A Rapid, Non-invasive Method for Anatomical Observations of Tadpole Vertebrae in Vivo
Guocheng SHU, Shan XIONG, Wenyan ZHANG, Jianping JIANG, Cheng LI* and Feng XIE*
1 Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China
2 University of Chinese Academy of Sciences, Beijing 100049, China
1. Introduction
The larval period is an important part of amphibian life history and plays a significant role during the transition from the aquatic to the terrestrial stage. However, at the end of the last century, the tadpoles of approximately two-thirds of the nearly 3 300 known anuran species with a larval phase had not been described (McDiarmid and Altig, 1999), and this knowledge gap is critical to our understanding of the diversity and complexity of the life history traits of amphibians, especially their developmental biology. Thus, tadpole biology is a discipline within amphibian research that needs to be strengthened. Some aspects of this lack of tadpole research are due to limited methodological approaches.
To obtain an accurate visualization of internal threedimensional (3D) structures, researchers mainly utilize conventional or modified methods, such as serial histological sectioning (Ro?ková and Ro?ek, 2005) and gross dissection (Zhanget al., 2016). For example, the bone-cartilage double-staining technique has been widely used in comparative skeletal anatomy studies of small vertebrates since the last century (Hanken and Wassersug,1981; Simons and Van Horn, 1971; Wassersug, 1976;Williams, 1941), but these methods are typically timeconsuming and destructive to the specimens under examination. Recently, non-invasive visualization methods have come to the forefront including X-ray microcomputed tomography (micro-CT), which can overcome these weaknesses and visualize the internal anatomy and structural complexity of organisms in the micrometer (μm) or nanometer (nm) ranges by relying on differences in the photon attenuation levels of these tissue types (Broeckhovenet al., 2017; Chaoet al.,2005; du Plessiset al., 2017; Ritman, 2004, 2011). This approach has been applied in biomedical studies, such as investigations of the skeleton, organs and vascular tree of live mammals, to obtain information on the status or progression of disease (Campbell and Sophocleous,2014; Ritman, 2004). Micro-CT also provides detailed anatomical interpretations to inform developmental,systematic, and functional morphological research in invertebrates and vertebrates (Gignacet al., 2016; Porro and Richards, 2017; Scherreret al., 2017). Applications include species identification (Boistelet al., 2011;Faulwetteret al., 2013; Paraparet al., 2017; Scherzet al.,2014) and exploring ecological or evolutionary questions in certain taxa (Broeckhoven and du Plessis, 2017). In herpetology, this tool has been widely used to conduct research in adult amphibians and reptiles (Chenet al.,2012; Fortunyet al., 2015; Kimet al., 2017; Lauridsenet al., 2011; Scherzet al., 2015; Vasquezet al., 2008),but non-invasive micro-CT has not yet been integrated into studies of the developmental biology and osteology of tadpoles. Therefore, we report the use of micro-CT for examining the structure of tadpole vertebrae with the hope of making this modern tool more accessible to the broadest range of morphological researchers across the widest range of fields.
The aims of this study are to 1) investigate the feasibility of micro-CT to examine the structure of tadpole vertebraein vivo, 2) compare the merits and defects of micro-CT with those of conventional methods(bone-cartilage double-staining) in the study of tadpole vertebrae, 3) determine the effects of scanning parameters on image quality, and 4) recommend guidelines for the use of micro-CT in the anatomical study of tadpole vertebrae.
2. Materials and Methods
2.1. Sample preparationWe used 53 tadpole specimens representing three anuran amphibian species from three genera of Megophryidae (Table S1) that were preserved at the Herpetological Museum of the Chengdu Institute of Biology (CIB), CAS. Specimens were first fixed in 10%formalin and then transferred to neutral buffered 10%formalin (20 individuals) or 70% ethanol (33 individuals)prior to scanning (Table S1). We staged the tadpoles based on the approach of Gosner (1960).
2.2. Micro-CT scanningA Quantum GX micro-CT Imaging System (PerkinElmer Health Sciences, USA)was used to acquire high-resolution 3D images of tadpole vertebral structure, located at the Chengdu Institute of Biology (Chengdu, China); this imaging system uses a cone beam X-ray source and a flat-panel X-ray detector to produce high-resolution 3D images of bone structures and the surrounding soft tissues (www.PerkinElmer.com). To prevent the specimens from drying during scanning, all samples were transferred into 2-ml polypropylene pipette tips (tube’s choosing was based on the tadpole size) with 10% formalin or 70% ethanol and then fastened to the sample bed for scanning. In the high-resolution mode,we chose a 72-mm acquisition field of view (FOV) and a 45-mm reconstruction FOV, which allowed for a 9-μm voxel size resolution under a small region (subvolume)reconstruction (www.PerkinElmer.com). When we compared the results of the two methods (micro-CT scan and double-staining), scanning was conducted for 4 min at a voltage of 70 kV and a current of 80 μA, which produced 458 projection images for every specimen.To detect the effects of scanning parameters (including voltage, current and scan time), we set different levels for each parameter according to the instrument design.Voltage levels were set at 30 kV, 50 kV, 70 kV or 90 kV;current levels were set at 20 μA, 40 μA, 60 μA or 80 μA;and scan times were set at 8 s, 18 s, 2 min, 4 min, 14 min and 57 min. Skeletal images were reconstructed using these projection images under the Quantum GX micro-CT Imaging System, and surface meshes of the skeleton were produced by regulating the threshold in the volume rendering control panel, which controlled voxel intensity in the 3D reconstruction. The images were exported in BMP (1024 × 1024 pixels) and AVI formats.
2.3. Bone-cartilage double-stainingPost-scanning, 32 of the 53 scanned specimens (Table S1) were eviscerated and then cleared and stained with alcian blue and alizarin red following the protocol of Hanken and Wassersug(1981).
2.4. Statistical analysisThe data set was tested for normality prior to analysis, and the Wilcoxon Signed-Rank test was applied to test for inter-method variations(micro-CT and bone-cartilage double-staining) in the determination of the number of tadpole vertebrae.Statistical tests were performed using R software 3.4.2 (R Development Core Team, 2017).
3. Results
3.1. Comparison between micro-CT and bone-cartilage double-stainingThe results showed that the two methods(micro-CT and bone-cartilage double-staining) could both clearly display the tadpole vertebrae in the sampled species and that there were no significant differences in the detected number of vertebrae of the tadpoles of the three species (Table 1, Figure 1). The bones were stained purplish red and the cartilage was stained dark blue (Figure 1A, 1C, 1E, 1G, 1I, 1K, 1M, 1O, 1Q and 1S), and after staining, more than half of the tadpoles were bent. Additionally, the bone staining was darker with advancement in developmental stage, especially in the bones (Figure 1 from K to S). The micro-CT could both visualize the bone and discriminate the incompletely ossified cartilage from other tissues (Figure 1 from B to J and from L to T), and the specimens remained in their original positions after scanning. However, the micro-CT seemed unable to distinguish the cartilage located in the head or arthrosis (Figure 1I, 1J, 1S and 1T).
The quality of the CT image differed with the developmental stages of the tadpoles, with the rendering quality of the vertebrae improving with development stage. For example, the images of the vertebrae of the tadpoles at stage 40 were more complete and clearer than those at stage 27 (Figure 1B and 1J). Nevertheless, the results of the double-staining technique showed little association with developmental stage (Figure 1A, 1C and 1I).
We also found that the degree of ossification of vertebrae varied with development stages within species and differed in species at the same stage. Normally, the later development stage is always with the higher degree of ossification within the species. That is, the bony staining color is redder in the higher degree of ossification of vertebrae in this study (Figure 1 from K to S).However, this trend seemed to be not true among species.For example, the degree of ossification of vertebrae inX.sangzhiensiswas higher than that inB. carinensisat the same developmental stage (Figure 1C and 1M), and even the degree of ossification of vertebrae inX. sangzhiensisat an early development stage was higher than that ofB.carinensisat a later development stage (Figure 1C and 1K). Similarly, the number of vertebrae changed with developmental stages. In general, the number of vertebrae increased first and then decreased within species (Figure 1 from L to K). However, there was a large variation among species, for instance, the number of vertebrae ofX. sangzhiensiswas more than that ofB. carinensisat the same developmental stage (Figure 1D and 1N).
In addition, it was easy to obtain clear 3D images of a tadpole skeleton using micro-CT, such as the three directional views of the vertebrae of theX. sangzhiensistadpole and to acquire detailed information about the vertebrae without destroying the specimens (Figure 2).
3.2. Factors affecting image qualityWe found that the voltage, current and scan time affected image quality.Generally, the image quality increased with increasing voltage (Figure 3A). For instance, at 30 kV, the micro-CT could not distinguish the vertebrae from polypropylene pipette tips (Figure 3, AI and A1), but at 70 kV, the vertebrae of the tadpole were clearly displayed. At 90 kV,the details of the vertebrae could be observed, but it was not possible to differentiate the vertebrae from ethanol(the CT values of the two objects were similar) (Figure 3,AIV and A4). Similar results were observed at different currents, but the impact of the current was less than that of the voltage setting. The image quality was similar under different currents when voltage and scan time were consistent (Figure 3B). Additionally, the image quality increased with scan time (from 8 s to 4 min), although the trend seemingly declined at 14 min (Figure 4IV).As shown in the results, the red arrows indicate that the boundaries of the vertebrae were more clearly displayed with increasing scan time (Figure 4 from 1 to 4), but the image quality did not be obviously improved after scanning for 14 min or longer (Figure 4V, 4VI, 4-5, 4-6).
But beyond that, a stark difference in image quality was revealed between the two preservation methods(70% ethanol or 10% formalin); the image was sharper when the specimens were preserved in 70% ethanol(Figure 5). Furthermore, micro-CT could hardly discern the skulls of tadpoles preserved in formalin, suggesting that preservation has an important effect on scan image quality.
4. Discussion
The tadpole skeleton consists of cartilage and bone,but the cartilage accounts for a larger portion during metamorphosis (McDiarmid and Altig, 1999). During this stage, the vertebrae are primarily composed of cartilage with little or no calcium, most of which cannot be stained by alizarin red, so alcian blue or other dyes (such as methylene blue and toluidine blue) have been applied in the double-staining procedure to reveal cartilage in the last several decades (Depew, 2014; Dingerkus and Uhler, 1977; Dinggerkus, 1981; Hanken and Wassersug,1981; Kelly and Bryden, 1983; Redfernet al., 2007;Wassersug, 1976; Yamada, 1991). However, this most popular and traditional method is destructive and can distort specimens. Our results show that micro-CT can discern the bone and cartilage from other soft tissues and can produce a 3D image of the vertebrae, although it cannot directly distinguish bone from cartilage without the help of contrast agents. Moreover, it is non-invasive and can allow researchers to reuse specimens for different research purposes, which is especially important for rare species. Obviously, the method provides an alternative approach to study tadpole vertebrae.
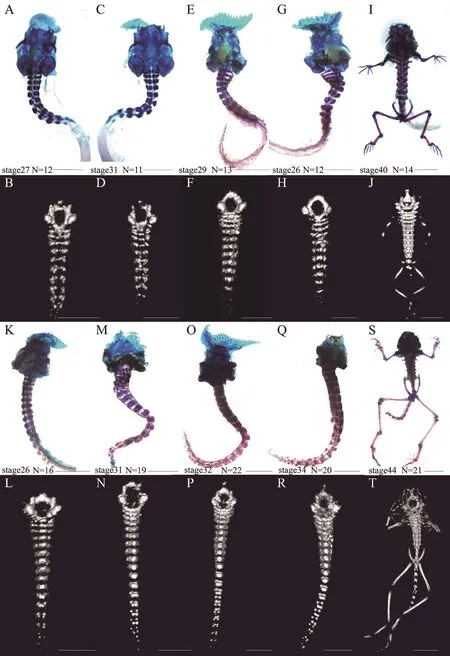
Figure 1 Comparison of the two methods for displaying tadpole vertebrae. The white backgrounds are the results of bone-cartilage doublestaining (upper), and the black backgrounds are the corresponding micro-CT results (lower) for the same specimens. Specimen cartilage was stained dark blue, and bone stained purplish red. All images present ventral views of the tadpoles. N represents the number of vertebrae. A, B,C, D, I and J: the tadpoles of B. carinensis; E , F, G and H: the tadpoles of A. shapingensis; and from K to T: the tadpoles of X. sangzhiensis.Scale bar: 5 mm.
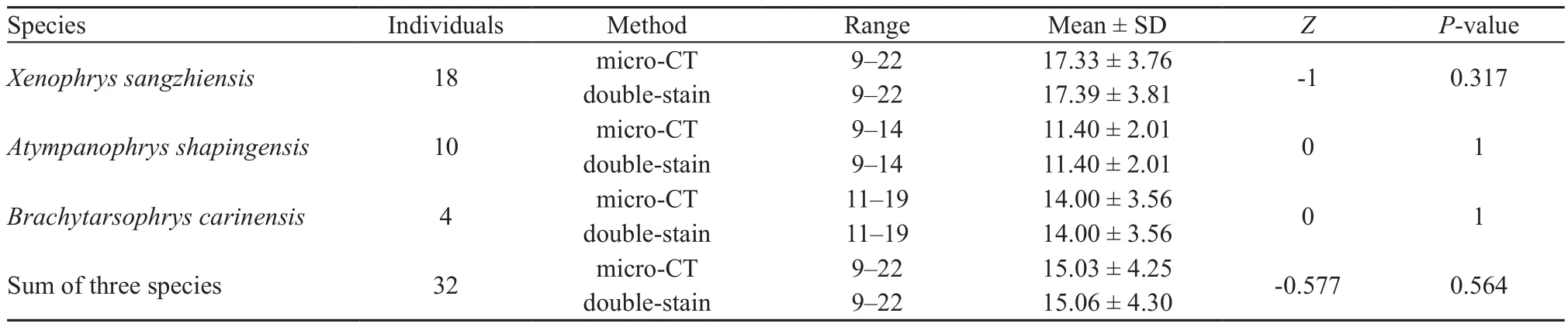
Table 1 Comparison of the two methods for examining the number of vertebrae in larval megophryids (Wilcoxon Signed-Rank test).

Figure 2 Micro-CT representations of the skeletal anatomy of the X. sangzhiensis tadpole (stage 35). A and B: dorsal view of tadpole vertebrae. C and D: ventral view of tadpole vertebrae. E and F: lateral view of tadpole vertebrae. Left-side scale bar: 5 mm. Right-side scale bar: 2 mm.
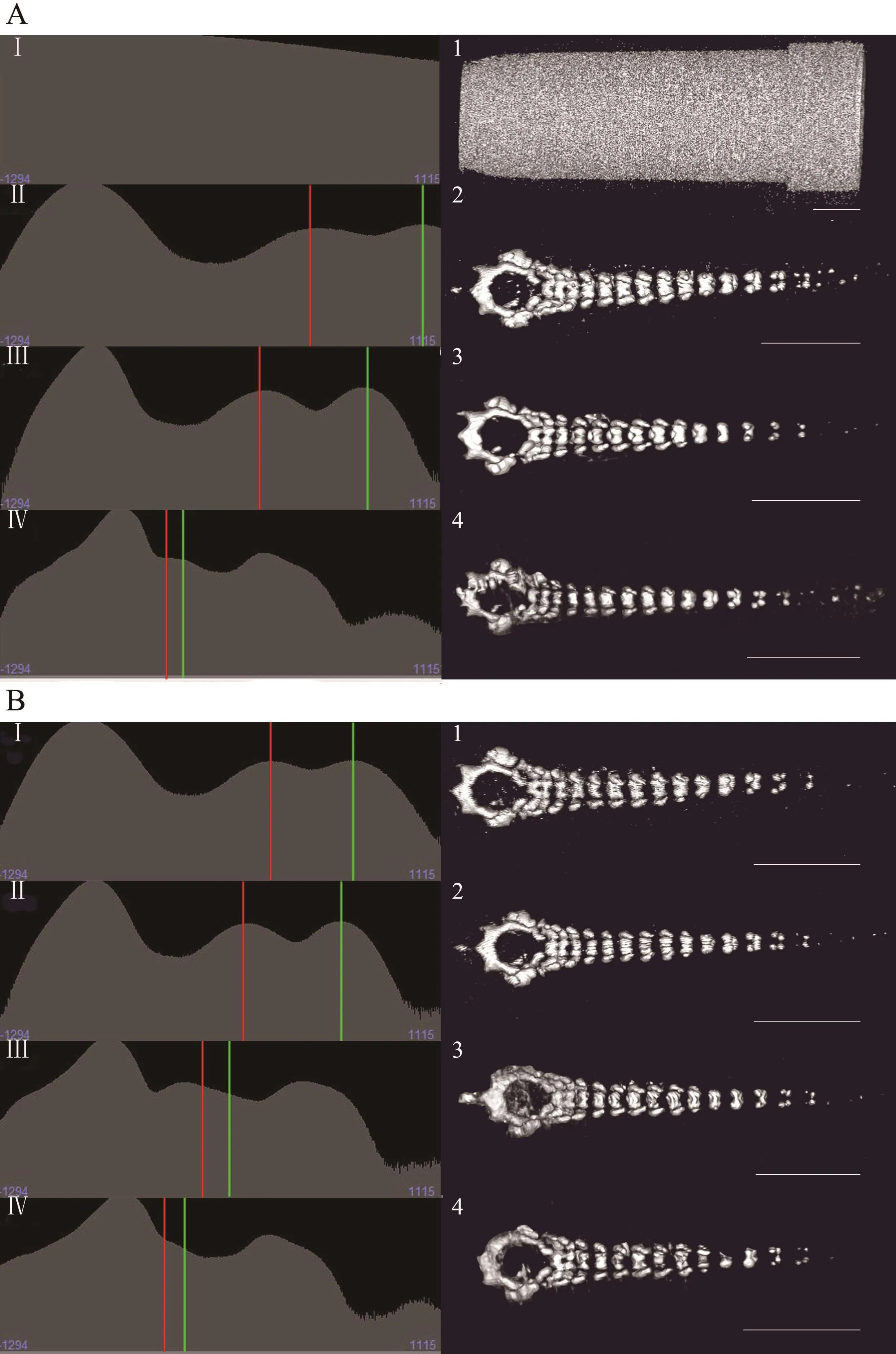
Figure 3 Renderings of the X. sangzhiensis tadpole (stage 34) vertebrae under different scanning voltages or currents. Left histograms(from I to IV) for each parameter show the distributions of voxel values on a relative linear scale. The X-axis represents the CT value (or voxel color table and opacity), and the Y-axis represents the voxel intensity. The ethanol and soft tissues background peak is marked by a solid vertical red line, and the mean of the object voxel distribution is marked by a solid vertical green line. The other peaks represent the voxel distributions of other objects, such as the peak to the left, which represents pore space and air. Images on the right (from 1 to 4) are the scanning results for each parameter. A) The effects of voltage on the scan image. From top to bottom, the respective parameters are 30 kV-88 μA-4 min, 50 kV-88 μA-4 min, 70 kV-88 μA-4 min and 90 kV-88 μA-4 min. B) The effects of current on scan image. From 1-4, the respective parameters are 90 kV-20 μA-4 min, 90 kV-40 μA-4 min, 90 kV-60 μA-4 min and 90 kV-80 μA-4 min. Scale bar: 5 mm.
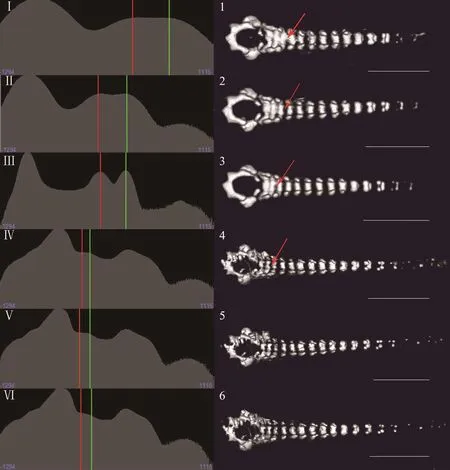
Figure 4 Renderings of the X. sangzhiensis tadpole (stage 34) vertebrae with different scan times. Left histogram (from I to VI) for each parameter shows the distribution of pixel grayscale values on a relative linear scale. The X-axis represents the CT value (or voxel color table and opacity), and the Y-axis represents the voxel intensity. The ethanol and soft tissues background peak is marked by a solid vertical red line, and the mean of the object voxel distribution is marked by a solid vertical green line. The other peaks represent the voxel distributions of other objects, such as the peak on the left, which represents pore space and air. The images on the right are the scanning results for each parameter. The red arrows indicate the boundary between the two vertebrae. From top to bottom, the respective parameters were 90 kV-88 μA-8 s, 90 kV-88 μA-18 s, 90 kV-88 μA-2 min, 90 kV-88 μA-4 min, 90 kV-88 μA-14 min and 90 kV-88 μA-57 min. Scale bar: 5 mm.
In this study, micro-CT seemingly could not render cartilage located in the head and appendages, and we speculate that the densities of these cartilages are much less than those of vertebrae due to the lack of calcium.In particular, articular cartilage is mainly composed of proteoglycans, collagens and chondrocytes (Karhulaet al., 2017), whose densities are similar to those of other soft tissues, so micro-CT cannot distinguish them. In addition, the main skeleton of the head in a tadpole is chondrocranium which is a cartilaginous case that protects the brain and supports the sense and jaw apparatus (Cannatella, 1999). Based on the results of both double-staining and micro-CT, the skeleton of the head showed a later ossification time than the vertebrae.In fact, the head has not completely ossified at the end of metamorphosis climax. And, the first sign of appendicular skeletal development usually appears after stage 37 in megophryids (Handriganet al., 2007). Thus, it is more effective to study the vertebrae than other parts of the skeleton by micro-CT in megophryid tadpole.Furthermore, the scan image quality increases with developmental stage, mainly due to the different degrees of vertebral calcification. Scherzet al. (2015) reported that micro-CT scanning can nicely render highly calcified structures, especially bone, because tadpoles have relatively higher ossification levels at later developmental stages. So, micro-CT scanning is more suitable for tadpoles at later stages.
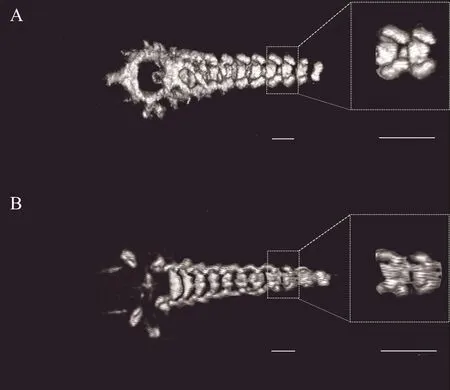
Figure 5 Scanned images of A. shapingensis tadpoles (stage 37) stored in 70% ethanol (A) and in 10% formalin (B). Scale bar: 2 mm.
Indeed, the non-mineralized structures (such as soft tissues) in small vertebrates or invertebrates can also be visualized by micro-CT with the help of contrast agents(Descampset al., 2014; Gignacet al., 2016; Metscher,2009a), such as PTA (1% (w/v) phosphotungstic acid in water) and IKI (1% iodine metal (I2) + 2% potassium iodide (KI) in water); Some soft tissues ofXenopus laevistadpoles have been successfully studied using micro-CT in combination with a contrast agent (PTA) (Descampset al., 2014; Metscher, 2009b). Thus, we can also combine with the contrast agent to explore the anatomy and osteology of tadpole when conducting CT scan.
The degree of ossification of vertebrae has drastic variation among species at the same developmental stage. As shown in this study, the degree of ossification ofX. sangzhiensisat the same or an earlier stage was higher than that inB. carinensis. Meanwhile, the number of vertebrae in the former is much more than that in the latter. This difference may be mainly related to the heterochronous arrest of bony development. Trueb(1973) reported that heterochrony is operational in the maintenance of osteological differences between the sexes in hylids. E. M. T. Stephenson (1960) and N. G.Stephenson (1965) also argued that the heterochronous changes would result in some types of osteological differences among closely related species. Thus, we speculate that the degree of ossification of vertebrae varied with species also resulted from the heterochrony of bony development. Furthermore, axial and appendicular skeletal development usually starts in quick succession and then proceeds together in anurans (Maglia 2003).However, Handriganet al. (2007) found that much of vertebral column development occurred before the onset of ossification in the limbs in megophryids. We also revealed a similar phenomenon that the degree of ossification of vertebrae is different between species at the same developmental stage (or the same development level of the external limb). So, the Gosner staging table that is based primarily on limb development is not always an appropriate standard for all species as megophryid tadpole (Handriganet al., 2007).
For tadpole scanning, the voltage played a key role among the scanning parameters, but it is inadvisable to choose an overly high or low voltage since these scanning parameters can increase the overlapping intensities between the void and solid phases, which will reduce the degree of differentiation. Similar results were observed when comparing different currents or scan time, so we recommend moderate scanning parameters(such as 70 kV-80 μA-4 min) when scanning tadpoles.In addition, micro-CT performed poorly when using specimens stored in formalin preservative because formalin can decalcify the skeleton, especially bone(Heyeret al., 1994), diminishing the contrast between the skeleton and soft tissues. Therefore, we suggest that specimens to scan should be preserved in 70% ethanol,or formalin preservative should be kept neutral to reduce decalcification.
Bone-cartilage double-staining is a critical tool for evolutionary and developmental biologists to evaluate the ontogeny of the skeleton (Depew, 2009) because it can clearly distinguish differences between bone and cartilage. However, this process is time-consuming and complex, requires specialized chemicals, and is ultimately destructive to the specimens, preventing future uses(Hanken and Wassersug, 1981; Simons and Van Horn,1971; Wassersug, 1976; Williams, 1941). These issues are especially impactful for rare specimens that must be utilized for a variety of studies, but micro-CT can avoid these drawbacks due to its non-invasive nature. First, we can dissect tadpole vertebraein vivowithout damaging the samples, which is very important for preserving rare specimens. Second, it is convenient and efficient to scan a large number of samples. Furthermore, we can reconstruct a particular structure or slice(s) at a higher resolution(du Plessiset al., 2017). It is also possible to repeatedly change the scanning parameters until a satisfactory image is obtained, and the multiple output files (including video format) from micro-CT can be viewed using different software.
5. Conclusion
This study demonstrated that micro-CT is a rapid, noninvasive, reliable and efficient method for studying the vertebrae of tadpoles and can increase specimen utilization. Correspondingly, it also provides an alternative approach to study vertebrae in tadpole biology.Ethanol preservative and moderate scanning parameters are recommended in tadpole scan. Furthermore, we suggest that micro-CT, alone or in combination with bone-cartilage double-staining, be more widely applied in herpetological research to promote the development of the field.
Acknowledgements The project is supported by the National Key Program of Research and Development,Ministry of Science and Technology (No. 2017YFC05 05202 granted to Jianping JIANG) and the National Natural Science Foundation of China (No. 31172055 granted to Cheng LI and No. 31172174 granted to Feng XIE). We are grateful to the Herpetological Museum of the Chengdu Institute of Biology for facilitating our examination of the specimens and to Nicholas C. WU for proof reading the manuscript.
Boistel R., Swoger J., Kr?i? U., Fernandez V., Gillet B., Reynaud E. G. 2011. The future of three-dimensional microscopic imaging in marine biology. Mar Biol,32(4): 438–452
Broeckhoven C., Plessis A., Roux S. G., Mouton P. L. F. N., Hui C. 2017. Beauty is more than skin deep: A non-invasive protocol for in vivo anatomical study using micro-CT. Methods Ecol Evol, 8(3): 358–369
Campbell G. M., Sophocleous A. 2014. Quantitative analysis of bone and soft tissue by micro-computed tomography:Applications toex vivoandin vivostudies. Bonekey Rep, 3: 564
Cannatella D. 1999. Architecture: Cranial and axial musculoskeleton. In McDiarmid R. W., Altig R. (Eds.), Tadpoles:the biology of anuran larvae. Chicago, USA: University of Chicago Press, 52–81
Chao W., Harteneck B. D., Liddle J. A., Anderson E. H.,Attwood D. T. 2005. Soft X-ray microscopy at a spatial resolution better than 15 nm. Nature, 435(7046): 1210–1213
Chen Y., Lin G., Chen Y., Fok A., Slack J. M. 2012. Microcomputed tomography for visualizing limb skeletal regeneration in youngXenopusfrogs. Anat Rec, 295(10): 1562–1565
Depew M. J. 2009. Analysis of skeletal ontogenesis through differential staining of bone and cartilage. In Westendorf (Eds.),Molecular Embryology:Methods and Protocols. Totowa, USA:Humana Press. 37–4
Descamps E., Buytaert J., De Kegel B., Dirckx J., Adriaens D.2012. A qualitative comparison of 3D visualization inXenopus laevisusing a traditional method and a non-destructive method.Belg J Zool, 142(2): 99–111
Dingerkus G., Uhler L. D. 1977. Enzyme clearing of Alcian blue stained whole small vertebrates for demonstration of cartilage.Stain Technol, 52: 229–232
Dingerkus G. 1981. The use of various alcohols for Alcian blue in toto staining of cartilage. Stain Technol, 56: 128–129
Dodd M. H. I., Dodd J. M. 1976. The biology of metamorphosis.Physiol Amphibia, 3: 467–599
Du Plessis A., Broeckhoven C., Guelpa A., Le Roux S. G. 2017.Laboratory X-ray micro-computed tomography: A user guideline for biological samples. GigaScience, 6(6): 1–11
Faulwetter S., Vasileiadou A., Kouratoras M., Dailianis T.,Arvanitidis C. 2013. Micro-computed tomography: Introducing new dimensions to taxonomy. ZooKeys, 263: 1
Fortuny J., Marcé-Nogué J., Heiss E., Sanchez M., Gil L.,Galobart à. 2015. 3D bite modeling and feeding mechanics of the largest living amphibian, the Chinese giant salamanderAndrias davidianus(Amphibia: Urodela). PLoS One, 10(4):e0121885
Gignac P. M., Kley N. J., Clarke J. A., Colbert M. W., Morhardt A. C., Cerio D., Cost I. N., Cox P. G., Daza J. D., Early C.M., Echols M. S., Henkelman R. M., Herdina A. N., Holliday C. M., Li Z., Mahlow K., Merchant S., Müller J., Orsbon C.P., Paluh D. J., Thies M. L., Tsai H. P., Echols M. S. 2016.Diffusible iodine based contrast enhanced computed tomography(diceCT): An emerging tool for rapid, high resolution, 3D imaging of metazoan soft tissues. J Anat, 228(6): 889–909
Gosner K. L. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica, 16(3):183–190
Handrigan G. R., Haas A., Wassersug R. J. 2007. Bony-tailed tadpoles: The development of supernumerary caudal vertebrae in larval megophryids (Anura). Evol Dev, 9(2): 190–202
Hanken J., Wassersug R. 1981. The visible skeleton. Funct Photo,16(4): 22–26
Heyer R., Donnelly M. A., Foster M., Mcdiarmid R. 1994.Measuring and monitoring biological diversity: Standard methods for amphibians. Washington, USA: Smithsonian Institution Press. 289–297
Karhula S. S., Finnil? M. A., Lammi M. J., Yl?rinne J. H.,Kauppinen S., Rieppo L., Pritzker K. P. H., Nieminen H. J.,Saarakkala S. 2017. Effects of articular cartilage constituents on phosphotungstic acid enhanced micro-computed tomography.PLoS One, 12(1): e0171075
Kelly W. L., Bryden M. M. 1983. A modified differential stain for cartilage and bone in whole mount preparations of mammalian fetuses and small vertebrates. Stain Technol, 58:131–134
Kim E., Sung H., Lee D., Kim G., Nam D., Kim E. 2017.Nondestructive skeletal imaging ofHyla suweonensisusing Micro-computed tomography. Asian Herpetol Res, 88(4): 235–243
Lauridsen H., Hansen K., Wang T., Agger P., Andersen J. L.,Knudsen P. S., Maglia, A. M. 2003. Skeletal development ofPelobates cultripesand comparisons of the osteology of pelobatoid frogs. Sci Pap Univ Kansas Nat Hist Mus, 30: 1–13
McDiarmid R. W., Altig R. 1999. Tadpoles: the biology of anuran larvae. Chicago, USA: University of Chicago Press. 52–90
Metscher B. D. 2009a. MicroCT for developmental biology:A versatile tool for high-contrast 3D imaging at histological resolutions. Dev Dyn, 238(3): 632–640
Metscher B. D. 2009b. MicroCT for comparative morphology:simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physio, 9(1): 11
Mizutani R., Suzuki Y. 2012. X-ray microtomography in biology.Micron, 43(2): 104–115
Parapar J., Candás M., Cunha-Veira X., Moreira J. 2017.Exploring annelid anatomy using micro-computed tomography:A taxonomic approach. Zool Anz, 270: 19–42
Porro L. B., Richards C. T. 2017, Digital dissection of the model organismXenopus laevisusing contrast-enhanced computed tomography. J Anat, 231: 169–191
Rasmussen A. S., Uhrenholt L., Pedersen M. 2011. Inside out:modern imaging techniques to reveal animal anatomy. PLoS One, 6(3): e17879
Redfern B. G., David W. L., Spence S. 2007. An alternative Alcian blue dye variant for the evaluation of fetal cartilage. Birth Defects Res B,80(3): 171–176
Ritman E. L. 2004. Micro-computed tomography–current status and developments. Annu Rev Biomed Eng, 6: 185–208
Ritman E. L. 2011. Current status of developments and applications of micro-CT. Annu Rev Biomed Eng, 13: 531–552
Ro?ková H., Ro?ek Z. 2005. Development of the pelvis and posterior part of the vertebral column in the Anura. J Anat,206(1): 17–35
R Development Core Team. 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: http://www.R-project.org (accessed 28 September 2017).
Scherrer R., Hurtado A., Machado E. G., Debiais-Thibaud M. 2017. MicroCT survey of larval skeletal mineralization in the Cuban garAtractosteus tristoechus(Actinopterygii;Lepisosteiformes). MorphoMuseuM, 3(3):e3
Scherz M. D., Ruthensteiner B., Vences M., Glaw F. 2014. A new microhylid frog, genusRhombophryne, from northeastern Madagascar, and a re-description ofR. serratopalpebrosausing micro-computed tomography. Zootaxa, 3860(6): 547–560
Scherz M. D., Ruthensteiner B., Vieites D. R., Vences M., Glaw F. 2015. Two new microhylid frogs of the genusRhombophrynewith superciliary spines from the Tsaratanana Massif in northern Madagascar. Herpetologica, 71(4): 310–321
Sephenson M. T. 1960. The skeletal characters of Leiopelma harniltoni McCulloch, with particular reference to the effects of heterochrony on the genus. Trans Roy Soc, 88 (3): 473–488
Sephenson N. G. 1965. Heterochronous changes among Australian leptodactylid frogs. Proc Zod Soc, 144 (3): 339–350
Simons E. V., Van Horn J. R. 1971. A new procedure for wholemount Alcian blue staining of the cartilaginous skeleton of chicken embryos, adapted to the clearing procedure in potassium hydroxide. Acta Morphol Neerl-Scand, 8: 281–292
Trueb L. 1973. Bones, frogs, and evolution. In Vial J. L. (Eds.)Evolutionary biology of anurans. Columbia: University of Missouri Press, 65–132
Vasquez S. X., Hansen M. S., Bahadur A. N., Hockin M. F.,Kindlmann G. L., Nevell L., Isabel Q. Wu., David J. G.,David M. W., Greg M. J., Christopher R. J., Johnl L. V.,Mario R. C., Johnson C. R. 2008. Optimization of volumetric computed tomography for skeletal analysis of model genetic organisms. Anat Rec. 291(5): 475–487
Wassersug R. J. 1976. A procedure for differential staining of cartilage and bone in whole formalin-fixed vertebrates. Stain Technol, 51(2): 131–134
Williams T. W. 1941. Alizarin red S and toluidine blue for differentiating adult or embryonic bone and cartilage. Stain Technol, 16: 23–25
Yamada T. 1991. Selective staining methods for cartilage of rat fetal specimens previously treated with alizarin red S.Teratology, 43(6): 615–619
Zhang M., Chen X., Chen X. 2016. Osteology ofQuasipaa robertingeri(Anura: Dicroglossidae). Asian Herpetol Res, 7(4):242–250
 Asian Herpetological Research2018年2期
Asian Herpetological Research2018年2期
- Asian Herpetological Research的其它文章
- Effects of Increased Salinity on Growth, Development and Survival in Early Life Stages of the Green Toad Bufotes variabilis (Anura:Bufonidae)
- Sexual Dimorphism, Female Reproductive Characteristics and Egg Incubation in an Oviparous Forest Skink (Sphenomorphus incognitus) from South China
- Amphibian Species Contribute Similarly to Taxonomic, but not Functional and Phylogenetic Diversity: Inferences from Amphibian Biodiversity on Emei Mountain
- Three New Ranidae Mitogenomes and the Evolution of Mitochondrial Gene Rearrangements among Ranidae Species
- A New Species of Gracixalus (Anura: Rhacophoridae) from West Guangxi, China
- A New Species of the Genus Sinomicrurus Slowinski, Boundy and Lawson, 2001 (Squamata: Elapidae) from Hainan Province, China
