F unction of the auxin-responsive gene TaSAUR75 under salt and drought stress
Yun Guo,Qiyn Jing,*,Zheng Hu,Xinjun Sun,Shoujin Fn,Hui Zhng,*
a Institute of Crop Science,National Key Facility for Crop Gene Resources and Genetic Improvement,Chinese Academy of Agricultural Sciences,Beijing 100081,China
b College of Life Science,Shandong Normal University,Jinan 250014,Shandong,China
1.Introduction
Extreme environmental conditions,such as salt,drought,and low-temperature stress,have been increasingly detrimental to crop production in past decades[1–8].The plasticity of cellular metabolism,physiology,and development determines whether a plant adapts to variable and diverse environments.Auxin influences almost all aspects of cell division,cell elongation,and cell differentiation and has a large influence on the final shape and function of cells and tissues in all higher plants.Auxin can also regulate and coordinate plant growth under stress[9].Seed germination is regulated by the membrane-bound transcription factor NTM2(NAC with transmembrane motif),which influences the auxin signal on germination under salt stress[10].
Auxin response to salt or drought stress in crop plants mediates a regulatory network that exists between auxin signaling and drought or salt stress[9,11].Drought and salt stress can influence auxin on metabolism and distribution.ROS(reactive oxygen species)generated by abiotic stresses may also influence auxin response[11].Iqbal and Ashraf[10]reported that priming seeds with different auxins,IAA(indole-3-acetic acid)and IBA(indole-3-butanoic acid),effectively decreased the reduction by salt stress of endogenous ABA(abscisic acid)concentration in a salt-sensitive wheat cultivar.Six of the seven YUC(YUCCA,flavin monooxygenase)auxin biosynthesis genes showed reduced expression in rice under drought stress[11].Arabidopsis overexpressing the iaaM(tryptophan monooxygenase)gene showed enhanced tolerance to drought by maintaining a higher level of IAA than that in wild-type plants.Additional experiments showed that auxin could enhance drought tolerance by modulating root architecture,ABA-responsive gene expression,and ROS metabolism[12].
Three gene families,the early or primary auxin-response families AUX/IAAs,GRETCHEN HAGEN3s(GH3s),and SAURs,are induced by auxin within a few minutes of receiving the signal.SAURs were first identified in soybean hypocotyls by differential hybridization[13].Most SAURs do not contain introns in their CDS(coding sequence),and SAUR proteins are small and located in nuclear,cytoplasmic,chloroplast,and plasma membrane subcellular locations[14].SAURs may be the largest family of early auxin response genes and are widespread among land plant species.There are 81 SAURs(with 2 pseudogenes)in Arabidopsis[15],58 (with 2 pseudogenes)in rice[16],58 in rice[17],134 in potato,99 in tomato[18],71 in sorghum[19],79 in maize[20],70 in citrus[21],and 38(with 1 pseudogene)in Moso bamboo[22].SAURs play multi-functional roles in plants.The AtSAUR19[23],AtSAUR41[24],AtSAUR63[25],and AtSAUR76-78[26]promote hypocotyl elongation,AtSAUR36[27]promotes leaf senescence,OsSAUR39 inhibits the growth and yield of rice plants,and overexpression of OsSAUR51[28]promotes bacterial blight resistance in plants.However,studies of SAUR s on stress are limited.SAUR family proteins are regulated by miR159,which promotes drought tolerance in wheat[29].In addition,many SAUR s are responsive to abiotic stresses such as salt and drought,indicating that SAUR s participate in the regulation of abiotic stress-tolerance responses[30–32].However,the functions of numerous SAUR s are largely unknown.
In our previous study,change in the transcriptome of Triticum.aestivum cv.Jimai 19 in response to high salt was assessed to characterize the salt-responsive mechanism in wheat.Seven TaSAUR s were found to be up-or downregulated after salt stress(unpublished).A salt stress-responsive SAUR gene,TaSAUR75(Gene ID,Traes_5DL_32B83E57E,Table 1)was selected for further analysis in the present study.
2.Materials and methods
2.1.Plant materials
The wheat cultivar Han 12 was used to isolate the TaSAUR75 gene.The wheat cultivars Han 12,Cha Dianhong,Chinese Spring,and Jimai 19 were used to examine the expression patterns of TaSAUR75 under salt stress.
2.2.Plant growth and salt tolerance evaluation
Wheat seeds were germinated on filter paper containing water,under white light(150 μmol photons m?2s?1;14-h light/10-h dark photoperiod)at 20°C in a growth chamber.When the coleoptiles were 1/3 or the radicle was 1/2 of the length of the seed,the seed was considered germinated.Germinated seedlings were immersed into Hoagland solution[33]until the fourth leaf was observed.The seedlings were then treated with a 2%(342 mmol L?1)NaCl Hoagland solution and the roots were collected at 0,2,6,12,24,48,and 72 h after treatment and stored at ?80 °C for further study.To keep the solution clean and pH-stable,it was changed daily.
Salt-tolerance analysis was performed following previous studies[34–38].Ten-day-old seedlings were treated with 2%NaCl in Hoagland solution for 7 days.The salt injury symptoms of seedlings were assigned a score of 0–5.The classification criteria of salt injury were as follows:level 0(no injury),level 1(damage on leaf tips),level 2(half of the leaf showing injury),level 3(full leaf showing injury),level 4(only the youngest leaf surviving),and level 5(death).The experiment was performed with three replications.The salt injury index(SI)was calculated using the following formula:
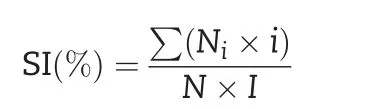
where Niis the number of plants assigned the salt injury score i(from 0 to 5),N is the total number of tested seedlings,and I is the highest score.
2.3.Quantitative real-time PCR(qRT-PCR)
Total RNA was isolated from different plant tissues using TRIzol reagent(Invitrogen,Carlsbad,CA,USA)according tothe manufacturer's instructions and was treated with RNase-free DNase I(Fermentas,Vilnius,Lithuania).Next,cDNA was synthesized from 100 ng of total RNA using RevertAid Reverse Transcriptase(Fermentas)and SYBR green qPCR Master Mix(Fermentas)was used for real-time PCR.The ACT2 gene of Arabidopsis was used to quantify the expression levels of TaSAUR75 and the target gene in transgenic Arabidopsis plants.TaTubulin was used to quantify the expression levels of TaSAUR75 in wheat.All primers used in this study are shown in Table 2.
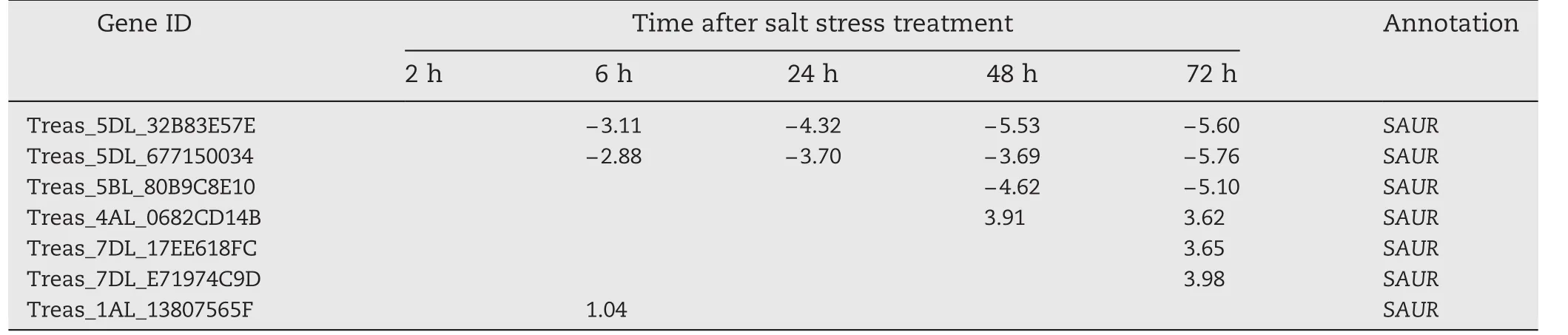
Table 1–Changes in the expression of candidate SAURs in transcriptome analysis of wheat roots.Numbers in table are log2 of gene expression in Jimai 19 roots under salt stress relative to that before salt stress.
Table 2–Primers used in this study.
2.4.Subcellular localization
To assess the subcellular localization of the TaSAUR75 protein,the TaSAUR75 ORF(open reading frame)without the termination codon was amplified using primers(TaSAUR75 GFP-F and TaSAUR75 GFP-R)that incorporated Sall and Bam HI sites and was fused to the N terminus of the hGFP gene under the control of the CaMV 35S promoter.Transient expression of the TaSAUR75:hGFP fusion construct and hGFP control vector was induced by introduction of the vectors separately into wheat protoplasts using PEG-mediated transfection.The transformed wheat protoplast cells were incubated at 25°C for 15 h.The transformed cells were cultured in the darkness for 16–24 h and were observed under a confocal microscope(Leica Microsystem,Heidelberg,Germany).
2.5.Generation of TaSAUR75 transgenic Arabidopsis plants
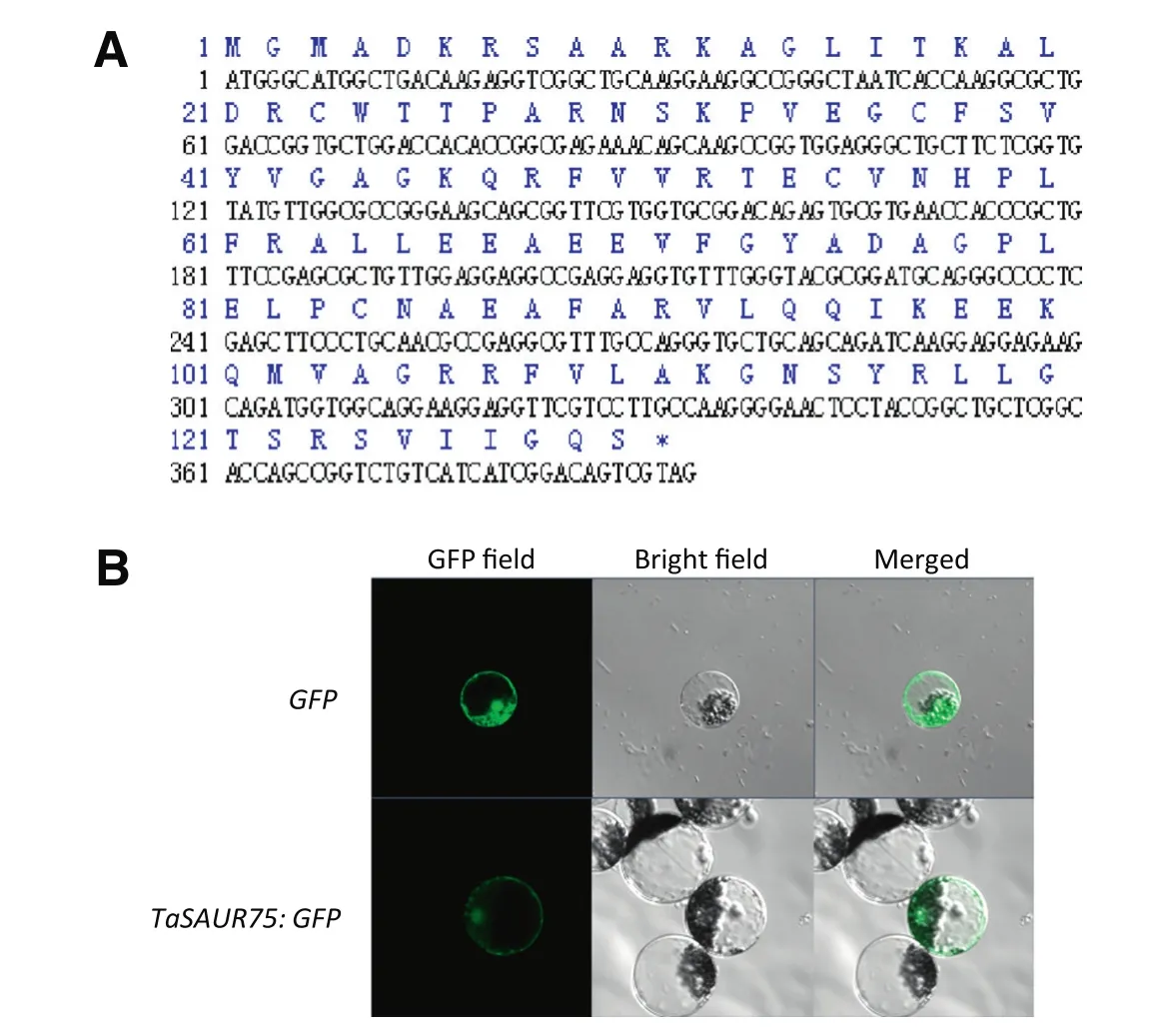
Fig.1–Characterization and subcellular localization of TaSAUR75.A,Translated amino acid sequence and cDNA sequence of TaSAUR75;B.TaSAUR75 was located in both the cytoplasm and the nucleus of wheat protoplasts.
To generate an overexpression construct in transgenic Arabidopsis plants that constitutively overexpressed TaSAUR75 from a cauliflower mosaic virus 35S promoter,a 393-bp fragment sequence was amplified from wheatgenomic DNAusing primers TaSAUR75-cds-F and TaSAUR75-cds-R.The amplified fragment contained Xba I sites and was introduced into the pEASY-Blunt Cloning Kit vector(TransGen,Beijing,China)for sequencing confirmation and then subcloned into the pGFPGUSplus vector.The construct was introduced into Agrobacterium tumefaciens GV3101,from which it was transformed into Columbia(Col-0)ecotype Arabidopsis plants by vacuum infiltration.The agrobacterium strain GVC3101 containing the pGFPGUSplus-TaSAUR75 plasmid was grown overnight and harvested.The bacteria were resuspended at 0.8 OD at 600 nm in transformation media(1/2 Murashige and Skoog media(MS),containing 5%sucrose and 0.005%Silwet L-77).Four-week-old,soil-grown and flowering Arabidopsis thaliana ecotype Col-0 plants under normal conditions were dipped in transformation medium.The plants were then covered with black bag for 2 days and harvested 3 weeks later.The T3 generation plants were used for further analysis.
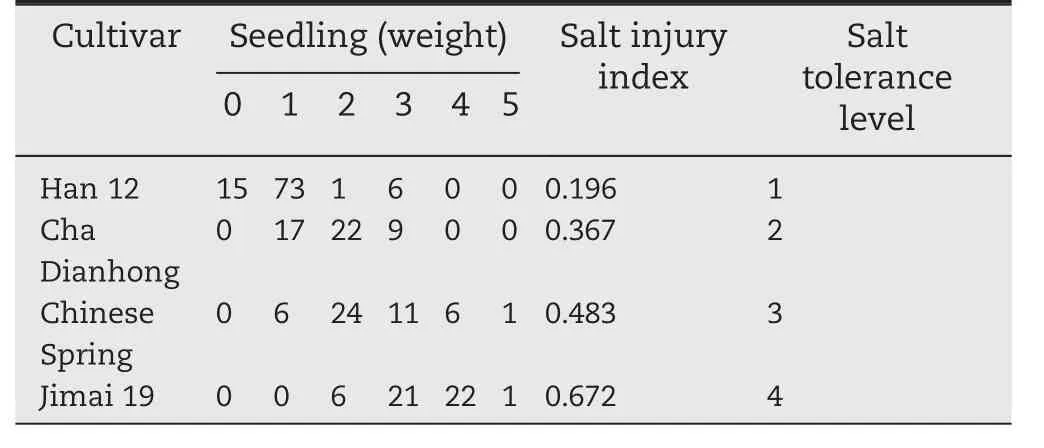
Table 3–Salt-tolerance evaluation of four wheat cultivars.
2.6.Germination assay
Seeds were surface-sterilized in 75%ethanol solution and 0.01%Triton X-100 three times and then were washed three times in 75%ethanol solution.Sterile seeds were used in this assay.MS plates saturated with 100 mmol L?1NaCl were used to assay the sensitivity of seed germination to salt,and MS plates with 300 mmol L?1mannitol were used to assay the sensitivity of seed germination to drought.Wild-type(WT)or overexpression line seeds were placed on MS agar plates with distilled water,100 mmol L?1NaCl or 300 mmol L?1mannitol and were cultured at 4°C for 3 days before being incubated at 22°C for germination.Seeds were considered germinated when the radicles had pierced the seed coat.Germination was scored daily after the seeds were placed at 25°C.Each treatment contained 30 seeds per line with three replications.
2.7.Root growth assay
Sterile overexpression lines and WT seeds were germinated on MS agar plates.Fifteen-day-old seedlings of each line were transferred to MS agar plates supplemented with 300 mmol L?1mannitol or 100 mmol L?1NaCl.The seedlings were examined after 7 days of erect growth in the treatment medium(22°C,12 h light/12 h dark cycle).
2.8.Drought-and salt-stress treatments of transgenic Arabidopsis
For drought tolerance assays,sterile overexpression lines and WT seeds were germinated on MS medium.One-week-old seedlings were planted in identical pots containing mixed soil(vermiculite:humus,1:1)and were well watered.The seedlings were cultured in a growth chamber(22°C,70%humidity,12 h light/12 h dark cycle)for 3 weeks and then without watering for 3 weeks.After 3 weeks of drought treatment,the seedlings were rewatered,and 3 days later the survival rate of each line was calculated.For salt stress,three-week-old seedlings grown under normal conditions were treated with 250 mmol L?1NaCl daily for 3 days and then cultured under normal conditions for 3 weeks.Each treatment was applied to 30 seeds per line with three replications.
2.9.Measurement of the chlorophyll content
The chlorophyll content assay was performed according to Zhou et al.[39].Ten leaves were harvested,weighed and incubated with 10 mL of acetone(80%solution)in the dark for 36 h at room temperature.The absorbance of the supernatant was detected with a spectrophotometer(DU800;Beckman,Fullerton,CA,USA)at 633 nm and 645 nm.The concentration of chlorophyll was calculated using the following formula:
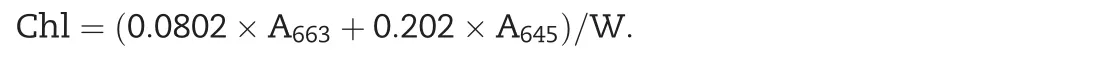
where A is the absorbance at specific wavelengths and W is the fresh weight of tissue extracted.
2.10.Detection of cell death
Programmed cell death was detected using the Trypan blue method,which dyes dead cells blue.Leaves from the overexpression and control plants were soaked in a 0.4%Trypan blue solution(MYM Biological Technology Company),boiled for 2 min in a water bath,and incubated for 8 h.The leaves were transferred to a 1.25 g mL?1chloral hydrate solution(MYM Biological Technology Company,Beijing,China)to fade for 3 days,and the solution was changed once daily.Finally,the seedlings were photographed.
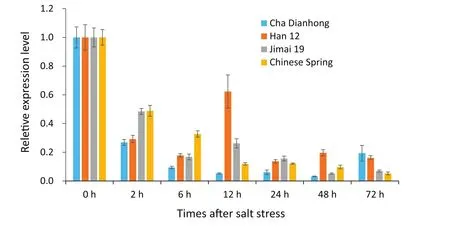
Fig.2–Expression of TaSAUR75 was reduced in wheat roots of different cultivars under salt stress.
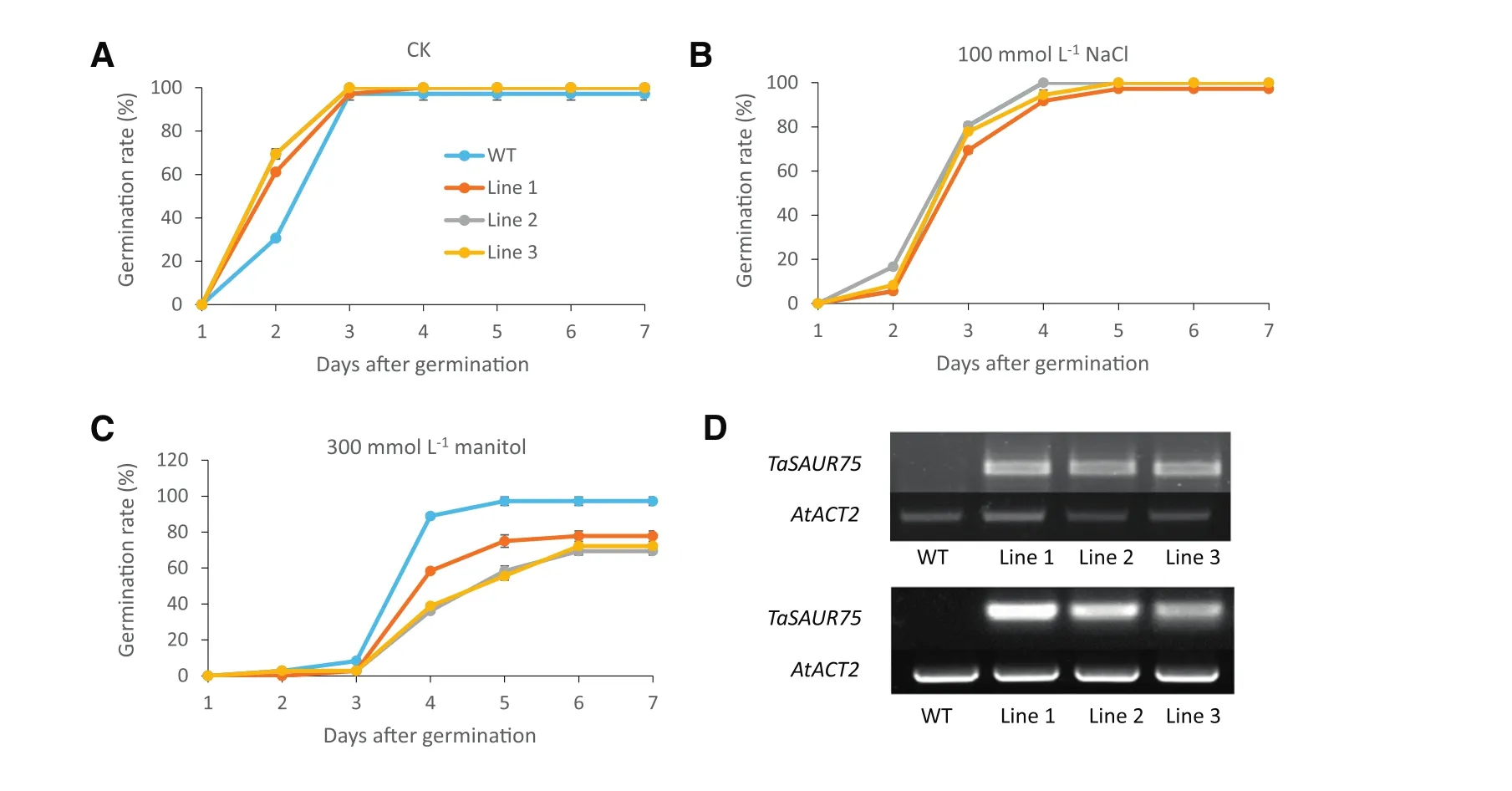
Fig.3–TaSAUR75 promotes germination under normal condition but inhibited germination under osmotic conditions.A–C.Germination rates of 35S:TaSAUR75 lines and wild-type plants on MS(A),MS media with 100 mmol L?1NaCl(B),MS media with 300 mmol L?1mannitol(C);D.PCR(top)and RT-PCR(bottom)confirmation of TaSAUR75 transfection in Arabidopsis.Each repetition contained 30 seeds and each test was repeated three times.
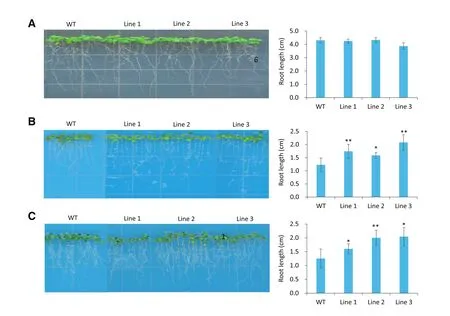
Fig.4–TaSAUR75 promotes roots elongation under salt and osmotic condition.A–C.35S:TaSAUR75 lines and wild-type plants grown erectly on MS(A),100 mmol L?1NaCl(B),and 300 mmol L?1mannitol(C)media.Error bars represent the SD of three independent experiments and asterisks indicate a significant difference(*P<0.05;**P<0.01)compared with the corresponding controls.
2.11.Hydrogen peroxide and superoxide staining
During salt stress,some plants generate hydrogen peroxide(H2O2)and superoxide(O2?)to mediate numerous physiological and biochemical processes.H2O2and O–2can be visually detected with 3,3′-diaminobenzidine(DAB,Beijing Biodee Biotechnology,Beijing,China)and nitro blue tetrazolium(NBT),respectively.Leaves from overexpression and control plants were infiltrated with 5 mg mL?1DAB at pH 3.8 for 20 h or 0.5 mg mL?1NBT at pH 7.5 for20 h in the dark to detect H2O2and O?2,respectively.The leaves were transferred to a glycerol and ethanol solution(95%glycerol:ethanol 3:7)and boiled for 15 min in a water bath.After cooling,the leaves were extracted at room temperature with the same solution until completely decolorized.
3.Results
3.1. Characterization and subcellular localization of TaSAUR75
Salt stress significantly(>48-fold)reduced the expression of TaSAUR75 at 72 h compared with that under normal conditions,indicating that TaSAUR75 is involved in salt response.We next cloned full-length TaSAUR75 from Han 12 for further study.
TaSAUR75 encodes a 130 aa polypeptide with a molecular weight of 14.3 kDa and an isoelectric point of 9.54.The TaSAUR75 protein contains an auxin-inducible domain(3–93,90 aa,Fig.1-A),which is the signature sequence of SAURs.
To determine the subcellular localization of TaSAUR75,GFP was fused to the C terminus of TaSAUR75 under the control of the CaMV35S constitutive promoter.The TaSAUR75 CDS was isolated from the Han 12 wheat cultivar.The recombination vector 35S:TaSAUR75-GFP and control vector 35S:GFP were transfected into wheat protoplast cells using a PEG-mediated procedure.Microscopic analyses detected fluorescence from the TaSAUR75-GFP protein in both the cytoplasm and the nucleus of wheat protoplasts(Fig.1-B).
3.2.Expression pattern of TaSAUR75 in different cultivars of wheat roots under salt stress
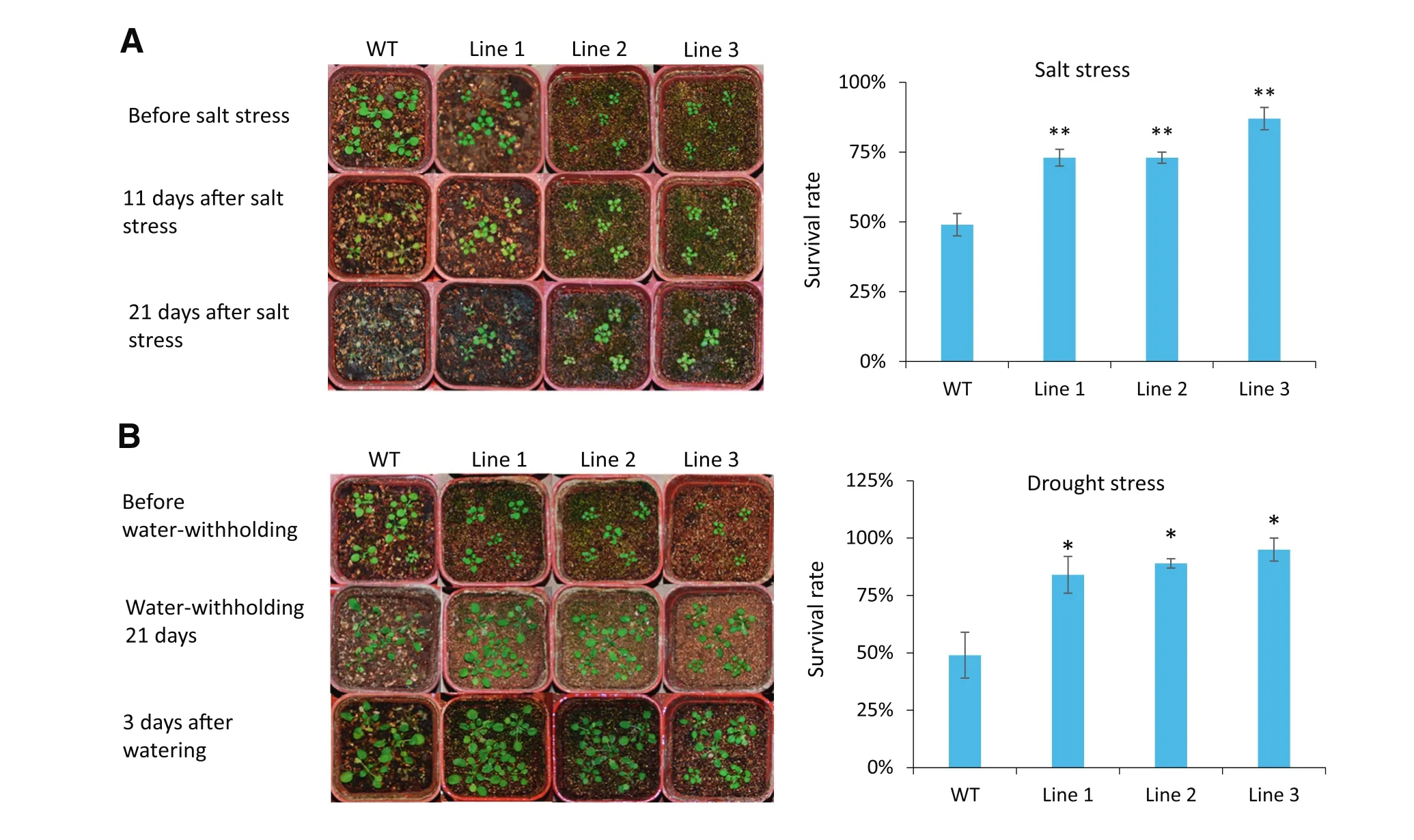
Fig.5–35S:TaSAUR75 promotes salt and drought tolerance in Arabidopsis.A.Salt tolerance of 35S:TaSAUR75 plants(lines 1,2,and 3).Healthy three-week-old wild-type and 35S:TaSAUR75 plants were treated with 250 mmol L?1NaCl followed by normal culturing for 3 weeks.B.Drought tolerance of 35S:TaSAUR75 plants(lines 1,2,and 3).Healthy 3-week-old wild-type and 35S:TaSAUR75 plants were grown for 21 days without water followed by rewatering for 3 days.Drought tolerance was assessed as the capability of plants to resume growth when returned to normal conditions following water stress.The survival rates of the wild-type and three independent transgenic lines under drought stress in soil are shown.Error bars represent the SD of three independent experiments and asterisks indicate a significant difference(*P<0.05;**P<0.01)compared with the corresponding controls.
To further study the expression of TaSAUR75 in wheat roots under salt stress,we selected two sensitive cultivars,Jimai 19 and Chinese Spring,and two tolerant cultivars,Han 12 and Cha Dianhong(Table 3)for evaluation.TaSAUR75 expression was decreased in wheat roots following 2%NaCl treatment in all four wheat cultivars,a finding that was consistent with the transcriptome analysis results.However,in the salt-sensitive cultivars Jimai 19 and Chinese Spring,TaSAUR75 expression continuously decreased and was maintained at a low level in response to salt stress.In contrast,in the salt-tolerant cultivars Cha Dianhong and Han 12,TaSAUR75 expression was partly restored during salt treatment,that of Han 12 was restored at 12 h,and that of Cha Dianhong was restored at 72 h.The expression of TaSAUR75 in Han 12 and Cha Dianhong remained at a higher level than that of the-salt sensitive cultivars,Jimai 19 and Chinese Spring(Fig.2).
3.3.TaSAUR75 increases plant salt and drought tolerance
Transgenic Arabidopsis plants overexpressing the full-length TaSAUR75 CDS were generated to validate the role of TaSAUR75 in mediating tolerance to salt stress.TaSAUR75 was integrated into the genome and was overexpressed in the three transgenic Arabidopsis lines(Fig.3-D).Homozygous transgenic lines overexpressing TaSAUR75 were selected for salt tolerance analysis.
The germination of the 35S:TaSAUR75 lines was faster than thatofwild-type plants under normalconditions on MS medium.On the second day,the proportion of wild-type plants that had germinated was 30%,whereas thatofthe 35S:TaSAUR75 lines that had germinated was>61%(Fig.3-A).There were no differences in germination rate between the 35S:TaSAUR75 lines and wild-type plants under the 100 mmol L?1NaCl stress conditions(Fig.3-B).Under an osmotic stress of 300 mmol L?1mannitol,the germination rate of 35S:TaSAUR75 lines was lower than that of wild-type lines(Fig.3-C).
After 7 days of erect growth on MS medium there was no difference in root length between the 35S:TaSAUR75 and wild-type lines,but the roots of the 35S:TaSAUR75 lines were much longer than those of the wild-type plants under both 100 mmol L?1NaCl and 300 mmol L?1mannitol stresses on MS medium(Fig.4).
At the seedling stage,when the 3-week-old Arabidopsis plants grown in pots were allowed to dry by withholding water for 21 days,followed by 3 days of watering or 21 days of 250 mmol L?1NaCl treatment,the survival rate of the WT plants was lower than that of the 35S:TaSAUR75 lines(Fig.5-A,B).These results indicated that TaSAUR75 increased plant tolerance to salt and drought stresses.
3.4.Expression pattern of stress-responsive genes in 35S:TaSAUR75 plants
The expression of stress-responsive genes was examined in wild-type plants and 35S:TaSAUR75 lines under normal and stress conditions by qRT-PCR.Multiple stress-responsive genes were selected for testing.However,there were no significant changes in the expression of RD29A,RD 17,SOS1,HSP70,MYB2,GolS1,and PR 1(data not shown).AtDREB2 was upregulated after 12 h of salt stress by 250 mm NaCl,and AtRD26 was upregulated after 1 h of drought stress(Fig.6-A,B).These results indicated that the abiotic tolerance of the 35S:TaSAUR75 plant lines was activated under salt and drought stress.
3.5.ROS and chlorophyll content of the 35S:TaSAUR75 lines and wild-type plants under stress
To further study the salt and drought stress tolerance mechanisms in the 35S:TaSAUR75 lines,we assessed the contents of O–2and H2O2present in the wild-type and 35S:TaSAUR75 plants after 7 days of growth on 100 mmol L?1NaCl or 300 mmol L?1mannitol MS plates by DAB,NBT and Trypan blue staining(Fig.7-A,B).Under salt stress,there was no difference between wild-type plants and 35S:TaSAUR75 lines regardless of O–2or H2O2content.However,when the leaves were soaked in 350 mmol L?1NaClfor36 h(Fig.7-C),the chlorophyllcontentofthe leaves ofthe wild-type plants,but not that of the 35S:TaSAUR75 lines,was significantly reduced.Under drought stress,more H2O2accumulated in wild-type plants than in 35S:TaSAUR75 lines.In addition,the lines were much lighter than wild-type plants dyed with Trypan blue,which acts in dead and notin live cells.These results indicated that the 35S:TaSAUR75 lines were tolerant to salt and drought stress.
4.Discussion
4.1.TaSAUR75 is inhibited by salt stress
Expression of TaSAUR75 rapidly decreased under salt stress in wheat roots in this study,indicating that TaSAUR75 was involved in the stress response.Furthermore,the expression of TaSAUR75 in salt-tolerant cultivars(Cha Dianhong and Han 12)was higher than that in salt-sensitive cultivars(Jimai 19 and Chinese Spring)after 72 h of salt treatment.
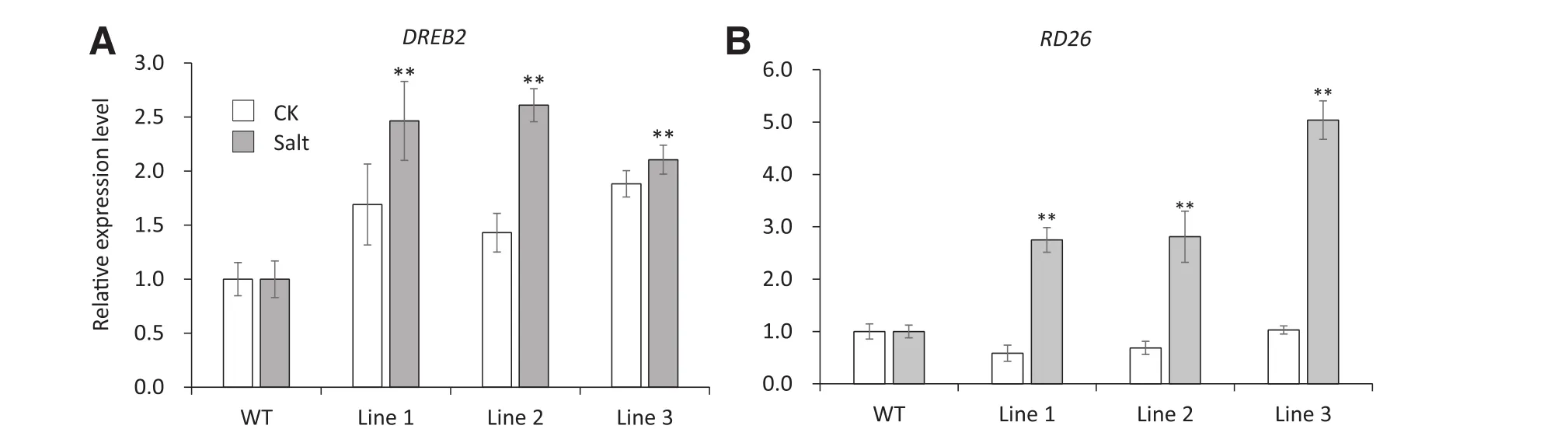
Fig.6–The relative transcript levels of stress responsive genes AtDREB 2 and AtRD 26 are increased by salt and drought stress treatment.A–B.Expression of AtDREB2(A)and AtRD26(B)in wild-type plants and 35S:TaSAUR75 lines under normal(CK)and salt-or drought-stress conditions.Asterisks indicate a significant difference(*P<0.05;**P<0.01)compared with the corresponding controls.
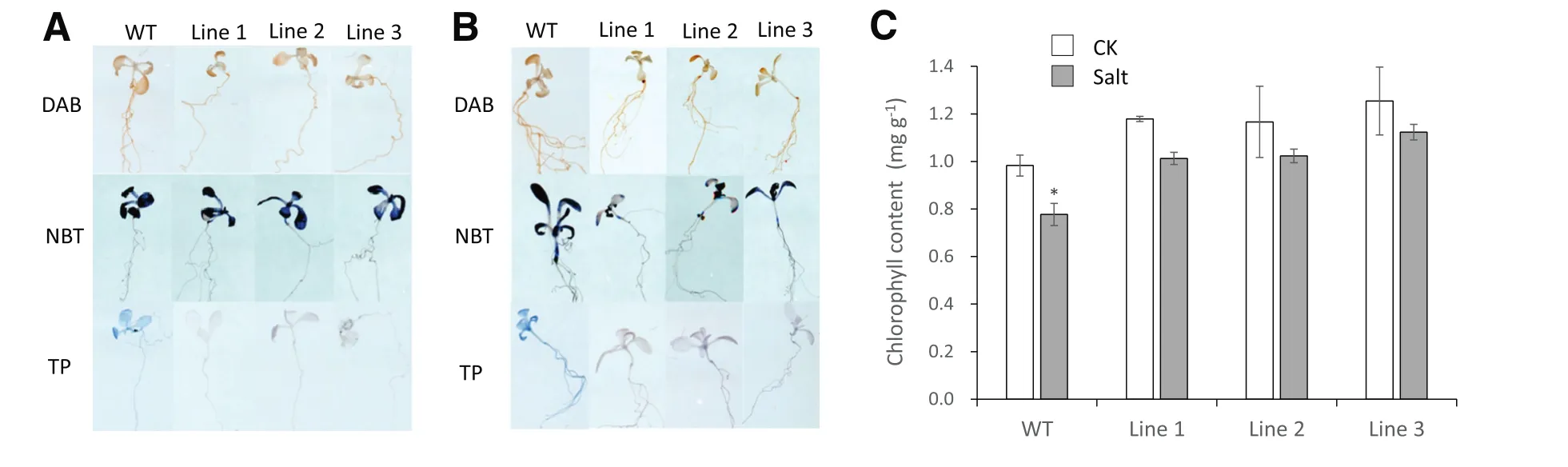
Fig.7–H2O2,O–2and chlorophyll content of 35S:TaSAUR75 lines and wild-type plants under stress.A,B.In situ detection of H2O2 and O–2 by DAB(3,3′-diaminobenzidine),NBT(nitro blue tetrazolium)and Trypan blue staining in WT and TaSAUR75 transgenic seedlings treated with 100 mmol L?1NaCl(A)or 300 mmol L?1mannitol(B).C.Relative chlorophyll content in leaves 36 h after NaCl treatment.The values are the means±SD of three independent experiments and asterisks indicate a significant difference(*P<0.05;**P<0.01)compared with the corresponding controls.
4.2.TaSAUR75 increase plant growth and development under abiotic stress
SAUR s are considered to promote plant growth and development[24–27].In this study,35S:TaSAUR75 lines grew faster than wild plants,and the roots of 35S:TaSAUR75 lines were longer than those of wild-type plants under salt stress.This result indicated that TaSAUR75 can maintain plantgrowth,reducing inhibition by abiotic stress.The survival rate of the 35S:TaSAUR75 lines was higher than that of wild-type plants after salt or drought treatment in soil,suggesting that TaSAUR75 might enhance plant tolerance to abiotic stress by promoting plant growth.The germination rate of 35S:TaSAUR75 lines was greater than that of wild plants on MS medium,also indicating that TaSAUR75 promotes plant growth.However,there was no significant difference between 35S:TaSAUR75 lines and wild plants on NaCl medium,suggesting that TaSAUR75 is not functional in the germination stage under salt stress.Interestingly,the germination rate of the 35S:TaSAUR75 lines was lower than that of wild-type plants on MS medium,showing that TaSAUR75 was sensitive to droughtstress.We suggestthatthe process leading to this phenomenon merits further study.
4.3.TaSAUR75 upregulates stress responsive genes
Multiple stress-responsive genes were selected for tests of their expression patterns in 35S:TaSAUR75 plants.AtRD26 and AtDREB2 were upregulated after drought stress and salt stress,respectively.RD26 belongs to the ATAF gene subfamily,which is involved in abiotic stress[40].Large-scale transcriptome analyses have shown that genes regulated by RD26 participate in ROS removal and defense[41].DREB2 is a transcription factor belonging to the AP2/ER F(DREB)family.DREB2 that is induced by osmosis and high salt stress,and overexpression of DREB2 increases plant tolerance to multiple abiotic stresses such as salt,drought and cold[42].Thus,overexpression of TaSAUR75 may play a direct or indirect role in the expression of downstream stress-associated genes.
4.4.TaSAUR75 promotes plant tolerance to abiotic stress
ROS are small molecules produced in response to many types of abiotic stress that damages tissues[42].In this study,35S:TaSAUR75 lines accumulated less H2O2than wild-type plants after drought stress,indicating that TaSAUR75 inhibited H2O2accumulation by activating stress-tolerance genes such as AtRD 26.AtRD 26 reduced ROS accumulation,which increased under drought in the 35S:TaSAUR75 line[43].
After salt stress,degradation of chlorophyll in the 35S:TaSAUR75 line was lower than that in wild-type plants,indicating that TaSAUR75 inhibited chlorophyll degradation under salt stress.The seedlings were dyed with Trypan blue to detect program cell death.
After Trypan blue staining,wild-type plant cells initiated programmed cell death,but 35S:TaSAUR75 lines did not do so under abiotic stress.Thus,TaSAUR75 may promote plant tolerance to abiotic stress by inhibiting H2O2accumulation and chlorophyll decrease under abiotic stress.
5.Conclusions
TaSAUR75 is downregulated under salt stress and is located in the cytoplasm and nucleus.Overexpression of TaSAUR75 increases tolerance to drought and salt stress in Arabidopsis.TaSAUR75 may increase tolerance to abiotic stress by promoting plant growth and development, upregulating stress-responsive genes,and inhibiting H2O2accumulation and chlorophyll decrease under abiotic stress.
This study was supported by the National Key Research and Development Program of China(2016YFD0100304),National Transgenic Key Project from the Ministry of Agriculture of China(2016ZX08002-002)and Agricultural Science and Technology Program for Innovation Team on the Identification and Excavation of Elite Crop Germplasm,CAAS.
[1]J.S.Boyer,P.Byrne,K.G.Cassman,M.Cooper,D.Delmer,T.Greene,F.Gruis,J.Habben,N.Hausmann,N.Kenny,R.Lafitte,S.Paszkiewicz,D.Porterf,A.Schlegel,J.Schussler,T.Setter,J.Shanahan,R.E.Sharp,T.J.Vyn,D.Warner,J.Gaffney,The U.S.drought of 2012 in perspective:a call to action,Glob.Food Sec.2(2013)139–143.
[2]Y.Hirabayashi,R.Mahendran,S.Koirala,L.Konoshima,D.Yamazaki,S.Watanabe,H.Kim,S.Kanae,Global flood risk under climate change,Nat.Clim.Chang.3(2013)816–821.
[3]S.C.Pryor,R.J.Barthelmie,J.T.Schoof,High-resolution projections of climate-related risks for the Midwestern USA,Clim.Res.56(2013)61–79.
[4]C.E.Bita,G.Tom,Plant tolerance to high temperature in a changing environment:scientific fundamentals and production of heat stress-tolerant crops,Front.Plant Sci.4(2013)273.
[5]S.M.Gourdji,A.M.Sibley,D.B.Lobell,Global crop exposure to critical high temperatures in the reproductive period:historical trends and future projections,Environ.Res.Lett.8(2013)02041.
[6]C.Rosenzweig,J.Elliott,D.Deryng,A.C.Ruane,C.Müllere,A.Arneth,K.J.Boote,C.Folberth,M.Glotter,N.Khabarov,K.Neumann,F.Piontek,T.A.M.Pugh,E.Schmid,E.Stehfest,H.Yang,J.W.Jones,Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison,Proc.Natl.Acad.Sci.U.S.A.111(2014)3268–3273.
[7]T.Iizumi,H.Sakuma,M.Yokozawa,J.J.Luo,A.J.Challinor,M.E.Brown,G.Sakurai,T.Yamagata,Prediction of seasonal climate-induced variations in global food production,Nat.Clim.Chang.3(2013)904–908.
[8]S.Furukawa,T.Fujita,M.Shimabukuro,M.Iwaki,Y.Yamada,Y.Nakajima,O.Nakayama,M.Makishima,M.Matsuda,I.Shimomura,Increased oxidative stress in obesity and its impact on metabolic syndrome,J.Clin.Invest.114(2004)1752–1761.
[9]V.Naser,E.Shani,Auxin response under osmotic stress,Plant Mol.Biol.91(2016)661–672.
[10]J.Park,Y.S.Kim,S.G.Kim,J.H.Jung,J.C.Woo,C.M.Park,Integration of auxin and salt signals by the NAC transcription factor NTM2 during seed germination in Arabidopsis,Plant Physiol.156(2011)537–549.
[11]N.Iqbal,S.Umar,N.A.Khan,M.I.R.Khan,A new perspective of phytohormones in salinity tolerance:regulation of proline metabolism,Environ.Exp.Bot.100(2014)34–42.
[12]P.Schopfer,A.Liszkay,M.Bechtold,G.Frahry,A.Wagner,Evidence that hydroxyl radicals mediate auxin-induced extension growth,Planta 214(2002)821–828.
[13]M.Iqbal,M.Ashraf,Alleviation of salinity-induced perturbations in ionic and hormonal concentrations in spring wheat through seed preconditioning in synthetic auxins,Acta Physiol.Plant.35(2013)1093–1112.
[14]D.Hao,W.Nai,Y.Chang,X.H.Li,J.H.Xiao,L.Z.Xiong,Carotenoid deficiency impairs ABA and IAA biosynthesis and differentially affects drought and cold tolerance in rice,Plant Mol.Biol.83(2013)475–488.
[15]H.T.Shi,L.Chen,T.T.Ye,X.D.Liu,K.J.Ding,Z.L.Chan,Modulation of auxin content in Arabidopsis confers improved drought stress resistance,Plant Physiol.Biochem.82(2014)209–217.
[16]G.Hagen,T.Guilfoyle,Auxin-responsive gene expression:genes,promoters and regulatory factors,Plant Mol.Biol.49(2002)373–385.
[17]M.Jain,A.K.Tyagi,J.P.Khurana,Genome-wide analysis,evolutionary expansion,and expression of early auxinresponsive SAUR gene family in rice(Oryza sativa),Genomics 88(2006)360–371.
[18]J.Wu,S.Y.Liu,Y.J.He,X.Y.Guan,X.F.Zhu,L.Cheng,J.Wang,G.Lu,Genome-wide analysis of SAUR gene family in Solanaceae species,Gene 509(2012)38–50.
[19]S.K.Wang,Y.H.Bai,C.J.Shen,Y.R.Wu,S.N.Zhang,D.A.Jiang,T.J.Guilfoyle,M.Chen,Y.H.Qi,Auxin-related gene families in abiotic stress response in Sorghum bicolor,Funct.Integr.Genomics 10(2010)533–546.
[20]Y.Z.Chen,X.Hao,J.Cao,Small auxin upregulated RNA(SAUR)gene family in maize:identification,evolution,and its phylogenetic comparison with Arabidopsis,rice,and sorghum,J.Integr.Plant Biol.56(2014)133–150.
[21]R.J.Xie,C.C.Dong,Y.Y.Ma,L.Deng,S.L.He,S.L.Yi,Q.Lv,Y.Q.Zheng,Comprehensive analysis of SAUR,gene family in citrus and its transcriptional correlation with fruitlet drop from abscission zone A,Funct.Integr.Genomics 15(2015)729–740.
[22]Q.S.Bai,D.Hou,L.Li,Z.C.Cheng,W.Ge,J.Liu,X.P.Li,S.H.Mu,J.Gao,Genome-wide analysis and expression characteristics of small auxin-up RNA(SAUR)genes in moso bamboo(Phyllostachys edulis),Genome 60(2017)325–336.
[23]A.K.Spartz,S.H.Lee,J.P.Wenger,N.Gonzalez,H.Itoh,D.Inzé,W.A.Peer,A.S.Murphy,P.J.Overvoorde,W.M.Gray,The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion,Plant J.70(2012)978–990.
[24]Y.Y.Kong,Y.B.Zhu,C.Gao,W.J.She,W.Q.Lin,Y.Chen,N.Han,H.W.Bian,M.Y.Zhu,J.H.Wang,Tissue-specific expression of SMALL AUXIN UP RNA41 differentially regulates cell expansion and root meristem patterning in Arabidopsis,Plant Cell Physiol.54(2013)609–621.
[25]K.Chae,C.G.Isaacs,P.H.Reeves,G.S.Maloney,G.K.Muday,P.Nagpal,J.W.Reed,Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation,Plant J.71(2012)684–697.
[26]Z.G.Li,H.W.Chen,Q.T.Li,J.J.Tao,X.H.Bian,B.Ma,W.K.Zhang,S.Y.Chen,J.S.Zhang,Three SAUR proteins SAUR76,SAUR77 and SAUR78 promote plant growth in Arabidopsis,Sci Rep 5(2015)12477.
[27]H.Kai,W.Wu,S.S.Gan,SAUR36,a small auxin up RNA gene,is involved in the promotion of leaf senescence in Arabidopsis,Plant Physiol.161(2013)1002–1009.
[28]H.Aoki,A.Onishi,M.Miyashita,H.Miyagawa,O.Yatou,K.Saito,Involvement of the rice OsSAUR51 gene in the auxinrelated field resistance mechanism against bacterial blight disease,JARQ 50(2016)219–227.
[29]O.P.Gupta,N.L.Meena,I.Sharma,P.Sharma,Differential regulation of microRNAs in response to osmotic,salt and cold stresses in wheat,Mol.Biol.Rep.41(2014)4623–4629.
[30]M.Sadder,A.Alsadon,M.Wahb-Allah,Transcriptomic analysis of tomato lines reveals putative stress-specific biomarkers,Turk.J.Agric.For.38(2015)108–114.
[31]M.Jain,J.P.Khurana,Transcript profiling reveals diverse roles of auxin-responsive genes during reproductive development and abiotic stress in rice,FEBS J.276(2009)3148–3162.
[32]A.K.Spartz,H.Ren,M.Y.Park,K.N.Grandt,S.H.Lee,A.S.Murphy,M.R.Sussman,P.J.Overvoorde,W.M.Gray,Saur inhibition of pp2c-d phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis,Plant Cell 26(2014)2129–2142.
[33]H.D.Robert,D.I.Arnon,Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients,Soil Sci.50(1940)463–485.
[34]Q.Y.Jiang,Z.Hu,H.Zhang,Y.Z.Ma,Overexpression of GmDREB1 improves salt tolerance in transgenic wheat and leaf protein response to high salinity,Crop J.2(2014)120–131.
[35]J.Zhu,Z.L.Bie,Y.N.Li,Physiological and growth responses of two different salt-sensitive cucumber cultivars to NaCl stress,Soil Sci.Plant Nutr.54(2008)400–407.
[36]U.Erturk,N.Sivritepe,C.Yerlikaya,M.Bor,F.Ozdemir,I.Turkan,Responses of the cherry rootstock to salinity in vitro,Biol.Plant.51(2007)597–600.
[37]J.Shaterian,D.Waterer,H.De Jong,K.K.Tanino,Differential stress responses to NaCl salt application in early-and latematuring diploid potato(Solanum sp.)clones,Environ.Exp.Bot.54(2005)202–212.
[38]A.Zhen,Z.L.Bie,Y.Huang,Z.X.Liu,Q.Li,Effects of scion and rootstock genotypes on the anti-oxidant defense systems of grafted cucumber seedlings under NaCl stress,Soil Sci.Plant Nutr.56(2010)263–271.
[39]H.P.Zhou,J.F.Zhao,Y.Q.Yang,C.X.Chen,Y.F.Liu,X.H.Jin,L.M.Chen,X.Y.Li,X.W.Deng,K.S.Schumaker,Y.Guo,UBIQUITIN-SPECIFIC PROTEASE16 modulates salt tolerance in Arabidopsis by regulating Na+/H+antiport activity and serine hydroxymethyl transferase stability,Plant Cell 24(2012)5106–5122.
[40]M.Kasuga,Q.Liu,S.Miura,K.Yamaguchi-Shinozaki,K.Shinozaki,Improving plant drought,salt and freezing tolerance by gene transfer of a single stress-inducible transcription factor,Nat.Biotechnol.236(2001)176–186.
[41]S.Balazadeh,M.Kwasniewski,C.Caldana,M.Mehrnia,M.I.Zanor,G.P.Xue,B.M.Roeber,ORS1,an H2O2-responsive NAC transcription factor,controls senescence in Arabidopsis thaliana,Mol.Plant 4(2011)346.
[42]Q.Liu,M.Kasuga,Y.Sakuma,H.Abe,S.Miura,K.Yamaguchi-Shinozaki,K.Shinozaki,Two transcription factors,DREB1 and DREB2,with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought and low temperature responsive gene expression,respectively,in Arabidopsis,Plant Cell 10(1998)1391–1406.
[43]K.Kazan,Auxin and the integration of environmental signals into plant root development,Ann.Bot.112(2013)1655–1665.
- The Crop Journal的其它文章
- Genetic diversity assessment of a set of introduced mung bean accessions(Vigna radiata L.)
- Near-infrared reflectance spectroscopy reveals wide variation in major components of sesame seeds from Africa and Asia
- Alternate phenotype–genotype selection for developing superior high-yielding irrigated rice lines
- Development and validation of simple sequence repeat markers from Arachis hypogaea transcript sequences
- Soybean hairy roots produced in vitro by Agrobacterium rhizogenes-mediated transformation
- Elevated temperature intensity, timing, and duration of exposure affect soybean internode elongation, mainstem node number, and pod number per plant

