γ-Ray Irradiation-Derived MnO/rGO Composites for High Performance Lithium Ion Batteries
Y-li Guo,Hong-chng Jin,Zhen-zhen Du,Xue-wu Ge,Heng-xing Ji?
a.Department of Materials Science and Engineering,CAS Key Laboratory of Materials for Energy Conversion,iChEM(Collaborative Innovation Center of Chemistry for Energy Materials),University of Science and Technology of China,Hefei 230026,China
b.Department of Polymer Science and Engineering,CAS Key Laboratory of Soft Matter Chemistry, University of Science and Technology of China,Hefei 230026,China
γ-Ray Irradiation-Derived MnO/rGO Composites for High Performance Lithium Ion Batteries
Ya-li Guoa,Hong-chang Jina,Zhen-zhen Dua,Xue-wu Geb,Heng-xing Jia?
a.Department of Materials Science and Engineering,CAS Key Laboratory of Materials for Energy Conversion,iChEM(Collaborative Innovation Center of Chemistry for Energy Materials),University of Science and Technology of China,Hefei 230026,China
b.Department of Polymer Science and Engineering,CAS Key Laboratory of Soft Matter Chemistry, University of Science and Technology of China,Hefei 230026,China
We report a γ-ray irradiation reduction method to prepare MnO/reduced graphene oxide (rGO)nanocomposite for the anode of lithium ion batteries.γ-Ray irradiation provides a clean way to generate homogeneously dispersed MnO nanoparticles with finely tuned size on rGO surface without the use of surfactant.The MnO/rGO composite enables a fully charge/discharge in 2 min to gain a reversible speci fic capacity of 546(mA·h)/g which is 45%higher than the theoretical value of commercial graphite anode.
γ-Ray irradiation reduction,MnO anode,Reduced graphene oxide,Nanocomposites,Lithium ion batteries
I.INTRODUCTION
Portable electronics and electrical vehicles require advanced lithium ion batteries(LIBs)that can deliver an energy density of>250 Wh/kg at a high rate of>10 C for>1000 cycles[1],therefore,electrode materials with both high speci fic capacity and rate capability are greatly demanded to meet these goals.However, the existent commercial graphite anode has a limited speci fic capacity(372(mA·h)/g),while anode materials like transition metal oxides and silicon,which possesses high theoretical speci fic capacities,suffer from poor rate capability and cycling life due to their low electrical conductivity and volume expansion during charge/discharge[2].For instance,MnO is an attractive anode material for LIBs because of its low conversion potential and voltage hysteresis of<0.8 V,high theoretical capacity of 756(mA·h)/g,and high mass density of 5.43 g/cm3coupled with low cost,environmental friendliness,and high nature abundance of Mn element [3],however,its high electrical resistivity of 106?·cm [4]and volume change[5]result in an inferior speci fic capacity,rate capability and cycling life.
To date,different strategies have been proposed to address these issues and conclude that an optimized MnO-based anode material(also valid for other transion metal oxides or silicon)should possess nanometersize MnO particles uniformly dispersed on conductive frameworks to facilitate Li+diffusion and electron conduction.The conductive framework also serves as a stable support to prevent the loss of pulverized MnO upon Li+insertion/extraction to extend the cycling life[6].Recently,reduced graphene oxide(rGO), a two-dimensional material with excellent conductivity,large surface area,structural and chemical stability[7],has been demonstrated as an optimal choice to address the above mentioned issues[3a,8],and a variety of methods have been reported to prepare MnO/rGO composites.The most popular synthesis methods include hydrothermal and wet chemical reduction which can yield ultra fine MnO nanocrystals on rGO microsheets with promising electrochemical properties for LIBs[3a,8a],whereas,high pressure conditions and poor scalability of hydrotheromal process or toxic reduction agent used in wet chemical method hinder their practical applications.Especially,surfactant is required in these synthesis methods to prevent the aggregation of MnO nanoparticles and restacking of rGO sheets which is sandwiched between the MnO and rGO,ultimately hinders the charge transfer during charge/discharge.To the best of our knowledge,MnO-based anode materials for LIBs with high reversible capacity(e.g.~2000(mA·h)/g,with respect to the mass of the MnO/rGO composite,both here and below)and rate capability(e.g.~800(mA·h)/g at 8 A/g,corresponding to a fully charge/discharge in 6 min)combined with long cycle life(e.g.>500 cycles)has rarely been reported.
γ-Ray is high energy electromagnetic radiation usually produced by the decay of high energy states of atomic nuclei(γ-decay),which can decompose water molecules to form both reductive(hydrogen radical andhydrated electron,·H and e?aq)and oxidative(hydroxyl radical·OH)species.These species concentrations are dependent on the radiation intensity and will be deactivated when radiation is shut down,thereby,providing well controlled and clean sources of oxidants or reductants for chemical reactions.When water is mixed with oxidant sacri fice agents(e.g.ethanol),·OH can be eliminated and transformed into reductive radicals(·OH+CH3CH2OH→·CH(CH3)OH+H2O)which can reduce GO.Radiolysis-induced reduction reaction can generate homogeneously distributed condense products of nanometer sizes because of the negative standard potential of e?aq(~2.77 V vs.standard hydrogen electrode). The shape and size of the nanoparticles can be readily controlled by adjusting the irradiation time and intensity[9].Especially,e?aqreduced rGO can be well dispersed in water without the assistance of surfactants[9d].
Here we report a γ-ray irradiation reduction method to prepare MnO/rGO composites carried out at room temperature and ambient pressure.The as prepared composite shows excellent dispersity in aqueous solution,and the MnO nanoparticles are well dispersed on rGO surface with a narrow particle-size dispersion of (20±3)nm without the assistance of any surfactant. Therefore,the MnO/rGO composite shows high speci fic capacities retention of 2175,775 and 546(mA·h)/g (with respect to the mass of the composite)at high charge/discharge current densities of 0.2,8,and 15 A/g, corresponding to a fully charge/discharge in 2 min yet with speci fic capacity 45%higher than the theoretical value of graphite.
II.RESULTS AND DISCUSSION
As the photographs in FIG.1(a)show,manganese oxide/rGO reduced by hydroiodic acid(HI)precipitates gradually after keeping for 2 h in the solvent,while the sample produced by γ-ray still disperses evenly in the solvent,indicating a more homogeneous dispersion of the γ-ray reduced product.We studied the effect of irradiation time on the chemical composition of the manganese oxide/rGO composite. As shown in the X-ray diffraction(XRD)patterns(FIG.1(b)),crystal structure is non-detectable after the KMnO4/GO mixture was exposed to γ-ray for 4 h,and Mn3O4crystals are found with extended exposure time of more than 8 h.The X-ray photoelectron spectroscopy(XPS)of C 1s(FIG.S1 in supplementary materials)shows strong peak at 284.8 eV that can be assigned to graphitic carbon(C=C)in graphene,while the satellite peaks at higher binder energies arise from the oxygenated carbon atoms(C?O at 286.2 eV,C=O at 287 eV,O?C=O at 288.5 eV).The analysis of C 1s spectra indicates that with the increase of γ-ray irradiation time from 4 h to 32 h,the ratio of carbon-carbon bonds increases from~54%to~71%,and the contents of oxygenatedcarbon(O?C=O,O?C,and O=C)decrease to~29% (FIG.1(c)),indicating an increased reduction level of GO with extended γ-ray irradiation.
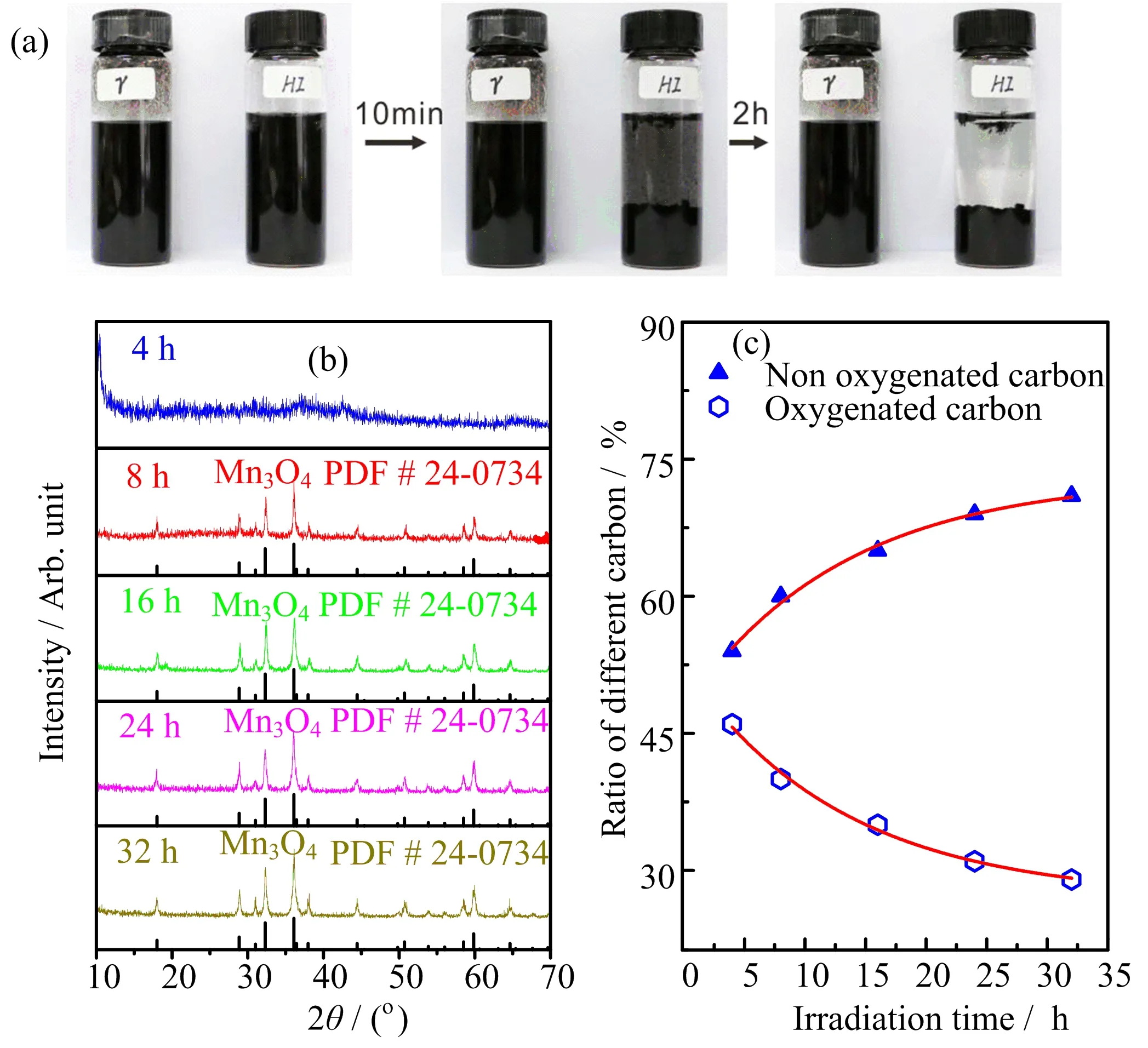
FIG.1(a)Photographs of the manganese oxide/rGO reduced by γ-ray and HI.(b)XRD patterns and(c)the contents of carbon species acquired by XPS spectra of C 1s for the manganese oxide/rGO composites prepared by γ-ray irradiation.
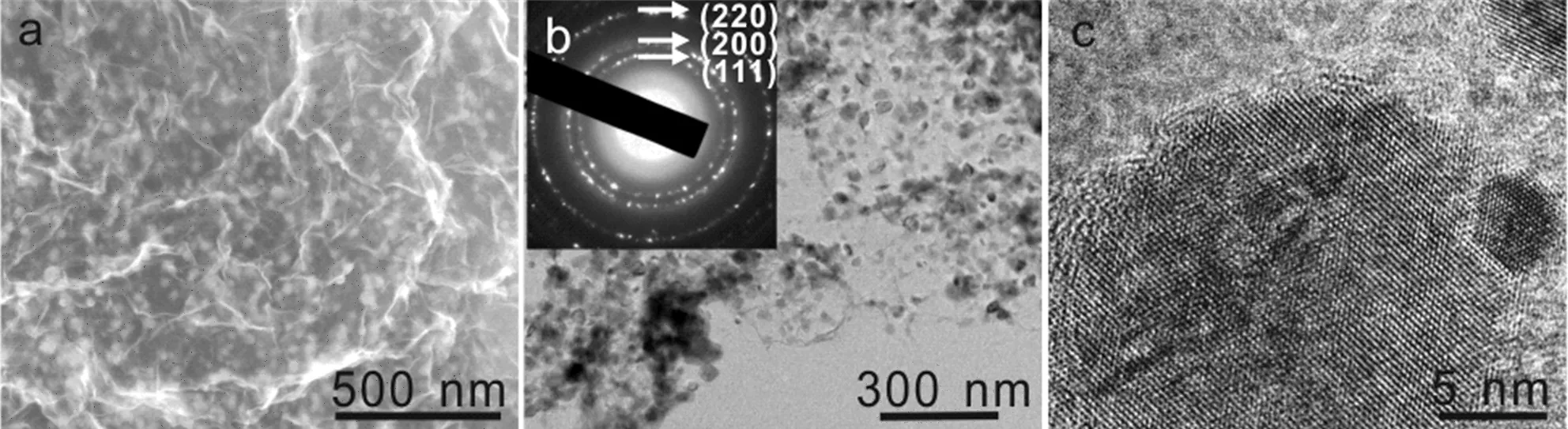
FIG.2(a)SEM and(b,c)TEM images of the MnO/rGO composites.The inset in panel(b)is the indexed selected area electron diffraction pattern.
The Mn3O4/rGO composites were subject to thermal annealing.The XRD pattern(FIG.S2 in supplementary materials,PDF#07-0230),Raman spectrum(FIG.S3 in supplementary materials),and XPS (FIG.S4 in supplementary materials)of the annealed composite indicate that the Mn3O4was further reduced to MnO.The scanning electron microscope(SEM)image(FIG.2(a))and electron dispersion X-ray(EDX, FIG.S5 in supplementary materials)mapping show a uniform dispersion of MnO particles on graphene sheets. These MnO nanoparticles present crystal nature with size in the range of tens of nanometers(FIG.2(b)and (c)).We studied the MnO particle size distribution by measuring and counting the particles in the SEM images of MnO/rGO composites reduced by γ-ray irradiation for different times(FIG.S6 in supplementary materials),and the statistic values are summarized in FIG.3(a).The MnO nanoparticles on rGO sheets have an average size of(27±6)nm when the γ-ray irradiation time was 8 h,which drops to(20±3)nm and subsequently increased to(28±5)and(33±6)nm when the γ-ray irradiation time was increased to 16,24,and 32 h. The larger MnO particle size for the composites irradiated for 8 h than that of 16 h may be due to the insufficient reduction of KMnO4(FIG.1(b)and(c)).And the further increase of the MnO particle size with the irradiation time indicates the γ-ray irradiation induced MnO growth,which shows as a slow rate of~0.8 nm/h. Therefore,the size of MnO nanoparticles can be finely tuned by γ-ray irradiation without the assistant of surfactant,which is very important for high-rate electrode materials as smaller MnO nanoparticles and direct attachment on rGO is crucial for charge transfer during the charge/discharge.Moreover,the mass ratio of MnO in composite that was studied by thermal gravimetric analysis(FIG.S7 in supplementary materials)can be readily tuned by varying the content of the KMnO4/GO mixture.The highest MnO mass ratio of 73.5 wt%was achieved when a GO/KMnO4mass ratio of 0.05 was applied(FIG.3(b)).
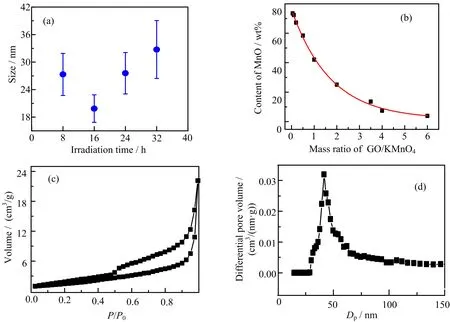
FIG.3(a)MnO particle size distribution for the MnO/rGO prepared with a KMnO4/GO mass ratio of 2 and γ-ray irradiation time of 8,16,24,and 32 h.(b)The content of MnO in composite prepared with KMnO4/GO mixture of different mass ratios.(c)Nitrogen adsorption-desporption isotherms of MnO/rGO composites.(d)Pore size distribution calculated by density functional theory.
For the further studies,we focus on the MnO/rGO composite prepared by γ-ray irradiation on KMnO4/GO(weight ratio of 2)for 16 h because of the smallest MnO particle size and moderate MnO mass loading of 58.5 wt%,which are favored for the LIBs. The pore structures of the MnO/rGO was detected by N2isotherms(FIG.3(c)).The calculated speci fic surface area of the MnO/rGO is 160 m2/g with average pore size of 41.5 nm.The porous structure of the MnO/rGO could be ascribed to the stacking of 20 nm-large MnO decorated rGO microsheets.
The electrochemical performance of MnO/rGO composites was investigated with R2032 coin type cell with Li metal as the counter/reference electrode.The galvanic charge/discharge(GCD)curves of MnO/rGO composite at different current densities are plotted in FIG.4(a).The discharge curve shows a plateau at 0.5 V vs.Li/Li+and a speci fic capacity of 2175(mA·h)/g at a low current density of 0.2 A/g.Note that the speci fic capacity value was calculated with respect to the mass of MnO/rGO composite.The subsequent charge curves show two plateaus at around 2.1 and 1.3 V vs.Li/Li+. These plateaus match well with the faradaic peaks in cyclic voltammograms curves(FIG.S8 in supplementary materials).These revisable cathodic and anodic peaks can be assigned to the revisable electrochemical reaction[10]:
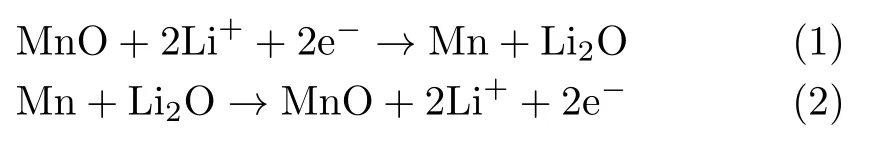
The discharge speci fic capacity gradually decreases to 546(mA·h)/g at the current density of 15 A/g with the discharge plateau at around 0.25 V clearly visible, indicative of excellent kinetics of the MnO lithiation [11].The rate capability of the MnO/rGO composite is shown in FIG.4(b).The speci fic capacities measuredat current densities of 0.2,0.5,1.0,2.0,4.0,6.0,8.0, 10,12 and 15 A/g are 1937,1684,1605,1301,1124, 971,775,714,634 and 546(mA·h)/g,respectively,with respect to the mass of composite.After changing the current density back to 0.2 A/g,the reversible capacity recovers to 2175(mA·h)/g,indicating an excellent electrochemical reversibility.The high reversible speci fic capacity of 546(mA·h)/g at a current density of 15 A/g corresponds to a fully charge/discharge in 2 min to gain energy that is 45%higher than the commercial graphite anode.This result is superior to previous MnO based anode materials(FIG.4(c))[3a,8b,12],which can be ascribed to the γ-ray irradiation induced wellcontrolled MnO particle size and direct attachment of MnO on rGO sheet without surfactant in between.
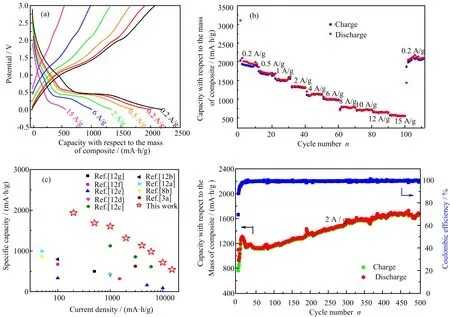
FIG.4(a)GCD curves,(b)rate capability at different current densities,(c)comparison of the speci fic capacity with other high-performance LIBs shown in references.(Refs.[12e-g]correspond to N-doped graphene,Refs.[12b?d]correspond to Fe3O4/graphene,SnO2/N-doped graphene and ZnO/graphene composites,respectively;Refs.[3a,8b,12a]correspond to MnO/graphene composites),(d)cycling performance at the current density of 2 A/g.
The cycling performance of MnO/rGO composites under 2 A/g is displayed in FIG.4(d). It is interesting to see that the speci fic capacity and coulombic efficiency increase at the initial cycles which has also been observed in previously reported Mn-based materials[3a,11,13]for LIBs.This phenomenon is owing to the activation process of MnO particles. At the initial cycles,only the top surface of the MnO can take into lithiation/delithiation reaction because of the low electrical conductivity of MnO.Whereas,the lithiation/delithiation process gradually penetrates into the core of the MnO particles and generates smaller MnO particles,thereby,improves the speci fic capacity of the composite[14].This result is in accordance with the decreased charge transfer resistance measured by electrochemical impedance spectroscopy(FIG.S9 in supplementary materials),down shift of the anodic peak at 2.1 V with cycling(FIG.S10 in supplementary materials),and 2?5 nm MnO nanocrystals after 500 cycles charge/discharge that are signi ficantly smaller than the MnO at the initial state(FIG.S11 in supplementary materials).For comparison,the electrochemical properties of MnO/rGO composites reduced by HI are displayed in FIG.S12 in supplementary materials,which indicates a lower speci fic capacity and rate capability than the γ-ray reduced MnO/rGO composite.
CV tests with a scan rate from 0.1 mV/s to 20 mV/s are employed to study the charge storage mechanism of the MnO/rGO composite.The cathodic peaks at~0.3 V and anodic peaks at~1.3 V are chosen to study the relationship between the current density(i) and scan rate(v)according to the power law equation:i=avb[15],where a and b are adjustable val-ues. A b-value of 0.5 indicates the lithium storage is totally solid-state diffusion-controlled whereas a bvalue of 1.0 represents a totally capacitive-dominated process.FIG.5(a)plots lgi of both the cathodic and anodic peak current density with respect to lgv.The b-values calculated by linear fitting of v in the range of 0.1 mV/s to 3 mV/s are 0.75 and 0.71,respectively,for the cathodic and anodic peaks,indicating a capacitive-dominated lithium storage.The b-value decreases to 0.37 and 0.5 for the cathodic and anodic currents,respectively,when v>3 mV/s,indicating solidstate diffusion-controlled lithium storage.
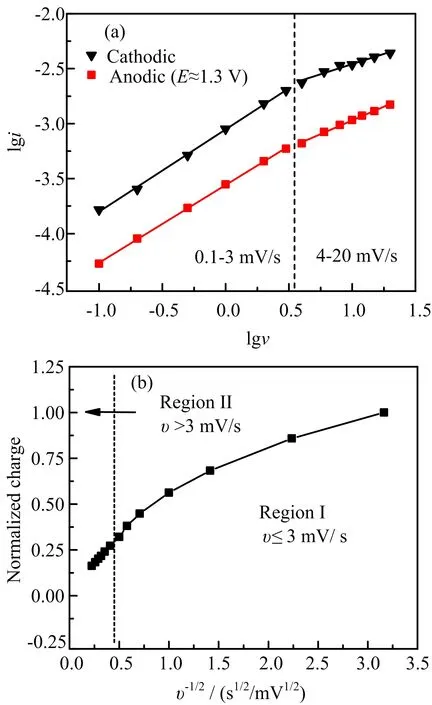
FIG.5(a)Linear relationship between logarithm current (lgi)and logarithm sweep rate(lgv)at the cathodic and anodic potentials.(b)Normalized charge versus v?1/2allows for the separation of diffusion-limited from capacitivelimited charge storage.
The total charge(Q)stored in MnO/rGO composite can be expressed as Q=Qd+Qc,where Qdand Qcare charges contributed by faradaic reaction and capacitive process,respectively.In the case of semi-in finite linear diffusion,Qdis dependent on v where Qd=cv?1/2[16].FIG.5(b)shows the plot of normalized charge versus v?1/2for MnO/rGO measured with scan rates from 0.1 mV/s to 20 mV/s.In the region I,where v≤3 mV/s,the Q is nonlinear with v?1/2,indicating that Q is mostly irrelevant with scan rate.In this scan rate region,the charge storage is mainly attributed to surface capacitive contribution.In region II,where the v>3 mV/s,the Q increases linearly with v?1/2,indicating that the charge storage is limited by diffusion process at high rates.
Based on the above analysis,the excellent rate capability of MnO/rGO can be attributed to the structure features yielded by the γ-ray irradiation synthesis.The e?aqinduces a homogeneous reduction of KMnO4and growth of Mn3O4on rGO surface,thereby,the particle size of MnO can be controlled in the rate of 0.8 nm/h with size variation of a few nanometers.The nanometer size MnO facilitate lithiation/delithiation reaction when charge/discharge. The γ-irradiation generates a stable suspension of Mn3O4/rGO without the assistance of surfactant,allowing for the direct condensing of manganese oxide on rGO surface.Such contact is favored for charge transfer between the MnO and rGO during charge/discharge.
III.CONCLUSION
We have demonstrated a γ-ray irradiation reduction method to synthesize MnO/rGO composites.The γray irradiation generated e?aqproduces homogeneously distributed MnO with finely tuned particle size on rGO without the assistance of surfactant.The MnO/rGO composites outputs a reversible gravimetric capacities of 2175 and 546(mA·h)/g with respect to the mass of the whole composite at current densities of 0.2 and 15 A/g,respectively,demonstrating a remarkable rate capability for the anode of LIBs.
IV.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.21373197),the 100 Talents Program of the Chinese Academy of Sciences, USTC Startup and the Fundamental Research Funds for the Central Universities(WK2060140018).
[1]L.Lu,X.Han,J.Li,J.Hua,and M.Ouyang,J.Power Sources 226,272(2013).
[2](a)X.Zheng,H.Wang,C.Wang,Z.Deng,L.Chen, Y.Li,T.Hasan,B.and L.Su,Nano Energy 22,269 (2016). (b)C.Chen,Y.Huang,H.Zhang,X.Wang,Y.Wang, L.Jiao,and H.Yuan,J.Power Sources 314,66(2016). (c)J.Hu,C.F.Sun,E.Gillette,Z.Gui,Y.Wang,and S.B.Lee,Nanoscale 8,12958(2016). (d)D.Y.Park,Y.K.Sun,and S.T.Myung,J.Power Sources 280,1(2015). (e)J.Wu,H.Chen,I.Byrd,S.Lovelace,and C.Jin, ACS Appl.Mat.Interfaces 8,13946(2016).
[3](a)Y.Sun,X.Hu,W.Luo,F.Xia,and Y.Huang,Adv. Funct.Mater.23,2436(2013). (b)X.Fang,X.Lu,X.Guo,Y.Mao,Y.S.Hu,J.Wang, Z.Wang,F.Wu,H.Liu,and L.Chen,Electrochem. Commun.12,1520(2010).
[4]M.Ali,M.Fridman,M.Denayer,and P.Nagels,Phys. Status Solidi B 28,193(1968).
[5](a)Y.Xia,Z.Xiao,X.Dou,H.Huang,X.Lu,R.Yan, Y.Gan,W.Zhu,J.Tu,and W.Zhang,ACS Nano 7, 7083(2013). (b)W.M.Chen,L.Qie,Y.Shen,Y.M.Sun,L.X. Yuan,X.L.Hu,W.X.Zhang,and Y.H.Huang,Nano Energy 2,412(2013). (c)H.Jiang,Y.Hu,S.Guo,C.Yan,P.S.Lee,and C. Li,ACS Nano 8,6038(2014).
[6]X.Liu,C.Chen,Y.Zhao,and B.Jia,J.Nanomater. 2013,1(2013).
[7]A.K.Geim and K.S.Novoselov,Nat.Mater.6,183 (2007).
[8](a)K.Zhang,P.Han,L.Gu,L.Zhang,Z.Liu,Q.Kong, C.Zhang,S.Dong,Z.Zhang,J.Yao,H.Xu,G.Cui, and L.Chen,ACS Appl.Mat.Interfaces 4,658(2012). (b)S.Zhang,L.Zhu,H.Song,X.Chen,and J.Zhou, Nano Energy 10,172(2014).
[9](a)L.M.Alrehaily,J.M.Joseph,A.Y.Musa,D.A. Guzonas,and J.C.Wren,Phys.Chem.Chem.Phys. 15,98(2013). (b)L.M.Alrehaily,J.M.Joseph,J.C.Wren,J.Phys. Chem.C 119,16321(2015). (c)A.Ans′on-Casaos,J.A.Pu′ertolas,F.J.Pascual, J.Hern′andez-Ferrer,P.Castell,A.M.Benito,W.K. Maser,and M.T.Martnez,Appl.Surf.Sci.301,264 (2014). (d)B.Zhang,L.Li,Z.Wang,S.Xie,Y.Zhang,Y. Shen,M.Yu,B.Deng,Q.Huang,C.Fan,and J.Li,J. Mater.Chem.A 22,7775(2012).
[10]Y.Xiao,X.Wang,W.Wang,D.Zhao,and M.Cao, ACS Appl.Mat.Interfaces 6,2051(2014).
[11]D.Kang,Q.Liu,R.Si,J.Gu,W.Zhang,and D.Zhang, Carbon 99,138(2016).
[12](a)Q.Sun,Z.Wang,Z.Zhang,Q.Yu,Y.Qu,J.Zhang, Y.Yu,and B.Xiang,ACS Appl.Mat.Interfaces 8,6303 (2016). (b)L.Li,A.Kovalchuk,H.Fei,Z.Peng,Y.Li,N.D. Kim,C.Xiang,Y.Yang,G.Ruan,and J.M.Tour, Adv.Energy Mater.5,1500171(2015). (c)R.Wang,C.Xu,J.Sun,L.Gao,and H.Yao,ACS Appl.Mat.Interfaces 6,3427(2014). (d)M.Yu,A.Wang,Y.Wang,C.Li,and G.Shi, Nanoscale 6,11419(2014). (e)T.Hu,X.Sun,H.Sun,G.Xin,D.Shao,C.Liu,and J.Lian,Phys.Chem.Chem.Phys.16,1060(2014). (f)Z.Y.Sui,C.Wang,Q.S.Yang,K.Shu,Y.W.Liu, B.H.Han,and G.G.Wallace,J.Mater.Chem.A 3, 18229(2015). (g)Z.S.Wu,W.Ren,L.Xu,F.Li,and H.M.Cheng, ACS Nano 5,5463(2011).
[13]H.Wang,Z.Xu,Z.Li,K.Cui,J.Ding,A.Kohandehghan,X.Tan,B.Zahiri,B.C.Olsen,C.M.Holt,and D.Mitlin,Nano Lett.14,1987(2014).
[14]Y.Xiao and M.Cao,ACS Appl.Mat.Interfaces 7, 12840(2015).
[15](a)H.Xiong,M.D.Slater,M.Balasubramanian,C.S. Johnson,and T.Rajh,J.Phys.Chem.Lett.2,2560 (2011). (b)J.Zhang,W.Zhang,T.He,I.S.Amiinu,Z.Kou, J.Li,and S.Mu,Carbon 115,95(2017).
[16]S.Ardizzone,G.Fregonara,and S.Trasatti,Electrochim.Acta 35,263(1990).
ceived on March 30,2017;Accepted on April 22,2017)
?Author to whom correspondence should be addressed.E-mail: jihengx@ustc.edu.cn,Tel./FAX:+86-551-63607290
 CHINESE JOURNAL OF CHEMICAL PHYSICS2017年4期
CHINESE JOURNAL OF CHEMICAL PHYSICS2017年4期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Laser-Assisted Stark Deceleration of Polar Molecules HC2n+1N(n=2,3,4) in High-Field-Seeking State
- Geometric Design of Anode-Supported Micro-Tubular Solid Oxide Fuel Cells by Multiphysics Simulations
- Fabricating Core-Shell WC@C/Pt Structures and its Enhanced Performance for Methanol Electrooxidation
- Highly Responsive and Selective Ethanol Gas Sensor Based on Co3O4-Modi fied SnO2Nano fibers
- Binding Mechanism and Molecular Design of Benzimidazole/Benzothiazole Derivatives as Potent Abl T315I Mutant Inhibitors
- Identi fication of Superoxide O2?during Thermal Decomposition of Molten KNO3-NaNO2-NaNO3Salt by Electron Paramagnetic Resonance and UV-Vis Absorption Spectroscopy
