long as eggs are not exposed to extreme temperatures for prolonged periods of time.
4. Conclusions
Sphenomorphus incognitusis a morphologically,zoogeographically and taxonomically well known species,but its ecology and biology remain sparsely studied. Here we used adults collected from South China to study sexual dimorphism, female reproduction and egg incubation in this species. From this study we know the following.First, the skink is a sexually monomorphic species in terms of adult SVL but shows sexual dimorphism in head size, abdomen length and limb length, with males being larger in head size (both length and width), longer in foreand hind-limb lengths and shorter in abdomen length than females of the same SVL. Second, females larger than 80 mm SVL lay a single clutch of 3–10 eggs per breeding season from early May to mid-August, with larger females generally laying more (but not always larger)eggs per clutch than do smaller ones. Third, the positive relationship between clutch mass and female SVL is not significant, and the offspring size-number tradeoff does not exist inS. incognitus. Fourth, embryonic stages at laying range from Dufaure and Hubert’s (1961)stage 31 to 32, and the mean incubation length at a given temperature is much longer inS. incognituscompared toS. modestawith nearly the same embryonic stage at laying. Last, eggs ofS. incognitusincubated at fluctuating temperatures take a shorter time to hatch than those incubated at stable temperatures with the same mean, and incubation temperature has no role in modifying hatchling morphology as long as eggs are not exposed to extreme temperatures for prolonged periods of time.
AcknowledgementsOur experimental procedures complied with the current laws on animal welfare and research in China. We thank Yijing CHEN, Kun GUO, Min SHAO, Yanqing WU, Guangzheng ZHANG and Liang ZHANG for assistance during the research. For funding,we thank the National Natural Science Foundation of China (31470471), the Priority Academic Development Program of Jiangsu Higher Education Institutions and the Innovation of Graduate Student Training Project of Jiangsu Province (KYLX15_0737).
Andrewartha S. J., Mitchell N. J., Frappell P. B. 2010. Does incubation temperature fluctuation influence hatchling phenotypes in reptiles? A test using parthenogenetic geckos.Physiol Biochem Zool, 83: 597–607
Auffenberg W., Auffenberg T. 1989. Reproductive patterns in sympatric Philippine skinks (Sauria: Scincidae). Bull FL State Mus Biol Sci Ser, 34: 201–247
Bra?a F. 1996. Sexual dimorphism in lacertid lizards: male head increase vs female abdomen increase.Oikos, 75: 511–523
Bra?a F., Ji X. 2007. The selective basis for increased egg retention: Early incubation temperature determines hatchling phenotype in wall lizards. Biol J Linn Soc, 91: 441–447
Bulté G., Irschick D. J., Blouin-Demerset G. 2008. The reproductive role hypothesis explains trophic morphology dimorphism in the northern map turtle. Funct Ecol, 22: 824–830
Charruau P. 2012. Microclimate of American crocodile nests in Banco Chinchorro biosphere reserve, Mexico: Effect on incubation length, embryos survival and hatchlings sex. J Therm Biol, 37: 6–14
Chen H. L., Ji X. 2002. The effects of thermal environments on duration of incubation, hatching success and hatchling traits in a colubrid snakeRhabdophis tigrinus lateralis(Boie). Acta Ecol Sin, 22: 1850–1858
Chen S. Y., Bi J. H., He Z. C., Li S. R., Liu R., Wang Y., Zhao X.2015. Sexual dimorphism and reproductive output ofEremias argusfrom Ordos. Chin J Zool, 50: 214–220
Chen X. J., Lin Z. H., Ji X. 2003. Further studies on influence of temperature on egg incubation in the Chinese skink,Eumeces chinensis. Zool Res, 24: 21–25
Chen Z. Q., Wei H. H., Liu J. L., Wu Y. K., Le X. G., Cheng S.L., Guo H. X., Ding G. H. 2017. New record ofSphenomorphus incognitusfrom Zhejiang and Jiangxi provinces. Sichuan J Zool,36: 479–480
Cooper W. E., Vitt L. J. 1989. Sexual dimorphism of head and body size in an iguanid lizard: paradoxical results. Am Nat, 133:729–735
Cox R. M., Skelly S. L., John-Alder H. B. 2003. A comparative test of adaptive hypothesis for sexual size dimorphism in lizards.Evolution, 57: 1653–1669
Du W. G., Hu L. J., Lu J. L., Zhu L. J. 2007. Effects of incubation temperature on embryonic development rate, sex ratio and post-hatching growth in the Chinese three-keeled pond turtle,Chinemys reevesii. Aquaculture, 272: 747–753
Du W. G., Ji X. 2001. Growth, sexual dimorphism and female reproduction of blue-tailed skinks,Eumeces elegans. Zool Res,22: 279–286
Du W. G., Ji X. 2003. The effects of incubation thermal environments on size, locomotor performance and early growth of hatchling soft-shelled turtles,Pelodiscussinensis. J Therm Biol, 28: 279–286
Du W. G., Wang L., Shen J. W. 2010. Optimal temperatures for egg incubation in two geoemydid turtles:Ocadia sinensisandMauremys mutica. Aquaculture, 305: 138–142
Dufaure J. P., Hubert J. 1961. Table de développement du lézard vivipare:Lacerta(Zootoca)viviparaJacquin. Arch Anat Microsc Morphol Exp, 50: 309–328
Fischer K., K?lzow N., H?ltje H., Karl I. 2011. Assay conditions in laboratory experiments: Is the use of constant rather than fluctuating temperatures justified when investigating temperature-induced plasticity? Oecologia, 166: 23–33
Hao Q. L., Liu H. X., Ji X. 2006. Phenotypic variation in hatchling Mongolian racerunners (Eremias argus) from eggs incubated at constant versus fl uctuating temperatures. Acta Zool Sin, 52:1049–1057
He N., Wu Z. J., Cai F. J., Wang Z. X., Yu H., Huang C. M.2011. Sexual dimorphism ofShinisaurus crocodilurus. Chin J Ecol, 30: 7–11
Hernández-Salinas U., Ramírez-Bautista A., Pavón N. P.,Pacheco L. F. R. 2014. Morphometric variation in island and mainland populations of two lizard species from the Pacific Coast of Mexico. Rev Chil Hist Nat, 87: 21
Hews, D. K. 1990. Examining hypotheses generated by field measures of sexual selection on male lizards,Uta palmeri.Evolution, 44: 1956–1966
Huang Q. Y. 1999. Scincidae. In Zhao E. M., Zhao K. T., Zhou K.Y. (Eds.), Fauna Sinica, Reptilia,Vol. 2. Beijing: Science Press,271–360
Huang W. S. 1996. Reproductive cycles and sexual dimorphism in the viviparous skink,Sphenomorphus indicus(Sauria:Scincidae), from Wushe, Central Taiwan. Zool Stud, 35: 55–61
Huang W. S. 1997. Reproductive cycle of the skink,Sphenomorphus taiwanensis, in central Taiwan. J Herpetol, 31:287–290
Huang W. S. 1998. Sexual size dimorphism and microhabitat use of two sympatric lizards,Sphenomorphus taiwanensisandTakydromus hsuehshanensis, from the central highlands of Taiwan. Zool Stud, 37: 303–308
Huang W. S. 2010. Ecology and reproductive characteristics of the skinkSphenomorphus incognituson an East Asian Island, with comments on variations in clutch size with reproductive modes inSphenomorphus. Zool Stud, 49: 779–788
Ji X, Bra?a F. 1999. Influence of thermal and hydric environments on embryonic use of energy and nutrients, and hatchling traits,in the wall lizards (Podarcismuralis). Comp Biochem Physiol,124A: 205–213
Ji X., Chen F., Du W. G., Chen H. L. 2003. Incubation temperature affects hatchling growth but not sexual phenotype in the Chinese soft-shelled turtlePelodiscus sinensis. J Zool, 261: 409–416
Ji X., Du W. G. 2000. Sexual dimorphism in body size and head size and female reproduction in a viviparous skink,Sphenomorphus indicus. Zool Res, 21: 349–354
Ji X., Huang H. Y., Hu X. Z., Du W. G. 2002. Geographic variation in female reproductive characteristics and egg incubation in the Chinese skink,Eumeces chinensis. Chin J Appl Ecol, 13: 680–684
Ji X., Lin C. X., Lin L. H., Qiu Q. B., Du Y. 2007. Evolution of viviparity in warm-climate lizards: An experimental test of the maternal manipulation hypothesis. J Evol Biol, 20: 1037–1045
Ji X., Lin L. H., Lin C. X., Qiu Q. B., Du Y. 2006. Sexual dimorphism and female reproduction in the many-lined sun skink (Mabuya multifasciata) from China. J Herpetol, 40: 353–359
Ji X., Lin L. H., Luo L. G., Lu H. L., Gao J. F., Han J. 2006.Gestation temperature affects sexual phenotype, morphology,locomotor performance and growth of neonatal brown forest skink,Sphenomorphus indicus. Biol J Linn Soc, 88: 453–463
Ji X., Qiu Q. B., Diong C. H. 2002. Sexual dimorphism and female reproductive characteristics in the oriental garden lizard,Calotes versicolorfrom a population in Hainan, southern China. J Herpetol, 36: 1–8
Ji X., Wang Y. Z., Wang Z. 2009. New species ofPhrynocephalus(Squamata, Agamidae) from Qinghai, Northwest China. Zootaxa,1988: 61–68
Ji X., Zhang C. H. 2001. Effects of thermal and hydric environments on incubating eggs, hatching success, and hatchling traits in the Chinese skink (Eumeces chinensis). Acta Zool Sin, 47: 250–259
Ji X., Zhou W. H., He G. B., Zhang X. D. 1998. Sexual dimorphism and reproduction in the grass lizard,Takydromus septentrionalis. Russ J Herpetol, 5: 44–48
Kratochvíl L., Fokt M., Rehák I., Frynta D. 2003.Misinterpretation of character scaling: A tale of sexual dimorphism in body shape of common lizards. Can J Zool, 81:1112–1117
Lau M. 2005. The occurrence ofSphenomorphus incognitusin Hong Kong with notes on its diagnostic features and distribution.Porcupine, 32: 9–10
Les H. L., Paitz R. T., Bowden R. M. 2007. Experimental test of the effects of fluctuating incubation temperatures on hatchling phenotype. J Exp Zool A, 307: 274–280
Li H., Ding G. H., Zhou Z. S., Ji X. 2013. Fluctuations in incubation temperature affect incubation duration but not morphology, locomotion and growth of hatchlings in the sand lizardLacerta agilis(Lacertidae). Acta Zool (Stockholm), 94:11–18
Li H., Ji X., Qu Y. F., Gao J. F., Zhang L. 2006. Sexual dimorphism and female reproduction in the multi-ocellated racerunner,Eremias multiocellata(Lacertidae). Acta Zool Sin,52: 250–255
Li H., Wang Z., Chen C., Ji X. 2012. Does the variance of incubation temperatures always constitute a selective force for the origin of reptilian viviparity? Curr Zool, 58: 812–819
Lin C. X., Du Y., Qiu Q. B., Ji X. 2007. Relatively high but narrow incubation temperatures in lizards depositing eggs in warm and thermally stable nests. Acta Zool Sin, 53: 437–445
Lin L. H., Li H., An H., Ji X. 2008. Do temperature fluctuations during incubation always play an important role in shaping the phenotype of hatchling reptiles? J Therm Biol, 33: 193–199
Lin L. H., Ma X. M., Li H., Ji X. 2010. Phenotypic variation in hatchling Chinese ratsnakes (Zaocys dhumnades) from eggs incubated at constant temperatures. J Therm Biol, 35: 28–33
Lin Z. H. 2004. Sexual dimorphism in head and body size and the growth during reproductive period in the lizard,Japalura splendida. Sichuan J Zool, 23: 277–280
Lin Z. H., Ji X. 2000. Food habits, sexual dimorphism and female reproduction of the skink (Eumeces chinensis) from a Lishui population in Zhejiang. Acta Ecol Sin, 20: 304–310
Lin Z. H., Ji X. 2004. Reproductive output and effects of incubation thermal environments on hatchling phenotypes of mucous rat snakesPtyas mucosus. Acta Zool Sin, 50: 541–550
Lin Z. H., Ji X., Luo L. G., Ma X. M. 2005. Incubation temperature affects hatching success, embryonic expenditure of energy and hatchling phenotypes of a prolonged egg-retaining snake,Deinagkistrodon acutus(Viperidae). J Therm Biol, 30:289–297
Linkem C. W., Diesmos A. C., Brown R. M. 2011. Molecular systematics of the Philippine forest skinks (Squamata: Scincidae:Sphenomorphus): Testing morphological hypotheses of interspecific relationships. Zool J Linn Soc, 163: 1217–1243
Liu Y, Shi L. 2009. Ontogenetic shifts of sexual dimorphism inPhrynocephalus grumgrzimailoi(Agamidae). Sichuan J Zool,28: 710–713
Lu H. L., Gao J. F., Ma X. H., Lin Z. H., Ji X. 2012. Tail loss affects fecundity but not offspring traits in the Chinese skink,Eumeces chinensis. Curr Zool, 58: 228–235
Lu H. L., Hu R. B., Ji X. 2009. The variance of incubation temperatures does not affect the phenotype of hatchlings in a colubrid snake,Xenochrophis piscator(Colubridae). J Therm Biol, 34: 138–143
Lu H. L., Ji X., Lin L. H., Zhang L. 2006. Relatively low upper threshold temperature in lizards using cool habitats. J Therm Biol, 31: 256–261
Lu H. L., Lin Z. H., Li H., Ji X. 2014. Geographic variation in hatchling size in an oviparous skink: Effects of maternal investment and incubation thermal environment. Biol J Linn Soc, 113: 283–296
Mouton P. F. N., Van Wyk J. H. 1993. Sexual dimorphism in cordylid lizards: A case study of the Drakensberg crag lizard,Pseudocordylus melanotus. Can J Zool, 71: 1715–1723
Nguyen T. Q., Schmitz A., Nguyen T. T., Orlov N. L., B?hme W.,Ziegler T. 2011. Review of the genusSphenomorphusFitzinger,1843 (Squamata: Sauria: Scincidae) in Vietnam, with description of a new species from northern Vietnam and southern China and the first record ofSphenomorphus mimicusTaylor, 1962 from Vietnam. J Herpetol, 45: 145–154
Nguyen T. Q., Tran T. T., Nguyen T. T., B?hme W., Ziegler T.2012. First record ofSphenomorphus incognitus(Thompson,1912) (Squamata: Scincidae) from Vietnam with some notes on natural history. Asian Herpetol Res, 3: 147–150
Olsson M., Shine R. 1997. The limits to reproductive output:offspring size versus number in the sand lizard (Lacerta agilis).Am Nat, 149: 179–188
Olsson M., Shine R., Wapstra E., Ujvari B., Madsen T. 2002.Sexual dimorphism in lizard body shape: The roles of sexual selection and fecundity selection. Evolution, 56: 1538–1542
Pi?a C. I., Larriera A., Cabrera M. R. 2003. Effect of incubation temperature on incubation period, sex ratio, hatching success, and survivorship inCaiman latirostris(Crocodylia,Alligatoridae). J Herpetol, 37: 199–202
Pincheira-Donoso D., Tregenza T. 2011. Fecundity selection and the evolution of reproductive output and sex-specific body size in theLiolaemuslizard adaptive radiation. Evol Biol, 38: 197–207
Qu Y. F., Gao J. F., Mao L. X., Ji X. 2011. Sexual dimorphism and female reproduction in two sympatric toad-headed lizards,Phrynocephalus frontalisandP. versicolor(Agamidae). Anim Biol, 61: 139–151
Ramírez-Bautista A., Cruz-Elizalde R., Hernández-Salinas U., Lozano A., Grummer J. A. 2017. Reproductive trait variation in theSceloporus scalarisspecies group (Squamata:Phrynosomatidae) from the Transvolcanic Belt, Mexico. Biol J Linn Soc, 122: 838–849
Reeve J. P., Fairbairn D. J. 2001. Predicting the evolution of sexual size dimorphism. J Evol Biol, 14: 244–254
Shen W., Pei J. C., Lin L. H., Ji X. 2017. Effects of constant versus fluctuating incubation temperatures on hatching success,incubation length and hatchling morphology in the Chinese skink (Plestiodon chinensis). Asian Herpetol Res, 8: 262–268
Shine R. 1992. Relative clutch mass and body shape in lizards and snakes: its reproductive investment constrained or optimized?Evolution, 46: 828–833
Shine R. 2004a. Incubation regimes of cold-climate reptiles: The thermal consequences of nest-site choice, viviparity and maternal basking. Biol J Linn Soc, 83: 145–155
Shine R. 2004b. Seasonal shifts in nest temperature can modify the phenotypes of hatchling lizards, regardless of overall mean incubation temperature. Funct Ecol, 18: 43–49
Sun B. J., Li S. L., Xu X. F., Zhao W. G., Luo L. G., Ji X., Du W. G. 2013. Different mechanisms lead to convergence of reproductive strategies in two lacertid lizards (Takydromus wolteriandEremias argus). Oecologia, 172: 645–652
Sun Y. Y., Du Y., Yang J., Fu T. B., Lin C. X., Ji X. 2012. Is the evolution of viviparity accompanied by a relative increase in maternal abdomen size in lizards? Evol Biol, 39: 388–399
Tang X. S., Huang S. 2014.Sphenomorphus incognitusfirstly found in Anhui province, China. Chin J Zool, 49: 609–612
Wang Z., Lu H. L., Ma L., Ji X. 2014. Viviparity in high altitudePhrynocephaluslizards is adaptive because embryos cannot fully develop without maternal thermoregulation. Oecologia,174: 639–649
Wang Z., Ma L., Shao M., Ji X. 2013. Differences in incubation length and hatchling morphology among five oviparousPhrynocephaluslizards (Agamidae) from China. Asian Herpetol Res, 4: 225–232
Xu D. D., Luo S. T., Liu W. H., Yao X. M., Wu H. X. 2014. The intersexual differences of sexual dimorphism, feeding habits and locomotor performance at different temperatures of southern grass lizard (Takydromus sexlineatus) in Zhaoqing, China.Sichuan J Zool, 33: 808–814
Xu X. F., Ji X. 2003. Ontogenetic shifts in sexual dimorphism in head size and food habits in the lacertid lizard,Eremias brenchleyi. Chin J Appl Ecol, 14: 557–561
Yang J., Sun Y. Y., Fu T. B., Xu D. D., Ji X. 2012. Selection for increased maternal body-volume does not differ between twoScincellalizards with different reproductive modes. Zoology,115: 199–206
Zeng Z. G., Zhao J. M., Sun B. J. 2013. Life history variation among geographically close populations of the toad-headed lizard (Phrynocephalus przewalskii): Exploring environmental and physiological associations. Acta Oecol, 51: 28–33
Zhang Y. P., Ji X. 2000. Ontogenetic changes of sexual dimorphism in head size and food habit in grass lizard,Takydromus septentrionalis. Zool Res, 21: 181–186
Zhang Y. P., Ji X. 2004. Sexual dimorphism in head size and food habits in the blue-tailed skink,Eumeces elegans. Acta Zool Sin,50: 745–752
Zhao W., Liu N. F. 2014. The proximate causes of sexual size dimorphism inPhrynocephalus przewalskii. PLoS One, 9:e85963
 Asian Herpetological Research2018年2期
Asian Herpetological Research2018年2期
- Asian Herpetological Research的其它文章
- Effects of Increased Salinity on Growth, Development and Survival in Early Life Stages of the Green Toad Bufotes variabilis (Anura:Bufonidae)
- Amphibian Species Contribute Similarly to Taxonomic, but not Functional and Phylogenetic Diversity: Inferences from Amphibian Biodiversity on Emei Mountain
- A Rapid, Non-invasive Method for Anatomical Observations of Tadpole Vertebrae in Vivo
- Three New Ranidae Mitogenomes and the Evolution of Mitochondrial Gene Rearrangements among Ranidae Species
- A New Species of Gracixalus (Anura: Rhacophoridae) from West Guangxi, China
- A New Species of the Genus Sinomicrurus Slowinski, Boundy and Lawson, 2001 (Squamata: Elapidae) from Hainan Province, China
