Bone marrow mesenchymal stem cell therapy in ischemic stroke: mechanisms of action and treatment optimization strategies
Guihong Li, Fengbo Yu, Ting Lei Haijun Gao Peiwen Li Yuxue Sun Haiyan Huang, Qingchun Mu,
1 Department of Neurosurgery, the First Hospital of Jilin University, Changchun, Jilin Province, China
2 Department of Neurosurgery, Hongqi Hospital of Mudanjiang Medical University, Mudanjiang, Heilongjiang Province, China
3 School of Pharmacy, Mudanjiang Medical University, Mudanjiang, Heilongjiang Province, China
REVIEW
Bone marrow mesenchymal stem cell therapy in ischemic stroke: mechanisms of action and treatment optimization strategies
Guihong Li1,2, Fengbo Yu3, Ting Lei1, Haijun Gao1, Peiwen Li1, Yuxue Sun1, Haiyan Huang1,*, Qingchun Mu2,*
1 Department of Neurosurgery, the First Hospital of Jilin University, Changchun, Jilin Province, China
2 Department of Neurosurgery, Hongqi Hospital of Mudanjiang Medical University, Mudanjiang, Heilongjiang Province, China
3 School of Pharmacy, Mudanjiang Medical University, Mudanjiang, Heilongjiang Province, China
Animal and clinical studies have confirmed the therapeutic effect of bone marrow mesenchymal stem cells on cerebral ischemia, but their mechanisms of action remain poorly understood. Here, we summarize the transplantation approaches, directional migration, differentiation, replacement, neural circuit reconstruction, angiogenesis, neurotrophic factor secretion, apoptosis, immunomodulation, multiple mechanisms of action, and optimization strategies for bone marrow mesenchymal stem cells in the treatment of ischemic stroke. We also explore the safety of bone marrow mesenchymal stem cell transplantation and conclude that bone marrow mesenchymal stem cell transplantation is an important direction for future treatment of cerebral ischemia. Determining the optimal timing and dose for the transplantation are important directions for future research.
nerve regeneration; ischemia/reperfusion injury; animal model; mechanisms of action; clinical application; research progress; genetic modification; angiogenesis; replacement therapy; neural regeneration
orcid: 0000-0003-1082-2692 (Qingchun Mu)
Accepted: 2016-03-18
Introduction
Ischemic stroke is currently one of the main causes of death in adults worldwide, with an especially high incidence and ischemic stroke-related disability rate in the elderly (Donnan et al., 2008; Johnston et al., 2009). Because of the short therapeutic time window in ischemic stroke, many patients with ischemic stroke may suffer from severe long-term disability, even if they receive timely interventional and thrombolytic therapies (Pandya et al., 2011). However, nerve regeneration appears several days or weeks after ischemic stroke, offering potential for a second therapeutic time window (Gopurappilly et al., 2011). New treatment approaches to alleviate disability after stroke may be considered during this second window, including stem cell transplantation.
Bone marrow mesenchymal stem cells (BMSCs) have self-renewal potential. These cells express markers for mesenchymal or endothelial cells (CD105, CD73, and CD90) as well as adhesion molecules (CD106, CD166 and CD29) (Javazon et al., 2004; Dominici et al., 2006), but do not express hematopoietic stem cell markers (CD11, CD14, CD34, CD45, CD79, CD19 and HLA-DR). BMSCs can differentiate not only into mesodermal cells but also into endodermal and ectodermal cells (Sanchez-Ramos et al., 2000; Phinney and Prockop, 2007; Uccelli et al., 2008). Sufficient evidence has shown that BMSCs affect the pathological processes underlying ischemic stroke through multiple mechanisms of action, including inducing angiogenesis, secreting neurotrophic factor, inhibiting apoptosis, and modulating the immune system (Li et al., 2008; Tate et al., 2010; Liu et al., 2012; Jellema et al., 2013; Zhao et al., 2013; Mitkari et al., 2014). Thus, BMSCs have great potential in the treatment of stroke (Li et al., 2008; Tate et al., 2010; Liu et al., 2012; Jellema et al., 2013; Zhao et al., 2013; Mitkari et al., 2014). In addition, BMSCs are generally derived from autologous tissue, precluding ethical controversy. BMSCs are easily cultured in vitro, have weak immunogenicity and good safety, and have been considered ideal seed cells in the treatment of ischemic stroke (Guo et al., 2013; Ishizaka et al., 2013; Kawabori et al., 2013; Hess et al., 2014; Ha et al., 2015).
Considering the potential multiple mechanisms of action of BMSCs following transplantation, we sought to analyze the various transplantation approaches, differences in mechanisms of action, and effectiveness and safety of BMSCs in ischemic stroke therapy.
BMSC Transplantation Approaches
BMSC transplantation is conducted mainly using intracranial and intravascular deliveries (Guzman et al., 2008). The intracranial technique refers to stereotactic injection. Following direct injection into the corpus striatum, more BMSCsare able to reach the targeted brain damage and the number of cells used is small and the onset time is short (Jin et al., 2005). Another intracranial delivery method is intraventricular injection. Its success depends on the migration of transplanted cells and their abilities to adapt to the cerebrospinal fluid and survive transiting the blood-brain barrier (Wang et al., 2013). Because intracranial transplantation is invasive, multiple injections in the infarct zone cause mechanical damage of local tissue and cells (Walczak et al., 2008).
Intravascular techniques include intravenous and intra-arterial approaches. Compared with intracranial delivery, intravascular injection more widely distributes transplanted cells through blood vessels and may be better for large-area brain damage (Bliss et al., 2010). A number of inflammatory cytokines are released after tissue damage (Tuttolomondo et al., 2008; Ahmadian Kia et al., 2011). BMSCs express a variety of chemokine receptors (Ponte et al., 2007) and are attracted to the area of injury or inflammation. After cerebral ischemia, intravenously transplanted BMSCs are targeted to the center of the ischemic area and peri-infarct zone. However, because of the large volume of BMSCs (Lee et al., 2009), most BMSCs may be captured in the pulmonary vascular system after intravenous infusion. Detante et al. (2009) verified that infused99mTc-HMPAO-labeled BMSCs were transiently trapped in the rat lung in the first 2 hours after stroke before reaching the ischemic area. Thus, compared with intracranial injection, intravenous injection is relatively simple and less invasive (Wu et al., 2008), but the number of cells reaching the ischemic tissue is low. Intra-arterial transplantation can obtain better effects than intravenous transplantation. Intra-arterial transplantation diminishes the number of cells trapped in other tissues and delivers cells to the injury site in a short period. Injection through the internal carotid artery is a simple and effective way to transplant cells, as cells can distribute in the brain tissue where blood is supplied by the middle cerebral artery (Guo et al., 2013). Jiang et al. (2013) demonstrated that BMSCs reach the arterial end close to the injury site following intra-arterial transplantation, indicating that intra-arterial transplantation is a safe, feasible method for promoting the recovery of neurological function in patients with stroke.
Although the intra-arterial and intracranial approaches reduce the problems associated with delivery of BMSCs, the two methods are invasive. The safety or effectiveness of BMSC transplantation in stroke therapy should be determined. The optimal time window of transplantation is unclear, and it may be that sooner is better. In addition, the optimal injection dose will require further study (Keimpema et al., 2009; Komatsu et al., 2010; Ishizaka et al., 2013; Kawabori et al., 2013).
Mechanisms of Action for BMSCs
BMSCs participate in the treatment of cerebral ischemia through multiple mechanisms, including cell migration, angiogenesis, apoptosis inhibition, neurotrophic factor secretion, neural circuit reconstruction, and immunomodulation (Figure 1).
Directional migration of BMSCs
In vivo microscopy or autoradiography has revealed that transplanted BMSCs mainly gather in the ischemic penumbra and the subventricular zone (Yilmaz et al., 2011; Park et al., 2014). Microglia and astrocytes in the infarct zone secrete stromal cell-derived factor 1 (SDF-1). BMSCs express chemokine receptor 4 (CXCR-4), the physiological receptor for SDF-1. The interaction of SDF-1 and CXCR-4 may cause BMSC migration into the infarct zone (Wang et al., 2008, 2012; Yu et al., 2012). A lack of CXCR-4 or SDF-1α will significantly reduce the targeted migration of BMSCs (Shyu et al., 2008; Sun et al., 2009). Wang et al. (2014) determined that the synergistic effect of CXCR-4 and CXCR-7 expressed in BMSCs promotes BMSC migration, and concluded that the effect of CXCR-7 is better than that of CXCR-4. Zhang et al. (2015) confirmed that the chemotactic factor CX3CL1/ fractalkine activates the Jak2-Stat5alpha-ERK1/2 signaling pathway through CX3CR1, triggers integrin-dependent restructuring, and urges BMSC migration toward the ischemic tissue. These findings suggest that BMSC migration is the result of interactions among multiple factors. It remains poorly understood how BMSCs traverse the blood-brain barrier.
BMSC differentiation, replacement, and neural circuit reconstruction
In vitro study results have demonstrated that BMSCs can differentiate into neurons, glial cells, and endothelial cells (Woodbury et al., 2000; Phinney and Prockop, 2007). The markers for neurons and glial cells can be identified in the central nervous system (CNS) of animal models of ischemic stroke following BMSC transplantation (Eglitis et al., 1999; Li et al., 2000; Chen et al., 2001; Zhao et al., 2002; Skvortsova et al., 2008; Jiang et al., 2014). However, mesenchymal stem cells (MSCs) do not express the voltage-gated ion channels that are expressed in functional nerve cells (Hofstetter et al., 2002). The improvement in the behaviors of animals modeling ischemic stroke is likely based on the plasticity of nervous system as well as on activation and migration of endogenous neural stem cells (Ding et al., 2007; Song et al., 2013). Therefore, the possibility of MSCs directly differentiating into cells that replace the injured CNS cells after stroke is very small, and there is still a lack of definite evidence.
BMSCs enhance axonal plasticity and reconstruct neural circuits, which may be the basis for the recovery of neurological function after ischemic stroke (van Velthoven et al., 2012). After intravenous infusion of BMSCs, the numbers of axons and myelin sheaths increase in the rat corpus striatum, hippocampus, and corpus callosum. Axons in the ischemic zone grow along the extending direction of reactive astrocytes (Li et al., 2006; Shen et al., 2006; Liu et al., 2010; van Velthoven et al., 2012). BMSCs restore the connections of different brain regions through axonal sprouting, noticeably enhancing the survival of the motor cortex in the peri-infarct zone and contributing to functional recovery after stroke (Liu et al., 2010; van Velthoven et al., 2012; Song et al., 2013). BMSC transplantation repairs the neural network and reconstructs neural connections, and the recovery of the neural circuit may contribute to enhanced sensorimotor functions (Song et al., 2013). Nevertheless, the molecular mechanism of BMSC-induced synaptic plasticity remains unclear.
BMSCs enhance angiogenesis
Angiogenesis in the infarct and peri-infarct zones plays an important role in mediating neuronal survival and regeneration. BMSC transplantation enhances angiogenesis in the ischemic zone, increasing the number of new microvessels (Chen et al., 2003b) and ameliorating neurovascular injuries. BMSCs can also secrete vascular endothelial growth factor, basic fibroblast growth factor and placental growth factor (Wakabayashi et al., 2010; Vogelgesang and Dressel, 2011; Chuang et al., 2012). Liu et al. (2014) considered that mitochondrial transport through tunneling nanotubes may be the key mechanism used by BMSCs to protect mitochondrial function and promote angiogenesis. In addition to secreting bioactive molecules and promoting angiogenesis, BMSCs support the crosslinking of peripheral cells, astrocytes, and endothelial cells, maintain the integrity of the blood-brain barrier (Fisher, 2009), form a microenvironment supporting neurogenesis, and promote the recovery of neurological function (Honmou et al., 2012). Mitkari et al. (2014) verified that intra-arterial infusion of human BMSCs (hBMSCs) enhances microvascular regeneration in the infarct zone, but does not improve the behavioral ability of rats. BMSC transplantation can promote angiogenesis in the infarct area, thereby providing favorable conditions for nerve regeneration.
BMSCs facilitate neurotrophic factor secretion from neurons
In vitro test results show that BMSCs secrete 11 kinds of neurotrophic factors after coculture with cortical neurons under hypoxic conditions (Tate et al., 2010). To determine the effects of BMSC secretion on neurotrophic factors, rat BMSCs were cultured with complete medium in animal models of stroke; the complete medium enhanced connections between nerve cells and promoted functional recovery after stroke (Tsai et al., 2014). BMSCs play an active nutritional support role in the early stage of transplantation in rats with cerebral ischemia (Loseva et al., 2011). BMSCs also induce parenchymal cells in the CNS to secrete nerve growth factor, brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), epidermal growth factor, basic fibroblast growth factor, insulin-like growth factor 1, hepatocyte growth factor, and stem cell factor (Wakabayashi et al., 2010; Lin et al., 2011; Zhang et al., 2011; Ishizaka et al., 2013; Kaengkan et al., 2013; Song et al., 2013). These bioactive factors synergistically promote functional recovery after stroke. BMSCs positively regulate bone morphogenetic protein 2/4, and promote synaptic vesicle protein expression (Zhang et al., 2006). These factors accelerate the differentiation of astrocytes in the ischemic zone, elevate connexin 43 expression, promote small molecule exchange in the brain, and enhance synaptic efficacy (Xin et al., 2006). Bioactive molecules directly or indirectly produced by BMSCs accelerate neurogenesis, elevate white matter integrity, and induce synaptogenesis.
BMSCs suppress apoptosis
BMSC transplantation effectively inhibits apoptosis in the ischemic penumbra. Chen et al. (2003a) found that apoptosis is reduced and basic fibroblast growth factor expression is increased in rat models of stroke following BrdU-BMSC transplantation. The apoptotic response in astrocytes is reduced after BMSC transplantation (Leu et al., 2010; Darsalia et al., 2012; Jiang et al., 2014). A few apoptotic cells and many regulatory T lymphocytes are detected during intravenous infusion of hBMSCs (Li et al., 2002). MSCs diminish caspase-3 activity, reduce the Bax/Bcl-2 ratio (Leu et al., 2010; Li et al., 2012), decrease interleukin (IL)-1β, IL-6, and tumor necrosis factor-α levels (Zhu et al., 2014), suppress apoptosis, and accelerate the proliferation of endogenous neural stem cells and glial cells (Mora-Lee et al., 2012) by activating an Akt-dependent anti-apoptotic cascade (Scheibe et al., 2012).
Immunomodulatory effects of BMSCs
BMSCs produce immunomodulatory effects, simultaneously weakening the innate and adaptive immune responses and mitigating the injury to the CNS. Ischemic stroke leads to a strong inflammatory response, resulting in leukocyte recruitment to the infarct zone (Iadecola and Anrather, 2011). In vitro test results have demonstrated that leukocyte proliferation is reduced and differentiation becomes abnormal after coculture with BMSCs (Bartholomew et al., 2002; Sato et al., 2007). The transforming growth factor beta secreted by MSCs diminishes monocyte chemoattractant protein-1 levels in the ischemic zone, reduces the number of circulating CD68+immune cells in the infarct zone by traversing the damaged blood-brain barrier, and suppresses immune responses in the ischemic zone (Yoo et al., 2013). In addition, MSCs diminish IL-23/IL-17 expression (Ma et al., 2013), decrease IL-1β, IL-6, and tumor necrosis factor-α levels (Zhu et al., 2014), and suppress the immune response by reducing STAT3 expression and phosphorylation in microglia (McGuckin et al., 2013). Liu et al. (2009) have confirmed that BMSCs increase IL-10 levels and decrease tumor necrosis factor-α expression to inhibit ischemic injury. Transplanted BMSCs inhibit T-cell proliferation, promote Treg cell expression, and nonspecifically suppress the production of CD4+and CD8+T cells (Di Nicola et al., 2002; Meisel et al., 2004; Aggarwal and Pittenger, 2005; Nasef et al., 2007). Moreover, MSCs can suppress the inflammatory reaction by downregulating macrophages, B cells, natural killer cells, and antigen-presenting cells (Beyth et al., 2005; Corcione et al., 2006; Krampera et al., 2006; Maggini et al., 2010; Marigo and Dazzi, 2011; Ribeiro et al., 2011). Although intravenously infused MSCs are captured in the lung, and intra-arterially infused MSCs gather in the spleen, MSCs still have immunomodulatory effects on the brain (Li and Chopp, 2009; Ankrum and Karp, 2010; Oh et al., 2010). These results indicate that transplanted MSCs have a long-term effect on immune function, but the immunomodulatory mechanisms remain poorly understood.
Modification of BMSCs
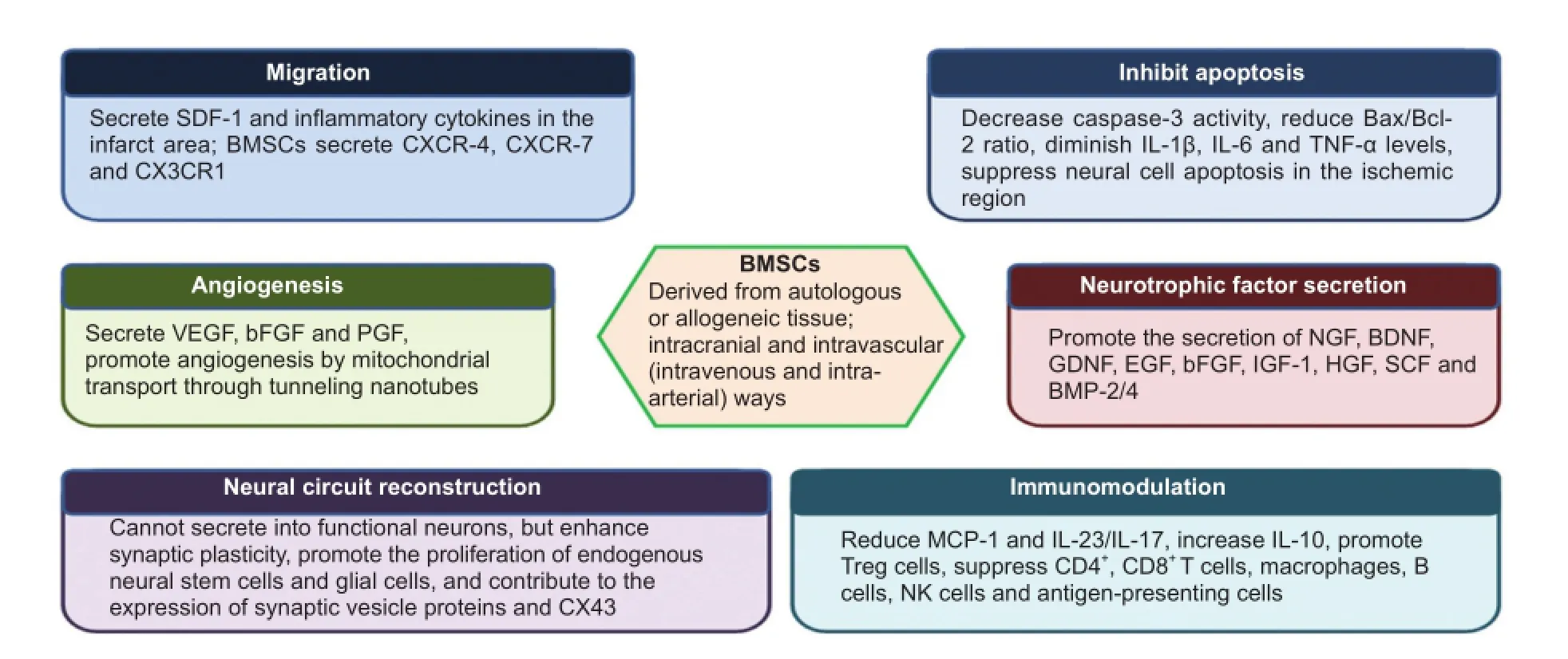
Figure 1 Mechanisms for the therapeutic effects of BMSC transplantation in cerebral ischemia.
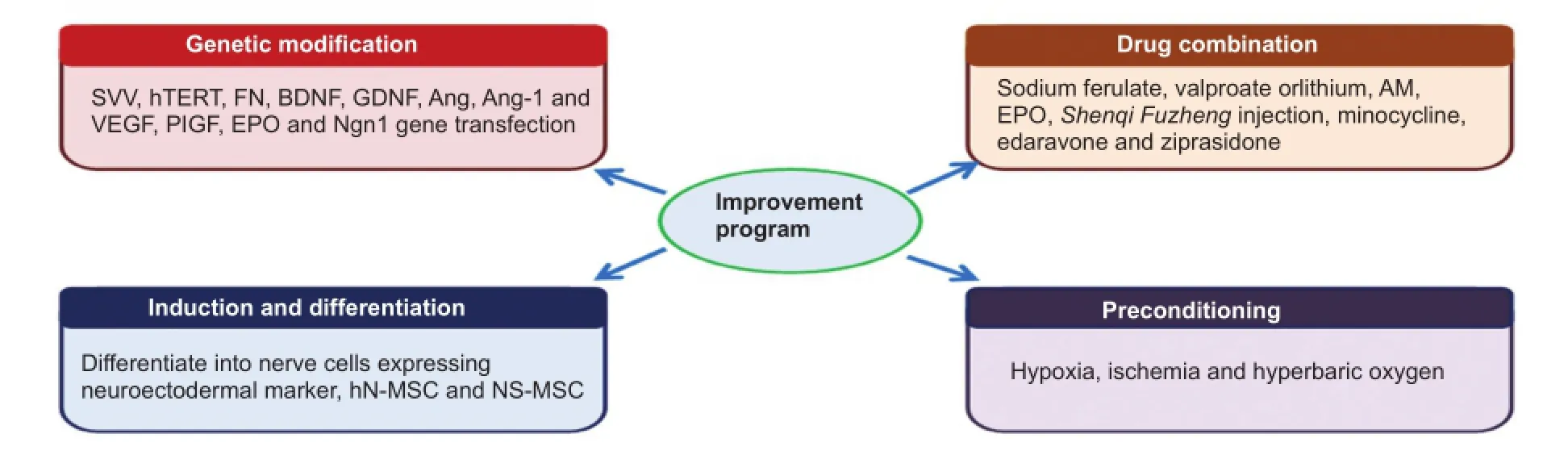
Figure 2 Modification of BMSCs.
Genetic modification uses a variety of biotechnology and bioengineering tools and techniques to modify the genetic makeup of organisms. BMSCs may be used as genetic carriers, combining cell therapy and gene therapy by introducing target genes. Genetic modification has a unique advantage in the transfection of an exogenous gene and can directionally increase the expression of beneficial biological factors. After transfection, BMSCs still retain their original therapeutic effects, including promoting migration and survival, increasing neurotrophic factor expression, accelerating angiogenesis in the ischemic zone, and resisting inflammation and apoptosis. Thus, BMSCs transfected with an exogenous gene offer a broad prospect in the treatment of cerebral infarction. In the clinic, fine manipulation for gene transfection and screening is necessary, which may delay the treatment process. By contrast, combining BMSCs and drug treatment is a simple, highly efficient, and feasible treatment method. Such combinations generate not only the cumulative effect of dual therapy but also synergistic effects, and effectively promote the recovery of neurological function. However, this method needs suitable drug collocation, and the time window for administration warrants further investigation. Inducing BMSCs to differentiate into neural cells or dedifferentiate into primitive stem cells exerts the efficiency of BMSCs in the treatment of stroke. Preconditioning, such as hypoxia, enhances the ability of BMSCs to survive. In addition, preconditioning allows for a greater therapeutic effect by increasing cell number and the expression of some biological factors. Thus, preconditioning increases therapeutic efficacyby changing the physical environment (Figure 2).
Genetic modification
Gene transfection-induced MSC differentiation into neural cells or gene transfection-induced endogenous neural stem cell proliferation and differentiation to reconstruct neural pathways increase the therapeutic value of BMSCs. In vitro and in vivo test results demonstrate that more survivin-modified BMSCs survive, infarct size becomes small after BMSC transplantation, and neurological function after stroke is noticeably restored (Liu et al., 2011a). After transplantation with human telomerase reverse transcriptase-MSCs, cell survival increases, infarct size is reduced, and behavioral function recovers (Honma et al., 2006). Fibronectin-modified MSCs increase therapeutic value by increasing stem cell survival and paracrine secretion of pro-survival or anti-inflammatory molecules (Garbayo et al., 2011).
BDNF preconditioning mitigates brain injury after focal cerebral ischemia. High BDNF expression in the ischemic zone may be able to achieve a better therapeutic effect. After BDNF-BMSC transplantation, more neuron-like cells are detected, fewer terminal deoxynucleotidyl transferase (TdT) dUTP nickend labeling (TUNEL)-positive cells are evident in the peri-infarct zone, BDNF expression increases in the infarct zone, infarct size reduces, and neurological function is noticeably restored (Kurozumi et al., 2004; Hamada et al., 2005; Nomura et al., 2005; Huang et al., 2008). Three hours after middle cerebral artery occlusion (MCAO), rats were infused with GDNF-transfected hBMSCs. Their MRI and behavioral score results revealed a strong therapeutic effect of GDNF-hBMSCs (Horita et al., 2006). After intravenous infusion of BDNF-BMSCs and glial cell derived neurotrophic factor (GDNF)-BMSCs, ischemic injury was lessened and neurological function was significantly recovered in rats previously subjected to MCAO; however, the infusion of ciliary neurotrophic factor-overexpressing BMSCs or neurotrophin-3-BMSCs did not provide these positive effects (Kurozumi et al., 2005).
In rats undergoing permanent MCAO, intravenous administration of angiopoietin-1 gene-modified hMSCs improved angiogenesis at the lesion border and regional cerebral blood flow, reduced lesion volume, and improved functional recovery (Onda et al., 2008). Rats receiving angiopoietin-1-vascular endothelial growth factor-hMSCs presented the excellent structural-functional recovery (Toyama et al., 2009). Placental growth factor-hMSCs reduce lesion volume, induce angiogenesis, and elicit functional improvement (Liu et al., 2006). Transduction of the erythropoietin gene into MSCs induces secretion of various trophic factors, decreases infarct volume, and improves the recovery of neurological function (Cho et al., 2010).
Neurogenin-1-expressing MSCs express neuron-specific proteins, including NeuroD and voltage-gated Ca2+and Na+channels, neurofilament 200, microtubule-associated protein, and vesicular glutamate transporter 2. Moreover, neurogenin-1-MSCs functionally connect to host neurons and markedly ameliorate motor dysfunctions (Kim et al., 2008). A large fraction of the transplanted fibronectin-MSCs express βIII-tubulin and promote neuronal differentiation (Garbayo et al., 2011). Flk-1+hBMSCs enhance the proliferation of neural stem cells or neural progenitor cells in the subventricular zone and hippocampus; many neural stem/ progenitor cells migrated into the ischemic zone and differentiated into neural and glial cells, promoting the recovery of neurological function (Bao et al., 2011). BDNF-BMSCs induce cell proliferation in the regional ischemic zone, reduce infarct size, and markedly improve motor function. Epidermal growth factor-like domain 7-modified BMSCs improve motor function, but do not affect infarct size. Polysaccharopeptide-modified MSCs do not show therapeutic outcomes. Sonic hedgehog-modified BMSCs have a negative effect on functional recovery (van Velthoven et al., 2014). Taken together, these findings suggest that not all bioactive factor gene transfections achieve the desired results.
Drug combination
BMSC therapy combined with drug treatment for ischemic stroke is potentially a feasible and efficient therapeutic approach. Drugs and BMSCs exert synergistic effects through different paths, including accelerating stem cell migration and survival, resisting apoptosis, and promoting endogenous stem cell proliferation, neurotrophic factor secretion, and angiogenesis. Thus, their combination effectively contributes to the recovery of neurological function.
Sodium ferulate combined with BMSCs accelerates BMSC migration toward the ischemic zone in a rat model of MCAO by upregulating SDF-1α and CXCR-4, promotes glucose metabolism by increasing glucose transporter 1 expression in the peri-infarct zone and BMSCs, and markedly reduces infarct size (Zhao et al., 2013). Valproate- or lithium-pretreated BMSCs enhance cell migration and targeting ability, and promote functional recovery; the mechanism is likely associated with valproate-induced CXCR-4 overexpression and lithium-induced matrix metalloproteinase-9 upregulation (Tsai et al., 2011). Adrenomedullin plus MSCs inhibits MSC apoptosis, induces angiogenesis, and improves neurological function (Hanabusa et al., 2005).
Cellular proliferation and neurogenesis were increased along the lateral ventricle wall, and neurological function was recovered after the combined administration of erythropoietin and MSCs, indicating that erythropoietin acts synergistically with MSCs to potentiate neurogenesis (Esneault et al., 2008). Chinese medicine administered with MSCs induces stem cells to differentiate into neuron-like cells, promotes angiogenesis, and accelerates the expression of neuron-specific enolase, neurofilament, and GFAP (Yao et al., 2005; Guan and Zhao, 2011). Treatment with minocycline combined with BMSCs increased the number of GFAP- and NeuN-positive cells (Bilen et al., 2013) and improved neurogenesis and functional recovery by accelerating the activation and proliferation of endogenous neural stem cells.
BMSCs and edaravone administration improved cerebral ischemia by reducing matrix metalloproteinase activation in a rat model of transient MCAO induced by tissue-type plasminogen activator (Tian et al., 2013). Their combination may indeed provide improved neurological function, but these results need further investigation. The combined administration of ziprasidone and neural progenitor cells reducesthe number of TUNEL-positive cells in the ischemic zone so as to enhance the anti-apoptotic effect. Their combination diminishes microglial aggregation in the ischemic zone, increases the number of neural progenitor cells, induces the expression of endogenous neurotrophic factor, such as BDNF, nerve growth factor and GDNF, and promotes the recovery of neurological function (Kaengkan et al., 2013). Thus, the combination of ziprasidone and BMSCs will likely resist apoptosis as well as contribute to stem cell survival and endogenous neurotrophic factor secretion.
Induction and differentiation
MSC differentiation into nerve cells or dedifferentiation into primitive stem cells may generate a better therapeutic effect than undifferentiated MSCs on ischemic stroke by improving cell survival, neurotrophic factor secretion, and neurogenesis. In vitro and in vivo test results demonstrate that dedifferentiated BMSCs have a high survival rate and great potential to differentiate into nerve cells (Liu et al., 2011b). Their increase in bcl-2 protein and microRNA-34a expression indicates good potential therapeutic effects on cerebral ischemia (Liu et al., 2011b). Human trabecular bone-derived MSCs were transfected with the notch intracellular domain to induce their differentiation into neuronal cells, which were then stereotaxically transplanted into the local ischemic hemisphere of gerbils (Xu et al., 2010). The transplanted cells were distributed around the peri-infarct region 28 days later. The cell survival rate was high, with many cells positive for microtubule-associated protein 2, and the recovery of neurological function was good (Xu et al., 2010). MSCs coated with highly hydrophobic diphenylamino-s-triazine-bridged p-phenylene were efficiently converted into neurosphere-like cellular aggregates (Heo et al., 2013). The spherical cells were subsequently induced to differentiate into neural cells expressing neuroectodermal markers (Heo et al., 2013). These cells were intra-cerebrally administered to rats 48 hours after permanent MCAO (Heo et al., 2013). The results showed a marked attenuation of ischemic damage with significant functional recovery, and the effects were better than those of BMSCs alone (Heo et al., 2013).
Preconditioning
Hypoxia preconditioning improves BMSC survival, migration, and targeted migration. After intranasal delivery of BMSCs treated with hypoxia preconditioning in a mouse focal cerebral ischemia model, the expression of CXCR4, matrix metalloproteinase 2, and matrix metalloproteinase 9 increases, cell death decreases, infarct volume in the peri-infarct region is reduced, and neurological function is recovered (Wei et al., 2013). After preconditioned and non-preconditioned MSCs are exposed to 6 hours of lethal anoxia, the number of preconditioned cells is greater than that of non-preconditioned cells (Kim et al., 2012). Ischemia preconditioning induces activation of Akt/hypoxia-inducible factor-1α (Kim et al., 2012). Both miR-107 and miR-210 participate independently via their respective putative target genes Pdcd10 and Casp8ap2 (Kim et al., 2012). Lin et al. (2013) determined that hypoxia preconditioning upregulates hypoxia-inducible factor-1α-activated Epac1 expression through Epac1-to-matrix metalloprotease signaling. Cell transplantation improves cerebral blood flow into the ischemic brain via induction of angiogenesis, which leads to recovery from stroke (Lin et al., 2013).
A significant reduction in T cells and MSCs and a significant increase in CD34+and natural killer cells have been identified in poststroke Bone marrow-derived mononuclear cells (MNCs) compared with prestroke MNCs (Yang et al., 2012). Moreover, the concentrations of IL-10, IL-6, monocyte chemoattractant protein-1, vascular endothelial growth factor, and tumor necrosis factor-α are significantly increased in poststroke compared with prestroke MNCs (Yang et al., 2012). Poststroke MNCs in comparison with prestroke MNCs lead to greater recovery of neurological function and reduced lesion size (Yang et al., 2012). Therefore, the therapeutic effect of BMSCs from ischemic rats is likely higher than that of normal rats. Further comparative tests should be conducted to confirm this assertion, which is consistent with clinical study of autologous transplantation.
Hyperbaric oxygen promotes the proliferation and activation of BMSCs (Thom et al., 2006). Mobilization of BMSCs to an ischemic area is improved in long-term hyperbaric oxygen treatments, suggesting that the duration of therapy is crucial for promoting the homing of BMSCs to the ischemic brain by hyperbaric oxygen therapies (Lee et al., 2013). Hyperbaric oxygen also stimulates trophic factor expression and improves gliosis and neurogenesis (Lee et al., 2013).
In conclusion, hypoxia preconditioning of BMSCs, transplantation of BMSCs from ischemic rats, or hyperbaric oxygen preconditioning after transplantation enhances BMSC migration and survival, promotes angiogenesis, and effectively improves neurological function.
Effectiveness and safety of clinical trials
In a study of BMSC transplantation for ischemic stroke (Bang et al., 2005), ischemic stroke patients were randomly divided into experimental (BMSC transplantation) and control groups (no treatment). The study found that BMSCs markedly increased the modified Rankin score and Barthel index (Bang et al., 2005). No adverse effects, such as venous thromboembolism, abnormal cell proliferation, systemic cancer, systemic infection, or neurological decline, were identified after MSC transplantation (Bernardo et al., 2007; Bhasin et al., 2011; Hess et al., 2014). These findings provide support for the safety and poststroke function improvement of BMSC transplantation in ischemic stroke. Other studies have also been conducted, including clinical trials examining the safety and effectiveness of autologous and allogeneic BMSC transplantation, a method to shorten the cycle of BMSCs cultured in vitro, the optimum time after stroke for infusing BMSCs, the therapeutic effects of various doses, and protocols aimed at additional improvements (Keimpema et al., 2009; Komatsu et al., 2010; Ishizaka et al., 2013; Kawabori et al., 2013).
Summary
BMSCs migrate and survive in the ischemic hemisphere, creating a microenvironment conducive to survival and regeneration for the repair of injured nerve tissue. TheBMSC-induced anti-inflammatory response mitigates nerve edema. Immune modulation also relieves the apoptosis of nerve cells and glial cells in the infarct zone. BMSCs promote the release of cytokines and neurotrophic factors and provide nutritional support for the injured neurons in the ischemic penumbra. BMSCs induce angiogenesis, improve blood circulation in the brain, and contribute to nerve tissue repair. BMSCs may also stimulate axonal sprouting and myelin remodeling and promote endogenous neurogenesis. Although many achievements have been made in determining the therapeutic mechanisms of BMSCs, these mechanisms have not been fully clarified. In particular, additional research will be required to determine the molecular biological mechanisms of neural plasticity and angiogenesis.
BMSCs have been used as genetic carriers to combine cell therapy and gene therapy. The combination of BMSCs and drug treatment is a simple, highly efficient, and feasible treatment option that provides cumulative as well as synergistic effects. Inducing BMSCs to differentiate into neural cells or to dedifferentiate into primitive stem cells elicits the efficiency of BMSCs in the treatment of stroke. Preconditioning BMSCs, such as under hypoxic conditions, enhances the ability of BMSCs to survive. Preconditioning offers great therapeutic effects by increasing cell number and the expression of some biological factors. The evidence for the application of various optimized methods includes the cognition of the mechansim following cerebral ischemia/ reperfusion injury and stem cell therapy in cerebral ischemia. Different optimizations amplify the role of BMSCs in various biological pathways and promote the therapeutic efficacy of BMSCs in stroke. Thus, the therapeutic advantages of transplanted BMSCs become more prominent. Future investigations should focus on genetic modifications and drug combinations as well as on optimal timing and doses for BMSC transplantation. Additionally, clinical trials are needed to determine the effectiveness and safety of genetically modified BMSCs. Overall, BMSC transplantation is an important direction for future treatment of ischemic stroke.
Author contributions: GL wrote the paper. FY obtained the funding. TL ensured the integrity of the data. HG analyzed the data. PL participated in statistical analysis. YS served as a principle investigator. QM and HH conceived and designed the study. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815-1822.
Ahmadian Kia N, Bahrami AR, Ebrahimi M, Matin MM, Neshati Z, Almohaddesin MR, Aghdami N, Bidkhori HR (2011) Comparative analysis of chemokine receptor's expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J Mol Neurosci 44:178-185.
Ankrum J, Karp JM (2010) Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med 16:203-209.
Bang OY, Lee JS, Lee PH, Lee G (2005) Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 57:874-882.
Bao X, Feng M, Wei J, Han Q, Zhao H, Li G, Zhu Z, Xing H, An Y, Qin C, Zhao RC, Wang R (2011) Transplantation of Flk-1+human bone marrow-derived mesenchymal stem cells promotes angiogenesis and neurogenesis after cerebral ischemia in rats. Eur J Neurosci 34:87-98.
Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R (2002) Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30:42-48.
Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, Zuffardi O, Locatelli F (2007) Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 67:9142-9149.
Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J (2005) Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 105:2214-2219.
Bhasin A, Srivastava MVP, Kumaran SS, Mohanty S, Bhatia R, Bose S, Gaikwad S, Garg A, Airan B (2011) Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra 1:93-104.
Bilen S, Pinarli F, Ak F, Fadillioglu E, Albayrak A, Boyuk G, Guler OG, Erden G, Ulus AT, Delibasi T (2013) Treatment efficacy with bone marrow derived mesenchymal stem cells and minocycline in rats after cerebral ischemic injury. Stem Cell Rev 9:219-225.
Bliss TM, Andres RH, Steinberg GK (2010) Optimizing the success of cell transplantation therapy for stroke. Neurobiol Dis 37:275-283.
Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M (2003a) Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res 73:778-786.
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M (2001) Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32:1005-1011.
Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M (2003b) Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res 92:692-699.
Cho GW, Koh SH, Kim MH, Yoo AR, Noh MY, Oh S, Kim SH (2010) The neuroprotective effect of erythropoietin-transduced human mesenchymal stromal cells in an animal model of ischemic stroke. Brain Res 1353:1-13.
Chuang TJ, Lin KC, Chio CC, Wang CC, Chang CP, Kuo JR (2012) Effects of secretome obtained from normoxia-preconditioned human mesenchymal stem cells in traumatic brain injury rats. J Trauma Acute Care Surg 73:1161-1167.
Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A (2006) Human mesenchymal stem cells modulate B-cell functions. Blood 107:367-372.
Darsalia V, Mansouri S, Orts?ter H, Olverling A, Nozadze N, Kappe C, Iverfeldt K, Tracy Linda M, Grankvist N, Sj?holm ?, Patrone C (2012) Glucagon-like peptide-1 receptor activation reduces ischaemic brain damage following stroke in type 2 diabetic rats. Clin Sci (Lond) 122:473-483.
Detante O, Moisan A, Dimastromatteo J, Richard MJ, Riou L, Grillon E, Barbier E, Desruet MD, De Fraipont F, Segebarth C, Jaillard A, Hommel M, Ghezzi C, Remy C (2009) Intravenous administration of99mTc-HMPAO-labeled human mesenchymal stem cells after stroke: in vivo imaging and biodistribution. Cell Transplant 18:1369-1379.
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838-3843.
Ding DC, Shyu WC, Chiang MF, Lin SZ, Chang YC, Wang HJ, Su CY, Li H (2007) Enhancement of neuroplasticity through upregulation of β1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis 27:339-353.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315-317.
Donnan GA, Fisher M, Macleod M, Davis SM (2008) Stroke. Lancet 371:1612-1623.
Eglitis MA, Dawson D, Park KW, Mouradian MM (1999) Targeting of marrow-derived astrocytes to the ischemic brain. Neuroreport 10: 1289-1292.
Esneault E, Pacary E, Eddi D, Freret T, Tixier E, Toutain J, Touzani O, Schumann-Bard P, Petit E, Roussel S, Bernaudin M (2008) Combined therapeutic strategy using erythropoietin and mesenchymal stem cells potentiates neurogenesis after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab 28:1552-1563.
Fisher M (2009) Pericyte signaling in the neurovascular unit. Stroke 40:S13-S15.
Garbayo E, Raval AP, Curtis KM, Della-Morte D, Gomez LA, D'Ippolito G, Reiner T, Perez-Stable C, Howard GA, Perez-Pinzon MA, Montero-Menei CN, Schiller PC (2011) Neuroprotective properties of marrow-isolated adult multilineage-inducible cells in rat hippocampus following global cerebral ischemia are enhanced when complexed to biomimetic microcarriers. J Neurochem 119:972-988.
Gopurappilly R, Pal R, Mamidi MK, Dey S, Bhonde R, Das AK (2011) Stem cells in stroke repair: current success and future prospects. CNS Neurol Disord Drug Targets 10:741-756.
Guan Y, Zhao YH (2011) Treatment of cerebral ischemia with combination of bone marrow-derived mesenchymal stem cells and Chinese medicine. Chin J Integr Med 17:715-720.
Guo L, Ge J, Wang S, Zhou Y, Wang X, Wu Y (2013) A novel method for efficient delivery of stem cells to the ischemic brain. Stem Cell Res Ther 4:116.
Guzman R, Choi R, Gera A, De Los Angeles A, Andres RH, Steinberg GK (2008) Intravascular cell replacement therapy for stroke. Neurosurg Focus 24:E15.
Ha BC, Jung J, Kwak BK (2015) Susceptibility-weighted imaging for stem cell visualization in a rat photothrombotic cerebral infarction model. Acta Radiol 56:219-227.
Hamada H, Kobune M, Nakamura K, Kawano Y, Kato K, Honmou O, Houkin K, Matsunaga T, Niitsu Y (2005) Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapy. Cancer Sci 96:149-156.
Hanabusa K, Nagaya N, Iwase T, Itoh T, Murakami S, Shimizu Y, Taki W, Miyatake K, Kangawa K (2005) Adrenomedullin enhances therapeutic potency of mesenchymal stem cells after experimental stroke in rats. Stroke 36:853-858.
Heo JS, Choi SM, Kim HO, Kim EH, You J, Park T, Kim E, Kim HS (2013) Neural transdifferentiation of human bone marrow mesenchymal stem cells on hydrophobic polymer-modified surface and therapeutic effects in an animal model of ischemic stroke. Neuroscience 238:305-318.
Hess DC, Sila CA, Furlan AJ, Wechsler LR, Switzer JA, Mays RW (2014) A double-blind placebo-controlled clinical evaluation of MultiStem for the treatment of ischemic stroke. Int J Stroke 9:381-386.
Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L (2002) Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A 99:2199-2204.
Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD (2006) Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol 199:56-66.
Honmou O, Onodera R, Sasaki M, Waxman SG, Kocsis JD (2012) Mesenchymal stem cells: therapeutic outlook for stroke. Trends Mol Med 18:292-297.
Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD (2006) Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res 84:1495-1504.
Huang D, Zhang Z, Chen B, Wu X, Wang N, Zhang Y (2008) Therapeutic efficacy of lentiviral vector mediated BDNF gene-modified MSCs in cerebral infarction. Sheng Wu Gong Cheng Xue Bao 24:1174-1179.
Iadecola C, Anrather J (2011) The immunology of stroke: from mechanisms to translation. Nat Med 17:796-808.
Ishizaka S, Horie N, Satoh K, Fukuda Y, Nishida N, Nagata I (2013) Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 44:720-726.
Javazon EH, Beggs KJ, Flake AW (2004) Mesenchymal stem cells: paradoxes of passaging. Exp Hematol 32:414-425.
Jellema RK, Wolfs TG, Lima Passos V, Zwanenburg A, Ophelders DR, Kuypers E, Hopman AH, Dudink J, Steinbusch HW, Andriessen P, Germeraad WT, Vanderlocht J, Kramer BW (2013) Mesenchymal stem cells induce T-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS One 8:e73031.
Jiang W, Liang G, Li X, Li Z, Gao X, Feng S, Wang X, Liu M, Liu Y (2014) Intracarotid transplantation of autologous adipose-derived mesenchymal stem cells significantly improves neurological deficits in rats after MCAo. J Mater Sci Mater Med 25:1357-1366.
Jiang Y, Zhu W, Zhu J, Wu L, Xu G, Liu X (2013) Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transplant 22:2291-2298.
Jin K, Sun Y, Xie L, Mao XO, Childs J, Peel A, Logvinova A, Banwait S, Greenberg DA (2005) Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol Dis 18:366-374.
Johnston SC, Mendis S, Mathers CD (2009) Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol 8:345-354.
Kaengkan P, Baek SE, Kim JY, Kam KY, Do BR, Lee ES, Kang SG (2013) Administration of mesenchymal stem cells and ziprasidone enhanced amelioration of ischemic brain damage in rats. Mol Cells 36:534-541.
Kawabori M, Kuroda S, Ito M, Shichinohe H, Houkin K, Kuge Y, Tamaki N (2013) Timing and cell dose determine therapeutic effects of bone marrow stromal cell transplantation in rat model of cerebral infarct. Neuropathology 33:140-148.
Keimpema E, Fokkens MR, Nagy Z, Agoston V, Luiten PG, Nyakas C, Boddeke HW, Copray JC (2009) Early transient presence of implanted bone marrow stem cells reduces lesion size after cerebral ischaemia in adult rats. Neuropathol Appl Neurobiol 35:89-102.
Kim HW, Mallick F, Durrani S, Ashraf M, Jiang S, Haider KH (2012) Concomitant activation of miR-107/PDCD10 and hypoxamir-210/ Casp8ap2 and their role in cytoprotection during ischemic preconditioning of stem cells. Antioxid Redox Signal 17:1053-1065.
Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, Chang DY, Cho KG, Kim SU, Huh Y, Lee JE, Lee SY, Lee YD, Suh-Kim H (2008) Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells 26:2217-2228.
Komatsu K, Honmou O, Suzuki J, Houkin K, Hamada H, Kocsis JD (2010) Therapeutic time window of mesenchymal stem cells derived from bone marrow after cerebral ischemia. Brain Res 1334:84-92.
Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F (2006) Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24:386-398.
Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H (2005) Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther 11:96-104.
Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H (2004) BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther 9:189-197.
Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ (2009) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5:54-63.
Lee YS, Chio CC, Chang CP, Wang LC, Chiang PM, Niu KC, Tsai KJ (2013) Long course hyperbaric oxygen stimulates neurogenesis and attenuates inflammation after ischemic stroke. Mediators Inflamm 2013:512978.
Leu S, Lin YC, Yuen CM, Yen CH, Kao YH, Sun CK, Yip HK (2010) Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J Transl Med 8:63.
Li D, Fang Y, Wang P, Shan W, Zuo Z, Xie L (2012) Autologous transplantation of adipose-derived mesenchymal stem cells attenuates cerebral ischemia and reperfusion injury through suppressing apoptosis and inducible nitric oxide synthase. Int J Mol Med 29:848-854.
Li WY, Choi YJ, Lee PH, Huh K, Kang YM, Kim HS, Ahn YH, Lee G, Bang OY (2008) Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing. Cell Transplant 17:1045-1059.
Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M (2002) Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 59:514-523.
Li Y, Chopp M (2009) Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett 456:120-123.
Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z (2000) Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab 20:1311-1319.
Li Y, McIntosh K, Chen J, Zhang C, Gao Q, Borneman J, Raginski K, Mitchell J, Shen L, Zhang J, Lu D, Chopp M (2006) Allogeneic bone marrow stromal cells promote glial-axonal remodeling without immunologic sensitization after stroke in rats. Exp Neurol 198:313-325.
Lin CH, Lee HT, Lee SD, Lee W, Cho CW, Lin SZ, Wang HJ, Okano H, Su CY, Yu YL, Hsu CY, Shyu WC (2013) Role of HIF-1α-activated Epac1 on HSC-mediated neuroplasticity in stroke model. Neurobiol Dis 58:76-91.
Lin YC, Ko TL, Shih YH, Lin MY, Fu TW, Hsiao HS, Hsu JY, Fu YS (2011) Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke 42:2045-2053.
Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD (2006) Neuroprotection by PIGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain 129:2734-2745.
Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, Yan C (2014) Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia—reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res 92:10-18.
Liu N, Chen R, Du H, Wang J, Zhang Y, Wen J (2009) Expression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol 6:207-213.
Liu N, Deguchi K, Yamashita T, Liu W, Ikeda Y, Abe K (2012) Intracerebral transplantation of bone marrow stromal cells ameliorates tissue plasminogen activator-induced brain damage after cerebral ischemia in mice detected by in vivo and ex vivo optical imaging. J Neurosci Res 90:2086-2093.
Liu N, Zhang Y, Fan L, Yuan M, Du H, Cheng R, Liu D, Lin F (2011a) Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by Survivin on experimental stroke in rats. J Transl Med 9:105.
Liu Y, Jiang X, Zhang X, Chen R, Sun T, Fok KL, Dong J, Tsang LL, Yi S, Ruan Y, Guo J, Yu MK, Tian Y, Chung YW, Yang M, Xu W, Chung CM, Li T, Chan HC (2011b) Dedifferentiation-reprogrammed mesenchymal stem cells with improved therapeutic potential. Stem Cells 29:2077-2089.
Liu Z, Li Y, Zhang ZG, Cui X, Cui Y, Lu M, Savant-Bhonsale S, Chopp M (2010) Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J Cereb Blood Flow Metab 30:1288-1295.
Loseva EV, Podgorny? OV, Poltavtseva RA, Mare? MV, Loginova NA, Kurskaia OV, Cukhikh GT, Cha?lakhian RK, Aleksandrova MA (2011) Effects of human cultural neuronal and mesenchymal stem cells on the rat learning and brain state after acute hypoxia. Ross Fiziol Zh Im I M Sechenova 97:155-168.
Ma S, Zhong D, Chen H, Zheng Y, Sun Y, Luo J, Li H, Li G, Yin Y (2013) The immunomodulatory effect of bone marrow stromal cells (BMSCs) on interleukin (IL)-23/IL-17-mediated ischemic stroke in mice. J Neuroimmunol 257:28-35.
Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Ca?ones C, Raiden S, Vermeulen M, Geffner JR (2010) Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One 5:e9252.
Marigo I, Dazzi F (2011) The immunomodulatory properties of mesenchymal stem cells. Semin Immunopathol 33:593-602.
McGuckin CP, Jurga M, Miller AM, Sarnowska A, Wiedner M, Boyle NT, Lynch MA, Jablonska A, Drela K, Lukomska B, Domanska-Janik K, Kenner L, Moriggl R, Degoul O, Perruisseau-Carrier C, Forraz N (2013) Ischemic brain injury: a consortium analysis of key factors involved in mesenchymal stem cell-mediated inflammatory reduction. Arch Biochem Biophys 534:88-97.
Meisel R, Zibert A, Laryea M, G?bel U, D?ubener W, Dilloo D (2004) Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase—mediated tryptophan degradation. Blood 103:4619-4621.
Mitkari B, Nitzsche F, Kerkel? E, Kuptsova K, Huttunen J, Nystedt J, Korhonen M, Jolkkonen J (2014) Human bone marrow mesenchymal stem/stromal cells produce efficient localization in the brain and enhanced angiogenesis after intra-arterial delivery in rats with cerebral ischemia, but this is not translated to behavioral recovery. Behav Brain Res 259:50-59.
Mora-Lee S, Sirerol-Piquer MS, Gutiérrez-Pérez M, Gomez-Pinedo U, Roobrouck VD, López T, Casado-Nieto M, Abizanda G, Rabena MT, Verfaille C, Prósper F, García-Verdugo JM (2012) Therapeutic effects of hMAPC and hMSC transplantation after stroke in mice. PLoS One 7:e43683.
Nasef A, Mathieu N, Chapel A, Frick J, Fran?ois S, Mazurier C, Boutarfa A, Bouchet S, Gorin NC, Thierry D, Fouillard L (2007) Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation 84:231-237.
Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD (2005) I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience 136:161-169.
Oh JY, Roddy GW, Choi H, Lee RH, Yl?stalo JH, Rosa RH, Prockop DJ (2010) Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci U S A 107:16875-16880.
Onda T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD (2008) Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab 28:329-340.
Pandya RS, Mao L, Zhou H, Zhou S, Zeng J, Popp AJ, Wang X (2011) Central nervous system agents for ischemic stroke: neuroprotection mechanisms. Cent Nerv Syst Agents Med Chem 11:81-97.
Park JW, Ku SH, Moon HH, Lee M, Choi D, Yang J, Huh YM, Jeong JH, Park TG, Mok H, Kim SH (2014) Cross-linked iron oxide nanoparticles for therapeutic engineering and in vivo monitoring of mesenchymal stem cells in cerebral ischemia model. Macromol Biosci 14:380-389.
Phinney DG, Prockop DJ (2007) Concise review: mesenchymal stem/ multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells 25:2896-2902.
Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J (2007) The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25:1737-1745.
Ribeiro CA, Salgado AJ, Fraga JS, Silva NA, Reis RL, Sousa N (2011) The secretome of bone marrow mesenchymal stem cells-conditioned media varies with time and drives a distinct effect on mature neurons and glial cells (primary cultures). J Tissue Eng Regen Med 5:668-672.
Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR (2000) Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol 164:247-256.
Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K (2007) Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109:228-234.
Scheibe F, Klein O, Klose J, Priller J (2012) Mesenchymal stromal cells rescue cortical neurons from apoptotic cell death in an in vitro model of cerebral ischemia. Cell Mol Neurobiol 32:567-576.
Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, Chopp M (2006) Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience 137:393-399.
Shyu WC, Lin SZ, Yen PS, Su CY, Chen DC, Wang HJ, Li H (2008) Stromal cell-derived factor-1 alpha promotes neuroprotection, angiogenesis, and mobilization/homing of bone marrow-derived cells in stroke rats. J Pharmacol Exp Ther 324:834-849.
Skvortsova VI, Gubskiy LV, Tairova RT, Povarova OV, Cheglakov IB, Holodenko RV, Holodenko IV, Yarygin KN, Yarygin VN (2008) Use of bone marrow mesenchymal (stromal) stem cells in experimental ischemic stroke in rats. Bull Exp Biol Med 145:122-128.
Song M, Mohamad O, Gu X, Wei L, Yu SP (2013) Restoration of intracortical and thalamocortical circuits after transplantation of bone marrow mesenchymal stem cells into the ischemic brain of mice. Cell Transplant 22:2001-2015.
Sun Y, He D, Zhang Y (2009) The study of the migration mechanism of murine flMSCs in ischemic injured brain. Zhongguo Ying Yong Sheng Li Xue Za Zhi 25:12-16.
Tate CC, Fonck C, McGrogan M, Case CC (2010) Human mesenchymal stromal cells and their derivative, SB623 cells, rescue neural cells via trophic support following in vitro ischemia. Cell Transplant 19:973-984.
Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG (2006) Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol 290:H1378-1386.
Tian F, Yamashita T, Deguchi K, Omote Y, Kawai H, Ohta Y, Abe K (2013) In vivo optical imaging correlates with improvement of cerebral ischemia treated by intravenous bone marrow stromal cells (BMSCs) and edaravone. Neurol Res 35:1051-1058.
Toyama K, Honmou O, Harada K, Suzuki J, Houkin K, Hamada H, Kocsis JD (2009) Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp Neurol 216:47-55.
Tsai LK, Wang Z, Munasinghe J, Leng Y, Leeds P, Chuang DM (2011) Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke 42:2932-2939.
Tsai MJ, Tsai SK, Hu BR, Liou DY, Huang SL, Huang MC, Huang WC, Cheng H, Huang SS (2014) Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J Biomed Sci 21:5.
Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G (2008) Inflammatory cytokines in acute ischemic stroke. Curr Pharm Des 14:3574-3589.
Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8:726-736.
van Velthoven CT, Braccioli L, Willemen HL, Kavelaars A, Heijnen CJ (2014) Therapeutic potential of genetically modified mesenchymal stem cells after neonatal hypoxic-ischemic brain damage. Mol Ther 22:645-654.
van Velthoven CT, van de Looij Y, Kavelaars A, Zijlstra J, van Bel F, Huppi PS, Sizonenko S, Heijnen CJ (2012) Mesenchymal stem cells restore cortical rewiring after neonatal ischemia in mice. Ann Neurol 71:785-796.
Vogelgesang A, Dressel A (2011) Immunological consequences of ischemic stroke: immunosuppression and autoimmunity. J Neuroimmunol 231:105-110.
Wakabayashi K, Nagai A, Sheikh AM, Shiota Y, Narantuya D, Watanabe T, Masuda J, Kobayashi S, Kim SU, Yamaguchi S (2010) Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res 88:1017-1025.
Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, Pittenger MF, van Zijl PC, Huang J, Bulte JW (2008) Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke 39:1569-1574.
Wang L, Lin Z, Shao B, Zhuge Q, Jin K (2013) Therapeutic applications of bone marrow-derived stem cells in ischemic stroke. Neurol Res 35:470-478.
Wang Y, Deng Y, Zhou GQ (2008) SDF-1α/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res 1195:104-112.
Wang Y, Fu W, Zhang S, He X, Liu Z, Gao D, Xu T (2014) CXCR-7 receptor promotes SDF-1α-induced migration of bone marrow mesenchymal stem cells in the transient cerebral ischemia/reperfusion rat hippocampus. Brain Res 1575:78-86.
Wang Y, Huang J, Li Y, Yang GY (2012) Roles of chemokine CXCL12 and its receptors in ischemic stroke. Curr Drug Targets 13:166-172.
Wei N, Yu SP, Gu X, Taylor TM, Song D, Liu XF, Wei L (2013) Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant 22:977-991.
Woodbury D, Schwarz EJ, Prockop DJ, Black IB (2000) Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61:364-370.
Wu J, Sun Z, Sun HS, Wu J, Weisel RD, Keating A, Li ZH, Feng ZP, Li RK (2008) Intravenously administered bone marrow cells migrate to damaged brain tissue and improve neural function in ischemic rats. Cell Transplant 16:993-1005.
Xin H, Li Y, Chen X, Chopp M (2006) Bone marrow stromal cells induce BMP2/4 production in oxygen-glucose-deprived astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells. J Neurosci Res 83:1485-1493.
Xu H, Miki K, Ishibashi S, Inoue J, Sun L, Endo S, Sekiya I, Muneta T, Inazawa J, Dezawa M, Mizusawa H (2010) Transplantation of neuronal cells induced from human mesenchymal stem cells improves neurological functions after stroke without cell fusion. J Neurosci Res 88:3598-3609.
Yang B, Xi X, Aronowski J, Savitz SI (2012) Ischemic stroke may activate bone marrow mononuclear cells to enhance recovery after stroke. Stem Cells Dev 21:3332-3340.
Yao XL, Zhang C, Lu XL, Feng S, Deng Y, Liu Z (2005) Experimental research on effect of human mesenchymal stem cells induced by Shenqi Fuzheng injection in cerebral infarction. Zhongguo Zhong Xi Yi Jie He Za Zhi 25:629-632.
Yilmaz G, Vital S, Yilmaz CE, Stokes KY, Alexander JS, Granger DN (2011) Selectin-mediated recruitment of bone marrow stromal cells in the postischemic cerebral microvasculature. Stroke 42:806-811.
Yoo SW, Chang DY, Lee HS, Kim GH, Park JS, Ryu BY, Joe EH, Lee YD, Kim SS, Suh-Kim H (2013) Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-β. Neurobiol Dis 58:249-257.
Yu X, Chen D, Zhang Y, Wu X, Huang Z, Zhou H, Zhang Y, Zhang Z (2012) Overexpression of CXCR4 in mesenchymal stem cells promotes migration, neuroprotection and angiogenesis in a rat model of stroke. J Neurol Sci 316:141-149.
Zhang C, Li Y, Chen J, Gao Q, Zacharek A, Kapke A, Chopp M (2006) Bone marrow stromal cells upregulate expression of bone morphogenetic proteins 2 and 4, gap junction protein connexin-43 and synaptophysin after stroke in rats. Neuroscience 141:687-695.
Zhang L, Li Y, Zhang C, Chopp M, Gosiewska A, Hong K (2011) Delayed administration of human umbilical tissue-derived cells improved neurological functional recovery in a rodent model of focal ischemia. Stroke 42:1437-1444.
Zhang Y, Zheng J, Zhou Z, Zhou H, Wang Y, Gong Z, Zhu J (2015) Fractalkine promotes chemotaxis of bone marrow-derived mesenchymal stem cells towards ischemic brain lesions through Jak2 signaling and cytoskeletal reorganization. FEBS J 282:891-903.
Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC (2002) Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol 174:11-20.
Zhao Y, Lai W, Xu Y, Li L, Chen Z, Wu W (2013) Exogenous and endogenous therapeutic effects of combination Sodium Ferulate and bone marrow stromal cells (BMSCs) treatment enhance neurogenesis after rat focal cerebral ischemia. Metab Brain Dis 28:655-666.
Zhu Y, Guan YM, Huang HL, Wang QS (2014) Human umbilical cord blood mesenchymal stem cell transplantation suppresses inflammatory responses and neuronal apoptosis during early stage of focal cerebral ischemia in rabbits. Acta Pharmacol Sin 35:585-591.
Copyedited by Smith T, Frenchman B, Yu J, Qiu Y, Li CH, Song LP, and Zhao M
10.4103/1673-5374.184506
How to cite this article: Li G, Yu F, Lei T, Gao H, Li P, Sun Y, Huang H, Mu Q (2016) Bone marrow mesenchymal stem cell therapy in ischemic stroke∶ mechanisms of action and treatment optimization strategies. Neural Regen Res 11(6)∶1015-1024.
Funding: This work was supported by the Natural Science Foundation of Heilongjiang Province of China, No. H2015083; and a grant from Higher Education Reform Project of Mudanjaing Medical University of China, No. 2013016.
*Correspondence to: Qingchun Mu, M.D., Ph.D. or Haiyan Huang, M.D., Ph.D., muqc@qq.com or huanghy@jlu.edu.cn.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Optic radiation injury in a patient with intraventricular hemorrhage: a diffusion tensor tractography study
- Synergetic effects of ciliary neurotrophic factor and olfactory ensheathing cells on optic nerve reparation (complete translation)
- miR-148b-3p promotes migration of Schwann cells by targeting cullin-associated and neddylationdissociated 1
- Transplantation of human adipose tissue-derived stem cells for repair of injured spiral ganglion neurons in deaf guinea pigs
- Indirubin-3′-monoxime suppresses amyloid-betainduced apoptosis by inhibiting tau hyperphosphorylation
- ROCK inhibition enhances neurite outgrowth in neural stem cells by upregulating YAP expression in vitro

