Genome-wide association study of heat stresstolerance traits in spring-type Brassica napus L.under controlled conditions
Miznur Rhmn, Sujn Mmidi , Mukhlesur Rhmn,*
a Department of Plant Sciences, North Dakota State University, Fargo, ND 58102, USA
b HudsonAlpha Institute for Biotechnology, Huntsville, AL 35806, USA
Canola(Brassica napus,AACC,2n=4x=38)is an amphidiploid species of Brassica derived from two diploid species,Brassica rapa L.(2n=20,AA)and Brassica oleracea L.(2n=18,CC)[1].It is the second largest oil-producing crop in the world after soybean(Foreign Agricultural Service,USDA,October 2015,https://www.ers.usda.gov/topics/crops/soybeans-oil-crops/canola/).Canola is cultivated as a major oilseed crop in Canada,Europe,China,Australia,USA,and the Indian subcontinent.North Dakota is the largest canola-producing state,with about 84%of U.S.canola production,and annually contributes about U.S.$384 million to the national economy(five-year average from 2011 to 2015;USDA-NASS 2016,https://quickstats.nass.usda.gov/).
It is predicted[2]from various climate models that the global mean temperature will increase by 1–4 °C by the end of the 21st century.The increasing temperature will create an adverse environment that impairs agriculture and crop production[3].Abiotic stress changes the morphological,physiological,biochemical,and molecular properties of plants.Crop growth at flowering stage is highly sensitive to heat stress[4,5],and causes flower abortion,pollen sterility,and reduced pod development and seed set and reduces the assimilatory capacity and productivity of crops[6,7].Certain genotypes are more tolerant to heat stress and the tolerance is controlled by multiple genes[8,9].
Canola is strongly affected by heat stress.Temperatures of 15–20 °C are optimal for growth and development of canola.High temperatures(over 27°C)cause pollen sterility,pod abortion,and yield loss ofthe crop[10–15].Ithas been estimated[15]that every 1°C temperature increases from the range optimal for growth and development during pod setting of canola causes a 10%yield reduction.Heat stress during the pre-anthesis stage reduces pollen fertility, whereas post-anthesis heatdecreases the female fertility of B.juncea[16].
Heat-stress tolerance in plants is a complex phenomenon involving numerous biochemical and metabolic activities such as antioxidant activity,membrane lipid unsaturation,gene expression and translation,protein stability,and accumulation of compatible solutes[17].Heat stress negatively affects the developmental and physiological processes,reproduction,and adaptation of plants[18].Heat stress tolerance is a polygenic trait that makes it difficult to introgress multiple favorable alleles into cultivars[19,20].
Canola germplasm shows comprehensive linkage disequilibrium owing to its limited geographic range and intensive breeding[21].Genome-wide association study(GWAS)is a powerful tool for characterizing the genetic architecture of traits and identifying multiple candidate genes associated with the traits in many crop species[22–24].It is based on historical recombination events and genome scanning with high-density DNA markers to locate genetic loci associated with traits of interest at a relatively high level of resolution[25,26].An association mapping study was performed to identify genomic regions and quantitative trait loci(QTL)controlling three traits affected by heat stress in a collection of spring-type accessions that have practical applications to genotype selection and generation advance.
2.Materials and methods
2.1.Plant materials and phenotyping
Eighty-eight accessions of spring-type B.napus from the USDA-ARS Germplasm Resources Information Network(GRIN)(Table S1)were used in this study.These genotypes are a subset of 104 spring genotypes that are available in the diverse GRIN collection.Spring-type canola generally flowers in 40–60 days.The experiment was conducted in a greenhouse and a plant growth chamber at North Dakota State University(NDSU),Fargo,ND,USA during 2014–2015.Two sets of experiments(set 1 and set 2)were conducted using a randomized complete block design(RCBD)with three replications per set.The set 1 experiment(control)was conducted in the greenhouse at 22/18°C(day/night)temperature until desiccation.Plants in the greenhouse were grown with a 16-h photoperiod provided by natural sunlight supplemented with 400 W HPS PL 2000 lamps(P.L.Light Systems Inc.,Beamsville,Ontario,Canada).The plants in set 2 were grown in the same greenhouse and subjected for five days to heat stress in the growth chamber starting at the sixth day after flower initiation.All plants did not flower on the same day.Only the plants at the 6-day flowering stage were transferred into the plant growth chamber.The artificial heat simulation setup in the plant growth chamber was similar to that used in earlier experiments[11–13,27,28]with slight modifications.The heat-stress simulation condition,based on North Dakota weather,included a temperature of 18°C for 8 h from 22:00 to 6:00 followed by temperature ramping from 18 °C to 35 °C over 6 h from 6:00 to 12:00,maintenance at 35°C for 4 h from 12:00 to 16:00,and ramping down from 35 °C to 18 °C over 6 h from 16:00 to 22:00.The relative humidity in the growth chamber was maintained at 70%and light was provided for 14 h every day.The plants in the growth chamber were watered twice daily at 300 mL plant?1per application.After the heat treatment,the plants were returned to the original greenhouse room at 22/18°C day/night temperature and grown until desiccation.Flowering buds were tagged before and after heat stress to characterize pod development during the period of heat stress.Flower buds were collected from the heat-stressed plants on the third day of heat treatment and buds were collected from the control plants at the same time.Pollen sterility,sterile/aborted pods on main raceme,and number of pods on main raceme were recorded for all plants in set 1(control)and set 2(heat stress treatment).
2.2.Pollen sterility study
A pollen sterility study was conducted in the laboratory.Flower buds prior to opening were collected from the greenhouse and growth chamber.Ten buds per replication from each accession were collected in an Eppendorf tube containing water and placed on ice during bud collection.The water in the Eppendorf tube was replaced with 30%acetic acid solution(70%ethanol+30%acetic acid)and the tubes were refrigerated.The preserved buds were opened with forceps on precleaned microslides (75.00 mm×25.00 mm×1.00 mm thick)and 2–3 anthers were macerated and pressed with a scalpel on each slide with 1–2 drops of 1%acetocarmine solution.The slide was heated over a spirit lamp for 5–10 s to fix the acetocarmine dye in the pollen grain.Anther debris was removed with a needle and a cover slip was placed on the slide to prevent the entry of air bubbles between the slide and cover slip.Sterile(un-or lightly stained)and viable(stained)pollen were counted under an optical microscope(N-400M,110–115 V ~60/60 Hz;0.4 A,halogen lamp 60 V 20 W,AmScope,Irvine,CA,USA).One hundred randomly selected pollen grains per slide were counted and the percentages of fertile and sterile pollen grains were recorded(Fig.1).
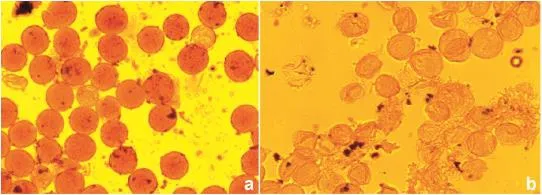
Fig.1–Pollen sterility of canola accession.Legend before(a)and after(b)heat stress.
2.3.Heat susceptibility index(HSI)
Heat stress for each trait was calculated using the equation suggested by Fischer and Maurer[29].
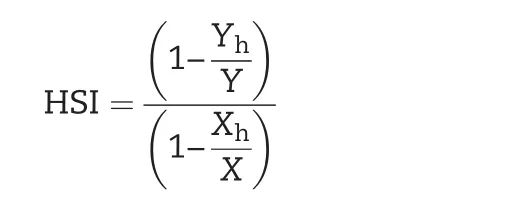
where Yhand Y are the phenotypic means of each genotype under heat stress and controlled conditions,respectively,and Xhand X are the phenotypic means for all lines under heat stress and controlled conditions,respectively.If the phenotypic mean for a genotype was zero,the value was recorded as 0.001 to avoid division by zero.
2.4.DNA extraction and genotyping
DNA was extracted using a Qiagen DNeasy kit(Qiagen,CA,USA).Genotyping was performed at the Institute of Genome Diversity(IGD),Cornell University,Ithaca,NY,USA for genotype-by-sequencing(GBS).The 88 genotypes used in the study are a subset of a core collection of the B.napus lines used for diversity study(unpublished data).Briefly,the quality-cleaned GBS data were aligned with canola reference genome[30]using bwa-mem[31].Multisample SNP calling was performed using VarScan[32].Missing alleles of SNPs were imputed using FastPHASE 1.3[33]to increase the power of the study and mapping causal variants.All markers with minor allele frequency(MAF)<5%were removed.
2.5.Genome-wide association analysis
Principal components(PCs)were estimated with TASSEL 5.0[34].PCs that accounted for 25%(PC1–PC3)and 50%(PC1–PC17)of cumulative variation were included in regression model to control for population structure.An identity-by-state matrix also estimated with TASSEL to control for relatedness among the genotypes[35].Six regression models(Na?ve,PC3,PC17,Kinship,PC3+Kinship,and PC17+Kinship)were used to estimate P-values for each of the polymorphic markers in TASSEL[34].Among the six models for each trait,the bestmodel was selected based on the smallestmean square difference(MSD)between the observed and expected P-values[36].A marker was considered significantifthe P-value ofthe marker was within the 0.1 and 0.01 percentile tail of 10,000 bootstraps[37,38].Stepwise regression was used to estimate the combined variation(r2)explained by these significant markers as well as to reduce the number of significant markers and to define major QTL[37,38].A P-value of 0.05 was required for both marker and model for stepwise inclusion of the marker in the REG procedure in SAS 9.3(SAS Institute,Cary,NC,USA).The positions of the markers,QTL,and candidate genes are based on the canola genome sequence reported by Chalhoub et al.[30].Genes that were 100 kb on either side of major QTL were considered candidate genes.The gene sequences of B.napus were blasted against Arabidopsis gene models[39]to obtain annotations for the gene models.
3.Results
3.1.Phenotypes
Phenotypic variation was observed for all three traits when the plants grown under normal greenhouse conditions were compared with plants exposed to 5-day heat stress during flowering time in the growth chamber.The heat-stress treatment increased pollen sterility(Fig.1)and the number of sterile or aborted pods and reduced the number of pods on the main raceme.The proportions of pollen sterility and sterile/aborted pods were sharply increased,by 84.4 and 26.1 times,respectively(Table 1),following heat stress.In contrast,the total numbers of pods on main racemes were reduced by 13.8%in heat-stressed plants.
3.2.Genotyping and association mapping
A total of 42,575 SNPs were obtained for the accessions used in this study.After filtering,a total of 37,539 SNPs with MAF>5%were further used for the subsequent analysis.Population structure was controlled and analyzed using principal component(PC)analysis.Three and 17 PCs accounted for cumulative variation of 25%and 50%,respectively,and were used for controlling population structure in the mixed model.Three continuous cluster groups were obtained with the first two principal components from PC analysis(Fig.2).Among the six models used in the analysis,the model with population structure(PC3)and kinship was the best model for pollen sterility,the model with PC17was the best for sterile/aborted pods,and the kinship model was the best model for total number of pods on the main raceme.

Table 1–Phenotypic variation in three traits studied under heat stress and control conditions.
3.3.Pollen sterility
Six markers were found significant at the 0.01 percentile(P≤6.38E?08,Table 2,Fig.3a),and were located on chromosomes C03,C04,and C08.Two other markers did not have a known position in the genome.Thirty-three additional markers were found significant in the 0.1 percentile tail of the empirical distribution(P≤5.86E?06;Table S2).These significant markers were found on 11 of the 19 chromosomes.Stepwise regression was performed to identify major QTL associated with these markers.Three significant QTL present on A02(1.481 Mb),C01(21.285 Mb)and C05(1.520 Mb)and two others whose locations were unknown were identified.These QTLs together explained 46.3% of the total phenotypic variation.The candidate genes identified included genes associated with male sterility,pollen tube growth,pollen abortion,and anther dehiscence(Table 3).
3.4.Sterile/aborted pods
For pod sterility,four markers were significant in the 0.01 percentile(P≤4.16E?05,Table 2,Fig.3b).These markers were located on chromosomes A01,A03,A08,and C01.Another 35 markers were significant at 0.1 percentile tail of the empirical distribution (P≤9.9E?04)(Table S2).Stepwise regression identified eight QTL on chromosomes A01(8.090 Mb),A03(16.335 Mb),A09(32.074 Mb),A10(12.793 Mb),C05(37.931 Mb and 42.411 Mb)and C07(27.295 Mb)and one with no known location,which together explained 60.5%of phenotypic variation(Table 3).Multiple candidate genes involved in pod abortion,floral organ development,pollen tube growth,and embryo and pollen abortion were identified in these QTL regions(Table 3).
3.5.Pods on main raceme
Five markers significant at 0.01(P≤1.39E?04,Table 2,Fig.3c)were associated with total number of pods on the main raceme.These markers were located on chromosomes A02,A06,and A10.Another 34 markers were found to be significant at 0.1 percentile tail of the empirical distribution(P≤1.73E?03)(Table S2).Seven QTL regions on A02(5.589 Mb),A05(15.015 Mb),A06(20.955 Mb),A10(1.645 Mb and 14.378 Mb)and C03(23.275 Mb)were identified.These QTL together explained 60.6%of the phenotypic variation(Table 3).Candidate genes in this QTL included genes involved in floral organ development,abortion of various organs during development,reduced flowering fertility,and other processes(Table 3).
4.Discussion
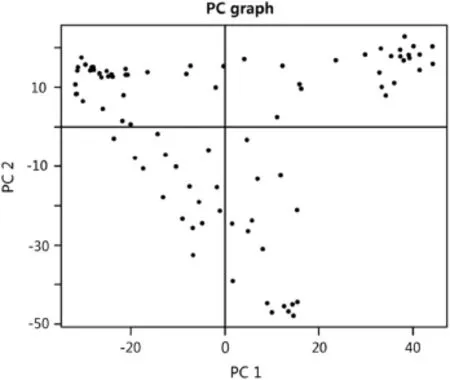
Fig.2–PCA plot showing distribution of two principal components of 37,269 SNPs.PC1 and PC2 explain respectively 13.42%and 9.50%of variation.
Improvement of several traits including heat stress tolerance is an important criterion to expand the cultivation of this crop in the United States beyond the north central region as well as worldwide.The identification of markers,genes,and QTL associated with heat stress-affected traits helps in selection of stress-tolerant genotypes in a breeding program.The spring-type germplasm accessions used in this study originated in 14 countries and represents a diverse collection in the U.S.that can be used for genome-wide association mapping studies.In this study,we simulated an artificial heat stress condition in a controlled plant growth chamber.This simulation allowed us to reliably control temperature,humidity,light,and moisture in the growth chamber to create artificial heat stress.Moreover,we evaluated the same germplasm in the greenhouse without heat stress,allowing comparison between normal growing and heat stress conditions to assess the effect of heat stress on traits.
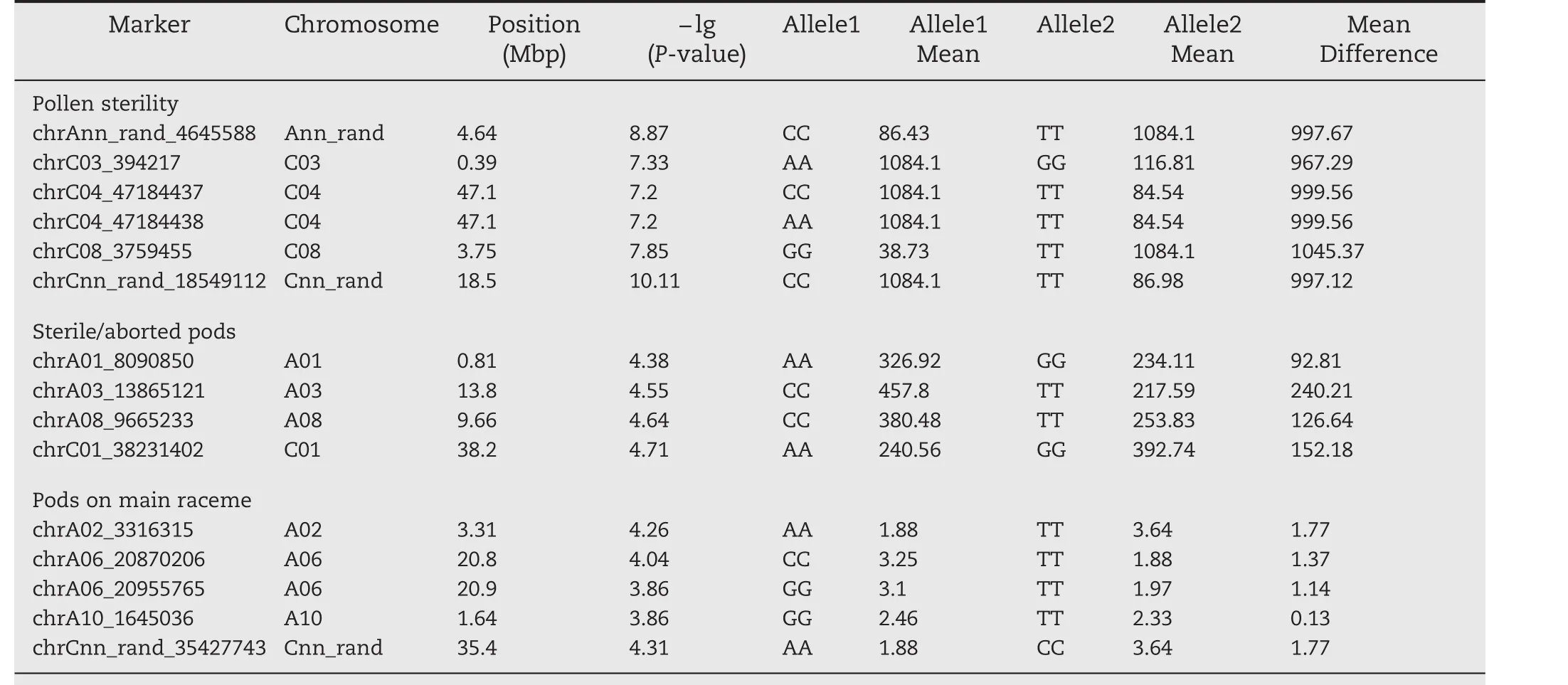
Table 2–Significant markers associated with different heat stress-tolerance traits at the 0.01 percentile tail.
4.1.Heat stress at plant growth stage
A heat stress-tolerance study in canola can be conducted at different developmental stages of the plant.In this study the heat stress was imposed on flowering plants starting six days after flower initiation.The early flowering stage of the crop is highly sensitive to heat stress and causes larger variation in a plant's physiological activity[13],and greatly reduces the seed yield[40].Heat stress during flower initiation or pod development of B.napus and B.juncea is more deleterious than heat stress during the bud initiation stage[14].It has been suggested[11]that heat stress after the pod development stage in Brassica does not significantly reduce crop yield.
4.2.Heat stress treatment
Different day/night temperatures and durations can be used for heat-stress studies.We conducted the study at 35/18°C(day/night)temperature for five days at the 6-day flowering stage in a growth chamber following Angadi et al.[11].However,studies have employed other parameters,such as 35/18°C (day/night)for 10 days at pod development,flowering,and bud formation stages[14],35/18°C(day/night)for 7 and 14 days during bolting to end flowering stages[13],and 38/23°C(day/night)for five days from 25 to 29 days after flowering[12].
4.3.Heat stress-affected traits
We studied pollen sterility,flower/pod abortion,and number of pods on main raceme,traits affected by heat stress.This study is in agreement with many findings of heat stress-induced pollen sterility,flower and pod abortion,and reduced pods per plant in several species including tomato[40],bean[41],and cotton[42].In cereals,pollen tube growth and pollen viability is markedly reduced by heat stress at reproductive stage[43].Heat stress affects the gametophytes and the tapetum layer,reducing water and nutrient transportation during microspore development[13].Heat stress also accelerates the early anther development process and progression of pollen mother cells,arrests cell proliferation,and leads to anther cell wall degradation,ultimately causing pollen abortion and male sterility in the flower[44].
4.4.Association mapping
Association mapping is a powerful tool for identifying marker–trait associations and genes associated with a trait[45].However,the results may be spurious if false positives are not controlled.To avoid these spurious associations,marker–trait associations are corrected for population structure and relatedness and a combination of these.Further,significant markers for each trait are selected based on an empirical distribution rather than a single P-value,following suggestions of Mamidi et al.[37]and Gurung et al.[38].However,the significant markers identified in our study had P-values<1E?03.To narrow the QTL peaks and identify markers for marker-assisted selection,stepwise regression was performed[37].Our analysis identified 20 QTL regions for the three traits combined.Even though the traits are physiologically related,we did not find common QTL(except one located on a scaffold that is not integrated with the chromosomes).This finding is because of the involvement of multiple physiological pathways into these trait developments.Gene models around these QTL were used to identify candidate genes.The canola accessions we studied had a lower LD(unpublished data),and therefore we selected markers around the 100 kb sequence on each side of the significant QTL.
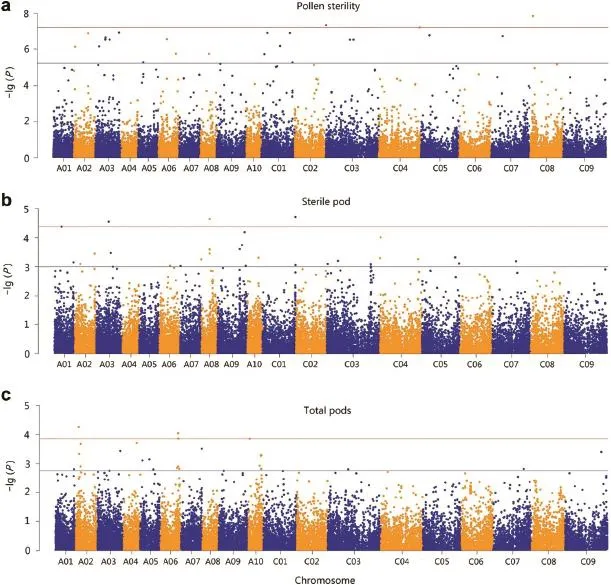
Fig.3–Manhattan plots for three major traits.(a)Pollen sterility,(b)sterile/aborted pods,and(c)pods on main raceme of B.napus associated with heat stress.Y-axis shows P-value on a ?lg scale and X-axis shows 19 chromosomes(A01–A10 and C01–09).Horizontal line in red shows percentile cutoff of 0.01 and in blue,percentile cutoff at 0.1.
4.5.Candidate genes
Flowering is one of the most important traits affected by heat stress.Multiple flowering-associated protein like F-box proteins[46,47],homeobox-leucine zipper protein 17[48]were identified in the QTL regions.Multiple proteins found in floral-organ those causes floral abortion are also affected by heat stress.They include adenosine kinase 2,which is related to impede flower and pod development through embryo abortion[49],the cytochrome P450 family,which is related to silique reduction and pollen abortion[50],and MATE efflux family proteins which is related to embryo abortion[51].In addition,a pentatricopeptide(PPR)repeat-containing family of proteins that also related to embryo abortion[52]and the protein kinase family of proteins that are known to induce pollen abortion in barley[53]were identified in the QTL regions.Cytokinin oxidase 7 and indole-3-acetic acid inducible 19,a proton pump interactor known to induce flower abortion,were also identified[54,55].Proteins known to induce embryo and seed abortion including the SET-domain containing protein lysine methyltransferase family protein[56],pyruvate kinase family protein[49],Galactosyltransferase family protein[57],GDSL proteins[51],phosphatidic acid phosphohydrolase 2[58],phosphoenolpyruvate carboxylase 3[58],and RGA-like protein 3[59]were also identified.
Pollen and flower sterility are also affected by heat stress.We identified many candidate genes associated with flower and pollen sterility,which ultimately reduce the yield of this crop.Among the candidate genes,pentatricopeptide repeat(PPR)
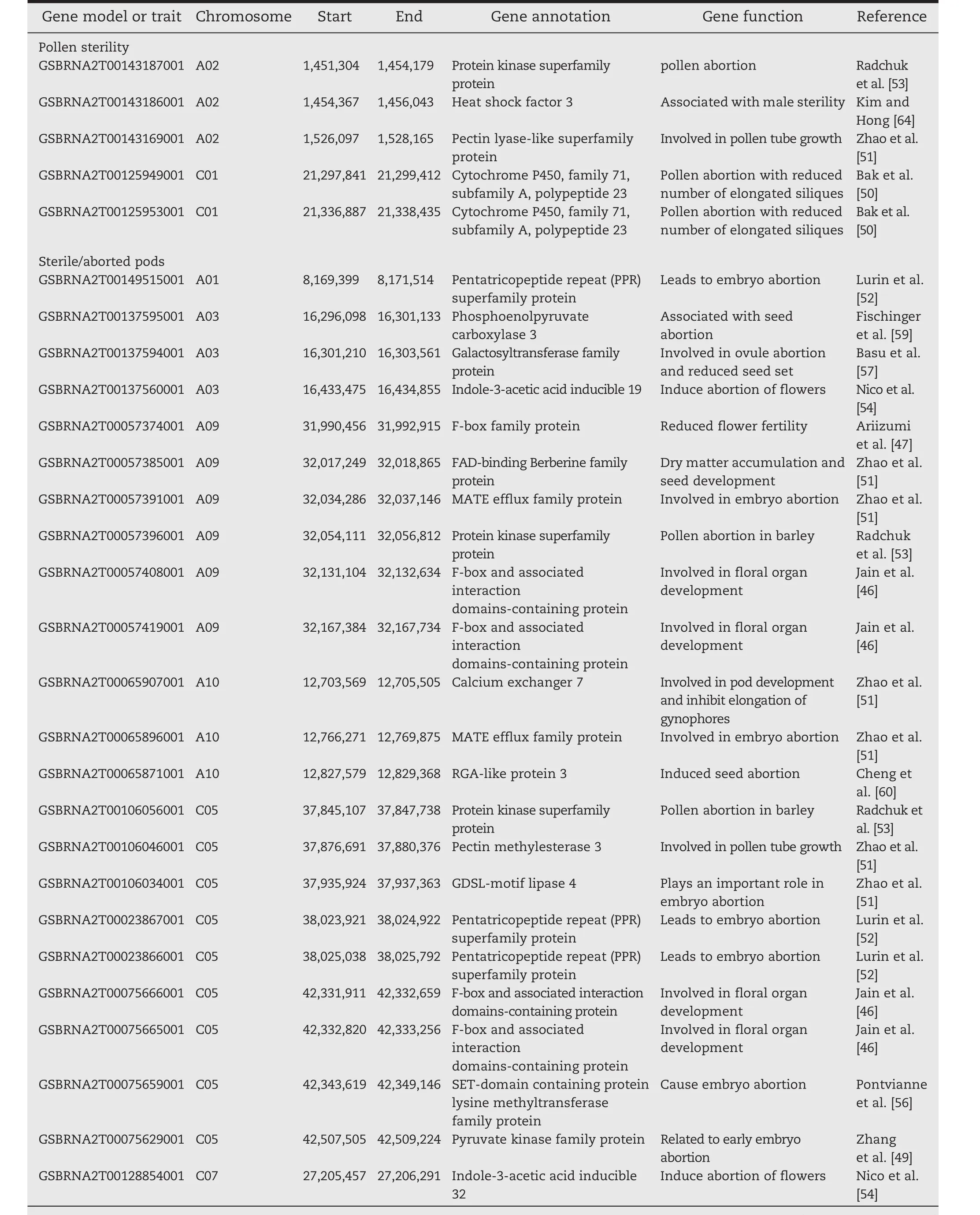
Table 3–Candidate genes associated with QTL for three traits under artificial heat stress conditions.
(continued on next page)superfamily protein,which leads to embryo abortion,that produces sterile pods[52]is one.The other candidate genes include protein 2-oxoglutarate(2OG)and Fe(II)-dependent oxygenase superfamily protein,which increase oxidative stress to reduce flowering and pod formation[60],and coatomer and beta subunit protein,which is involved in tapetum cell development and fertility restoration[61].A protein kinase superfamily that is involved in pollen abortion in barley[53],peptide transporter 5 that controls plant cell differentiation and nutrient supply and impedes flower development[62].In addition,adenosine kinase 2 protein,which involved in delay flower and pod development through embryo abortion[49],heat shock factor 3 which is associated with male sterility[63],pectin lyase-like superfamily protein that is involved in pollen tube growth[51],and cytochrome P450 that is associated with pollen abortion with reduced number of elongated siliques in Arabidopsis[50].
The number of pods per plant varied owing to the detrimental effects of heat stress.High temperature reduces pollen fertility,stigma receptivity,and pollen tube growth,leading to reduced pod development in plants.A set of genes associated with total number of pods per plant was identified in this study.For example,P450 reductase 1 is responsible for pollen abortion with reduced number of elongated siliques in Arabidopsis[50],Pectin methylesterase 31 is involved in pollen tube growth[51],and basic helix-loop-helix(bHLH)DNA-binding family protein is associated with the development and dehiscence of the seed and pod[64].A translocon at the inner envelope membrane of chloroplasts is involved in tapetum function and microspore development in Brassica[65],and a 17.6 kDa class II heat shock protein is associated with heat stress tolerance in crop plants[66].
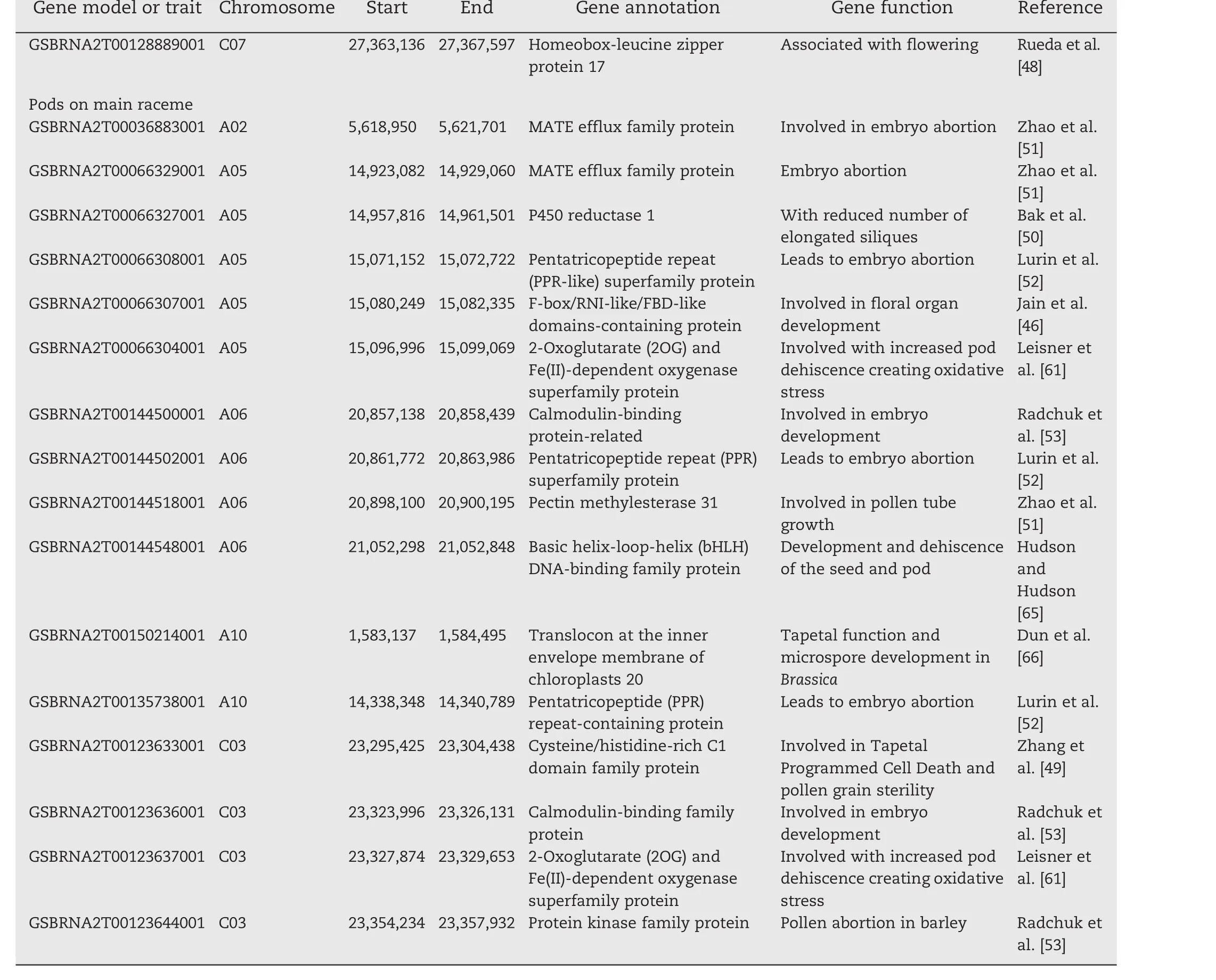
Table 3(continued)
5.Conclusions
GWAS using a diverse collection ofspring types B.napus and SNPs was able to identify 115 significant markers and 20 QTL linked to heat stress-affected traits during flowering stage in B.napus,and to define candidate genes near by the QTL.This study was conducted with spring-type cultivars that have direct application to breeding programs in the USA.The markers and QTL can be used for heat tolerance screening the various stages involved during cultivardevelopment.The candidate genes identified here are involved in various molecular and biochemical pathways.The accessions identified in this study carrying heat stress tolerance to pollen sterility,sterile/aborted pod,number of pods on main raceme could be excellent choices as parental lines in breeding programs.This study is the first step towards an understanding of the genetics of heat stress,which has an important role in developing cultivars to withstand in global growing temperatures.
Supplementary data
for
this
article can
be found online at https://doi.org/10.1016/j.cj.2017.08.003.
The study was funded by the Northern Canola Growers Association(grant number NCGA-2014-10),ND,USA.
[1]U.Nagaharu,Genome-analysis in Brassica with special reference to the experimental formation of B.napus and its peculiar mode of fertilization,Jpn.J.Bot.7(1935)389–452.
[2]N.Driedonks,I.Rieu,W.H.Vriezen,Breeding for plant heat tolerance at vegetative and reproductive stages,Plant Reprod.29(2016)67–79.
[3]M.L.Parry,O.F.Canziani,J.P.Palutikof,P.J.van der Linden,C.E.Hanson,Climate Change 2007:Impacts,Adaptation and Vulnerability:Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change,Cambridge University Press,Cambridge,UK,2007.
[4]N.Kaushal,K.Bhandari,K.H.M.Siddique,H.Nayyar,M.T.Moral,Food crops face rising temperatures:an overview of responses,adaptive mechanisms,and approaches to improve heat tolerance,Cogent Food Agric.2(2016)1134380.
[5]C.E.Bita,T.Gerats,Plant tolerance to high temperature in a changing environment:scientific fundamentals and production of heat stress-tolerant crops,Front.Plant Sci.4(2013)273.
[6]R.K.M.Hay,J.R.Porter,The Physiology of Crop Yield,2nd edition Blackwell Publishing,Oxford,UK,2007.
[7]B.Barnabas,K.Jager,A.Feher,The effect of drought and heat stress on reproductive processes in cereals,Plant Cell Environ.31(2008)11–38.
[8]P.V.V.Prasad,K.J.Boote,L.H.Allen,J.E.Sheehy,J.M.G.Thomas, Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress, Field Crop Res. 95 (2006) 398–411.
[9]A.J.Challinor,T.R.Wheeler,P.Q.Craufurd,C.A.T.Ferro,D.B.Stephenson,Adaptation of crops to climate change through genotypic responses to mean and extreme temperatures,Agric.Ecosyst.Environ.119(2007)190–204.
[10]M.J.Morrison,Heat stress during reproduction in summer rape,Can.J.Bot.71(1993)303–308.
[11]S.V.Angadi,H.W.Cutforth,P.R.Miller,B.G.McConkey,M.H.Entz,S.A.Brandt,K.M.Volkmar,Species to high temperature stress during reproductive growth,Can.J.Plant Sci.80(2000)693–701.
[12]N.M.Aksouh,B.C.Jacobs,F.L.Stoddard,R.J.Mailer,Response of canola to different heat stresses,Aust.J.Agric.Res.52(2001)817–824.
[13]L.W.Young,R.W.Wilen,P.C.Bonham-Smith,High temperature stress of Brassica napus during flowering reduces microand megagametophyte fertility,induces fruit abortion,and disrupts seed production,J.Exp.Bot.55(2004)485–495.
[14]Y.Gan,S.V.Angadi,H.Cutforth,D.Potts,V.V.Angadi,C.L.McDonald,V.V.Mcdonald,Canola and mustard response to short periods of temperature and water stress at different developmental stages,Can.J.Plant Sci.84(2004)697–704.
[15]W.F.Nuttall,A.P.Moulin,L.J.Townley-Smith,Yield response of canola to nitrogen,phosphorus,precipitation,and temperature,Agron.J.84(1992)765–768.
[16]G.U.Rao,A.Jain,K.T.Shivanna,Effects of high temperature stress on Brassica pollen:viability,germination and ability to set fruits and seeds,Ann.Bot.69(1992)193–198.
[17]H.Kaya,K.Ichi-Shibahara,K.I.Taoka,M.Iwabuchi,B.Stillman,T.Araki,FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems,Cell 104(2001)131–142.
[18]J.L.Hall,Cellular mechanisms for heavy metal detoxification and tolerance,J.Exp.Bot.53(2002)1–11.
[19]C.Frova,M.Sari-Gorla,Quantitative expression of maize HSPs:genetic dissection and association with thermotolerance,Theor.Appl.Genet.86(1993)213–220.
[20]E.Ottaviano,M.S.Gorla,E.Pe,C.Frova,Molecular markers(RFLPs and HSPs)for the genetic dissection of thermotolerance in maize,Theor.Appl.Genet.81(1991)713–719.
[21]M.Hasanuzzaman,K.Nahar,M.M.Alam,R.Roychowdhury,M.Fujita,Physiological,biochemical,and molecular mechanisms of heat stress tolerance in plants,Int.J.Mol.Sci.14(2013)9643–9684.
[22]X.H.Huang,Y.Zhao,X.H.Wei,C.Y.Li,A.H.Wang,Q.Zhao,W.J.Li,Y.L.Guo,L.W.Deng,C.R.Zhu,D.L.Fan,Y.Q.Lu,Q.J.Weng,K.Y.Liu,T.Y.Zhou,Y.F.Jing,L.Z.Si,G.J.Dong,T.Huang,T.T.Lu,Q.Feng,Q.Qian,J.Y.Li,B.Han,Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm,Nat.Genet.44(2012)32–39.
[23]H.Li,Z.Y.Peng,X.H.Yang,W.D.Wang,J.J.Fu,J.H.Wang,Y.J.Han,Y.C.Chai,T.T.Guo,N.Yang,J.Liu,M.L.Warburton,Y.B.Cheng,X.M.Hao,P.Zhang,J.Y.Zhao,Y.J.Liu,G.Y.Wang,J.S.Li,J.B.Yan,Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels,Nat.Genet.4(2013)43–50.
[24]N.Li,J.Q.Shi,X.F.Wang,G.H.Liu,H.Z.Wang,A combined linkage and regional association mapping validation and fine mapping of two major pleiotropic QTLs for seed weight and silique length in rapeseed(Brassica napus L.),BMC Plant Biol.1(2014)114.
[25]M.Nordborg,D.Weigel,Next-generation genetics in plants,Nature 456(2008)720–723.
[26]X.H.Huang,B.Han,Natural variations and genome-wide association studies in crop plants,Annu.Rev.Plant Biol.65(2014)531–551.
[27]P.L.Polowick,V.K.Sawhney,High temperature induced male and female sterility in canola(Brassica napus L.),Ann.Bot.62(1988)83–86.
[28]M.J.Morrison,D.W.Stewart,Heat stress during flowering in summer Brassica,Crop Sci.42(2002)797–803.
[29]R.A.Fischer,R.Maurer,Drought resistance in spring wheat cultivars.I.Grain-yield responses,Aust.J.Agric.Res.29(1978)897–912.
[30]B.Chalhoub,F.Denoeud,S.Y.Liu,I.A.Parkin,H.B.Tang,X.Wang,J.Chiquet,H.Belcram,C.B.Tong,B.Samans,M.Corréa,C.Da Silva,J.Just,C.Falentin,C.S.Koh,M.Isabelle Le Clainche,P.Bernard,B.Bento,K.Noel,A.Labadie,M.Alberti,D.Charles,H.Arnaud,C.Guo,S.Daviaud,K.Alamery,M.X.Jabbari,P.P.Zhao,H.Edger,D.Chelaifa,G.Tack,I.Lassalle,N.Mestiri,M.C.Schnel,G.Y.Le Paslier,V.Fan,P.E.Renault,A.A.Bayer,S.Golicz,T.H.Manoli,V.H.D.Lee,S.Thi,Q.Hu Chalabi,C.C.Fan,R.Tollenaere,Y.H.Lu,C.Battail,J.X.Shen,C.H.D.Sidebottom,X.F.Wang,A.Canaguier,A.Chauveau,A.Bérard,G.Deniot,M.Guan,Z.S.Liu,F.M.Sun,Y.P.Lim,E.Lyons,C.D.Town,I.Bancroft,X.W.Wang,J.L.Meng,J.X.Ma,J.C.Pires,G.J.King,D.Brunel,R.Delourme,M.Renard,J.M.Aury,K.L.Adams,J.Batley,R.J.Snowdon,J.Tost,D.Edwards,Y.M.Zhou,W.Hua,A.G.Sharpe,A.H.Paterson,C.Y.Guan,P.Wincker,Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome,Science 345(2014)950–953.
[31]H.Li,R.Durbin,Fast and accurate short read alignment with Burrows Wheeler transform,Bioinformatics 25(2009)1754–1760.
[32]D.Koboldt,Q.Y.Zhang,D.Larson,D.Shen,M.McLellan,L.Lin,C.Miller,E.Mardis,L.Ding,R.Wilson,VarScan 2:somatic mutation and copy number alteration discovery in cancer by exome sequencing,Genome Res.22(2012)568–576.
[33]P.Scheet,M.Stephens,A fast and flexible statistical model for large-scale population genotype data:applications to inferring missing genotypes and haplotypic phase,Am.J.Hum.Genet.78(2006)629–644.
[34]P.J.Bradbury,Z.Zhang,D.E.Kroon,T.M.Casstevens,Y.Ramdoss,E.S.Buckler,TASSEL:software for association mapping of complex traits in diverse samples,Bioinformatics 23(2007)2633–2635.
[35]K.Y.Zhao,M.J.Aranzana,S.Kim,C.Lister,C.Shindo,C.L.Tang,C.Toomajian,H.G.Zheng,C.Dean,P.Marjoram,M.Nordborg,An Arabidopsis example of association mapping in structured samples,PLoS Genet.3(2007)71–82.
[36]S.Mamidi,S.Chikara,R.J.Goos,D.L.Hyten,D.Annam,S.M.Moghaddam,R.K.Lee,P.B.Cregan,P.E.McClean,Genomewide association analysis identifies candidate genes associated with iron deficiency chlorosis in soybean,Plant Genome 4(2011)154–164.
[37]S.Mamidi,R.K.Lee,J.R.Goos,P.E.McClean,Genome-wide association studies identifies seven major regions responsible for iron deficiency chlorosis in soybean(Glycine max),PLoS One 9(2014),e107469.
[38]S.Gurung,S.Mamidi,J.M.Bonman,M.Xiong,G.Brown-Guedira,T.B.Adhikari,Genome-wide association study reveals novel quantitative trait loci associated with resistance to multiple leaf spot diseases of spring wheat,PLoS One 9(2014),e108179.
[39]T.Z.Berardini,L.Reiser,D.H.Li,Y.Mezheritsky,R.Muller,E.Strait,E.Huala,The Arabidopsis information resource:making and mining the “gold standard”annotated reference plant genome,Genesis 53(2015)474–485.
[40]A.A.Abdul-Baki,Tolerance of tomato cultivars and selected germplasm to heat stress,J.Am.Soc.Hortic.Sci.116(1991)1113–1116.
[41]I.Konsens,M.Ofir,J.Kigel,The effect of temperature on the production and abscission of flowers and pods in snap bean(Phaseolus vulgaris L.),Ann.Bot.67(1991)391–399.
[42]K.R.Reddy,H.F.Hodges,V.R.Reddy,Temperature effects on cotton fruit retention,Agron.J.84(1992)26–30.
[43]H.S.Saini,D.Aspinall,Abnormal sporogenesis in wheat(Triticum aestivum L.)induced by short periods of high temperature,Ann.Bot.49(1982)835–846.
[44]F.Giorno,M.Wolters-arts,C.Mariani,I.Rieu,Ensuring reproduction at high temperatures:the heat stress response during anther and pollen development,Plants 2(2013)489–506.
[45]L.M.Jia,W.G.Yan,C.S.Zhu,H.A.Agrama,A.Jackson,X.B.Kathleen Yeater,B.H.Li,B.L.Hu Huang,A.McClung,D.X.Wu,Allelic analysis of sheath blight resistance with association mapping in rice,PLoS One 7(2012),e32703.
[46]M.Jain,A.Nijhawan,R.Arora,P.Agarwal,S.Ray,P.Sharma,S.Kapoor,A.K.Tyagi,J.P.Khurana,F-box proteins in rice.Genome-wide analysis,classification,temporal and spatial gene expression during panicle and seed development,and regulation by light and abiotic stress,Plant Physiol.143(2007)1467–1483.
[47]T.Ariizumi,P.K.Lawrence,C.M.Steber,The role of two f-box proteins,SLEEPY1 and SNEEZY,in Arabidopsis gibberellin signaling,Plant Physiol.155(2011)765–775.
[48]E.C.Rueda,C.A.Dezar,D.H.Gonzalez,R.L.Chan,Hahb-10,a sunflower homeobox-leucine zipper gene,is regulated by light quality and quantity,and promotes early flowering when expressed in Arabidopsis,Plant Cell Physiol.46(2005)1954–1963.
[49]D.D.Zhang,Y.P.Hua,X.H.Wang,H.Zhao,L.Shi,F.S.Xu,A high-density genetic map identifies a novel major QTL for boron efficiency in oilseed rape(Brassica napus L.),PLoS One 9(2014),e112089.
[50]S.Bak,F.Beisson,G.Bishop,B.Hamberger,R.H?fer,S.Paquette,D.Werck-Reichhart,Cytochromes P450,Arabidopsis Book,9,2011,p.e0144.
[51]C.Z.Zhao,S.Z.Zhao,L.Hou,H.Xia,J.S.Wang,C.S.Li,A.Q.Li,T.T.Li,X.Y.Zhang,X.J.Wang,Proteomics analysis reveals differentially activated pathways that operate in peanut gynophores at different developmental stages,BMC Plant Biol.15(2015)188.
[52]C.Lurin,C.Andre,M.Caboche,N.Peeters,J.Renou,B.Szurek,L.Taconnat,I.Small,Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis,Plant Cell 16(2004)2089–2103.
[53]R.Radchuk,V.Radchuk,W.Weschke,L.Borisjuk,H.Weber,Repressing the expression of the sucrose nonfermenting-1-related protein kinase gene in pea embryo causes pleiotropic defects of maturation similar to an abscisic acid intensive phenotype,Plant Physiol.140(2006)263–278.
[54]M.Nico,A.I.Mantese,D.J.Miralles,A.G.Kantolic,Soybean fruit development and set at the node level under combined photoperiod and radiation conditions,J.Exp.Bot.67(2016)365–377.
[55]J.C.Song,L.J.Jiang,P.E.Jameson,Expression patterns of Brassica napus genes implicate IPT,CKX,sucrose transporter,cell wall invertase and amino acid permease gene family members in leaf,flower,silique and seed development,J.Exp.Bot.66(2015)133–149.
[56]E.Pontvianne,T.Blevins,C.S.Pikaard,Arabidopsis histone lysine methyltransferases,Adv.Bot.Res.53(2010)1–22.
[57]D.Basu,L.Tian,W.D.Wang,S.Bobbs,H.Herock,A.Travers,A.M.Showalter,A small multigene hydroxyproline-O-galactosyltransferase family functions in growth and development in Arabidopsis,BMC Plant Biol.15(2015)295.
[58]P.J.Eastmond,A.L.Quettier,J.T.Kroon,C.Craddock,N.Adams,A.R.Slabas,Phosphatidic acid phosphohydrolase 1 and 2 regulate phospholipid synthesis at the endoplasmic reticulum in Arabidopsis,Plant Cell 22(2010)2796–2811.
[59]S.A.Fischinger,J.Schulze,The importance of nodule CO2fixation for the efficiency of symbiotic nitrogen fixation in pea at vegetative growth and during pod formation,J.Exp.Bot.61(2010)2281–2291.
[60]C.X.Cheng,C.Jiao,S.D.Singer,M.Gao,X.Z.Xu,Y.M.Zhou,Z.Li,Z.J.Fei,Y.J.Wang,X.P.Wang,Gibberellin-induced changes in the transcriptome of grapevine(Vitis labrusca×V.vinifera)cv.Kyoho flowers,BMC Genomics 16(2015)128.
[61]C.P.Leisner,R.Ming,E.A.Ainswort,Distinct transcriptional profiles of ozone stress in soybean(Glycine max)flowers and pods,BMC Plant Biol.14(2014)335.
[62]S.P.Singh,S.P.Singh,T.Pandey,R.R.Singh,S.V.Sawant,A novel male sterility-fertility restoration system in plants for hybrid seed production,Sci Rep 5(2015)11274.
[63]H.P.Yang,Y.Matsubayashi,H.Hanai,Y.Sakagami,Phytosulfokine-α,a peptide growth factor found in higher plants:its structure,functions,precursor and receptors,Plant Cell Physiol.41(2000)825–830.
[64]S.Y.Kim,C.B.Hong,Heat shock stress causes stage-specific male sterility in Arabidopsis thaliana,J.Plant Res.114(2001)301–307.
[65]K.A.Hudson,M.E.Hudson,A classification of basic helixloop-helix transcription factors of soybean,Int.J.Genomics 2015(2015)603182.
[66]X.L.Dun,Z.F.Zhou,S.Q.Xia,J.Wen,B.Yi,J.X.Shen,C.Z.Ma,J.X.Tu,T.D.Fu,BnaC.Tic40,a plastid inner membrane translocon originating from Brassica oleracea,is essential for tapetal function and microspore development in Brassica napus,Plant J.68(2011)532–545.
- The Crop Journal的其它文章
- Genetic diversity assessment of a set of introduced mung bean accessions(Vigna radiata L.)
- Near-infrared reflectance spectroscopy reveals wide variation in major components of sesame seeds from Africa and Asia
- Alternate phenotype–genotype selection for developing superior high-yielding irrigated rice lines
- F unction of the auxin-responsive gene TaSAUR75 under salt and drought stress
- Development and validation of simple sequence repeat markers from Arachis hypogaea transcript sequences
- Soybean hairy roots produced in vitro by Agrobacterium rhizogenes-mediated transformation

