Transplantation of human adipose tissue-derived stem cells for repair of injured spiral ganglion neurons in deaf guinea pigs
Sujeong Jang, Hyong-Ho Cho, Song-Hee Kim Kyung-Hwa Lee, Yong-Bum Cho, Jong-Seong Park Han-Seong Jeong
1 Department of Physiology, Chonnam National University Medical School, Gwangju, Republic of Korea
2 Department of Otolaryngology, Chonnam National University Medical School, Gwangju, Republic of Korea
3 Department of Pathology, Chonnam National University Medical School, Gwangju, Republic of Korea
RESEARCH ARTICLE
Transplantation of human adipose tissue-derived stem cells for repair of injured spiral ganglion neurons in deaf guinea pigs
Sujeong Jang1,*, Hyong-Ho Cho2, Song-Hee Kim1, Kyung-Hwa Lee3, Yong-Bum Cho2, Jong-Seong Park1, Han-Seong Jeong1,*
1 Department of Physiology, Chonnam National University Medical School, Gwangju, Republic of Korea
2 Department of Otolaryngology, Chonnam National University Medical School, Gwangju, Republic of Korea
3 Department of Pathology, Chonnam National University Medical School, Gwangju, Republic of Korea
Excessive noise, ototoxic drugs, infections, autoimmune diseases, and aging can cause loss of spiral ganglion neurons, leading to permanent sensorineural hearing loss in mammals. Stem cells have been confirmed to be able to differentiate into spiral ganglion neurons. Little has been reported on adipose tissue-derived stem cells (ADSCs) for repair of injured spiral ganglion neurons. In this study, we hypothesized that transplantation of neural induced-human ADSCs (NI-hADSCs) can repair the injured spiral ganglion neurons in guinea pigs with neomycin-induced sensorineural hearing loss. NI-hADSCs were induced with culture medium containing basic fibroblast growth factor and forskolin and then injected to the injured cochleae. Guinea pigs that received injection of Hanks' balanced salt solution into the cochleae were used as controls. Hematoxylin-eosin staining showed that at 8 weeks after cell transplantation, the number of surviving spiral ganglion neurons in the cell transplantation group was significantly increased than that in the control group. Also at 8 weeks after cell transplantation, immunohistochemical staining showed that a greater number of NI-hADSCs in the spiral ganglions were detected in the cell transplantation group than in the control group, and these NI-hADSCs expressed neuronal markers neurofilament protein and microtubule-associated protein 2. Within 8 weeks after cell transplantation, the guinea pigs in the cell transplantation group had a gradually decreased auditory brainstem response threshold, while those in the control group had almost no response to 80 dB of clicks or pure tone burst. These findings suggest that a large amount of NI-hADSCs migrated to the spiral ganglions, survived for a period of time, repaired the injured spiral ganglion cells, and thereby contributed to the recovery of sensorineural hearing loss in guinea pigs.
nerve regeneration; adipose tissue-derived stem cells; spiral ganglion; neurons; hearing impairment; stem cell transplantation; brainstem auditory evoked potential; neural differentiation
orcid: 0000-0001-6921-6625 (Han-Seong Jeong) 0000-0001-8673-7887 (Sujeong Jang)
Accepted: 2015-12-22
Introduction
According to the World Health Organization (WHO, http:// www.who.int), more than 270 million people are affected by hearing loss, with estimates that up to 900 million people will experience some form of deficit by 2050. Hearing loss can be classified as either conductive or sensorineural. In particular, sensorineural hearing loss can be caused by the loss of hair cells, the loss of sound transducing sensory cells of the cochlea, or the loss of spiral ganglion neurons (SGNs). SGNs whose cell bodies lie in the spiral ganglion (SG) deliver auditory signals from cochlear hair cells to the central nervous system through the cochlear nerve. There are 30,000—40,000 SGNs in each inner ear, but these neurons are poorly regenerated in mammals (Holley, 2005; Schettino and Lauer, 2013; Geleoc and Holt, 2014; Wong and Ryan, 2015). Damage to SGNs can occur as the result of both genetic and environmental factors, and loss of these neurons, along with the associated damage to cochlear synapses, has been identified as a major cause of sensorineural hearing loss (Sergeyenko et al., 2013). The efficacy of cochlear implantation, a procedure whose main therapeutic benefit is replacement of nonfunctional cochlea, relies heavily on the degree of preservation of SGNs, and the restoration or replacement of degenerated SGNs is an important step in the treatment of sensorineural hearing loss (Incesulu and Nadol, 1998; Geleoc and Holt, 2014).
Stem cell replacement therapy is a prime candidate for the treatment of neurological disorders. Currently, several research strategies focus on developing viable means of stem cell transplantation, with the aim of replacing and restoring the neural elements that have degenerated as a result of hearing loss. To date, bone marrow-derived mesenchymal stem cells(MSCs) (Sharif et al., 2007; Jang et al., 2015b), neural stem cells (NSCs) (Okano et al., 2005; He et al., 2014), embryonic stem cells (ESCs) (Corrales et al., 2006), induced pluripotent stem cells (Ishikawa et al., 2015), and inner ear stem cells (Li et al., 2003) have all been studied in this regard. However, since the use of ESCs and NSCs is limited by various ethical and logistical constraints, adult peripheral tissues provide a more easily studied, alternative source of stem and progenitor cells. Adipose tissue provides a source of stem cells that can be easily obtained via lipoaspiration. Furthermore, adipose tissue-derived stem cells (ADSCs) have the ability to self-renew and can differentiate alongside several lineages of mesenchymal tissue, including adipocytes, osteoblasts, myocytes, chondrocytes, endothelial cells, and cardiomyocytes (Zuk et al., 2002; Gimble et al., 2007). ADSCs may also be induced into neurospheres, neural stem cell-like cells, and functional neural cells in vitro (Nagase et al., 2007; Jang et al., 2010; Zhang et al., 2014).
In the present study, we transplanted neural induced-human ADSCs (NI-hADSCs) into the inner ear of the deaf guinea pig and investigated whether NI-hADSCs have the potential to not only regenerate or replace the lost SGNs, but also to promote recovery of hearing ability.
Materials and Methods
Preparation of hADSCs and in vitro neural induction
According to the protocol described in our previously published study (Jang et al., 2010), we cultured hADSCs and differentiated them into NI-hADSCs, which have the potential to become functional neuronal cells when supplemented with basic fibroblast growth factor (bFGF; Invitrogen, Carlsbad, CA, USA) and forskolin (Sigma-Aldrich. St. Louis, MO, USA) in vitro. Fat tissue (50—100 μL) from the human earlobe, which could easily be taken during inner ear operations, was obtained from seven healthy donors 7—20 years of age according to the guidelines established by the Ethics Committee of the Chonnam National University Medical School (IRB:I-2009-03-016). The donors provided the written informed consent. There were no significant differences in fat tissue between children and adults. Human ADSCs were grown as adherent cultures in Dulbecco's modified Eagle's medium (Hyclone, Logan, UT, USA) with 10% fetal bovine serum (Hyclone) and 1% penicillin-streptomycin (Hyclone) in an incubator with 5% CO2. The cells were incubated with bFGF and forskolin for 2 weeks for neural induction and were then confirmed to have the function of neuron-like cells through molecular and immunocytochemical analyses.
Immunocytochemistry
After 2 weeks of neural induction, immnucytochemical determination of cell type-specific markers in hADSCs and NI-hADSCs was performed as previously described (Jang et al., 2015b). Cells were fixed for 15 minutes with 4% paraformaldehyde (Sigma-Aldrich) in phosphate-buffered saline (PBS; Amresco Inc., Solon, OH, USA) and blocked for 20 minutes with 0.5% Triton X-100 (Sigma-Aldrich) containing 10% normal goat serum (Vector Laboratories Inc., Burlingame, CA, USA) in PBS. The cells were then incubated for 2 hours in antiserum diluted in PBS at 1:300 for rabbit anti-neurofilament L polyclonal antibody (NFL), 1:300 for rabbit anti-neurofilament M polyclonal antibody (NFM), and 1:300 for rabbit anti-neurofilament H polyclonal antibody (NFH). These primary antibodies were purchased from Millipore (Billerica, MA, USA). After washing three times with PBS for 15 minutes each, appropriate biotinylated goat anti-rabbit IgG secondary antibody and VECTASTAIN ABC kits (Vector Lab, Burlingame, CA, USA) were used, and the immunoreaction was visualized with diaminobenzidine (Sigma-Aldrich). Staining was observed using a Zeiss AXIO Vert.A1 inverted microscope (Carl Zeiss, Jena, Germany).
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
To analyze the relative expression of different mRNAs between primary hADSCs and NI-hADSCs, RT-PCR analysis was performed after 2 weeks of neural induction as previously described (Jang et al., 2015a). Total RNA was extracted from cultured hADSCs using the TRI reagent (Takara Bio Inc., Shiga, Japan), and cDNA was synthesized using a Reverse Transcription System (Takara Bio Inc.). Amplification was carried out at 94°C for 2 minutes, followed by 35 cycles at 94°C for 1 minute, at appropriate annealing temperatures for each primer for 1 minute, and at 72°C for 1 minute. The forward and reverse PCR oligonucleotide primers chosen to amplify the cDNA are listed in Table 1. RT-PCR products were separated via electrophoresis using 2% agarose gels (Sigma-Aldrich). The amount of cDNA was normalized based on signals from the ubiquitously expressed β-actin in order to analyze the relative expression of different mRNAs. The density was quantified using ImageJ software (Jang et al., 2015a).
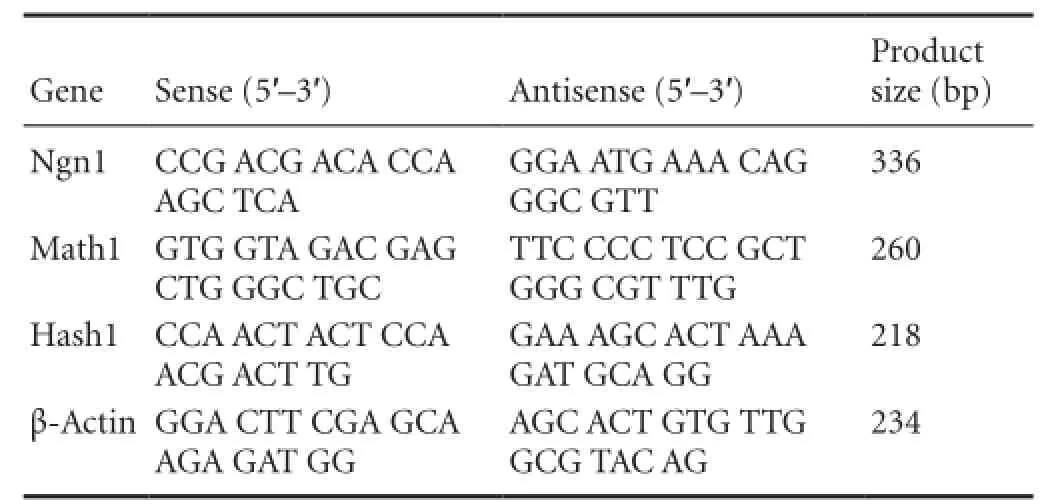
Table 1 Sequence of PCR primers
Animal model and NI-hADSCs transplantation
All procedures involving animals were approved by the Institutional Animal Care Committee of Research Institute of Medical Science at Chonnam National University and the Ethics Committee of the Chonnam National University Medical School, Republic of Korea. Sixteen guinea pigs were used and equally randomized to two groups: transplantation (NI-hADSCs were transplanted into the injured cochleae) and control groups (Hank's balanced salt solution (HBSS; Hyclone, Logan, UT, USA) was injected into the injuredcochleae).
All deafening procedures were conducted 7 days prior to stem cell transplantation as described in our previous study (Jang et al., 2015b). Guinea pigs had normal auditory function, which was confirmed by measuring auditory brainstem responses (ABRs) to broad click stimuli. Under general anesthesia with sodium pentothal (45 mg/kg, intraperitoneal injection, JW Pharmaceutical, Korea), the right postauricular region of each guinea pig was shaved and sterilized with 70% ethanol. The animal was then placed on a heating pad (37°C), and the right bulla was exposed and opened to visualize the basal cochlea. A small hole was made into the scala tympani using a dental burr at the most basal cochlear turn. To induce deafening, 5% neomycin solution (Sigma-Aldrich) was then administered with a Hamilton syringe (Hamilton Company, Reno, NV, USA) and infusion pump (KD Scientific Inc., Holliston, MA, USA) at the speed of 2 μL/min. This procedure generally causes severe loss of SGNs.
For the transplantation of NI-hADSCs, guinea pigs were anesthetized and placed on a heating pad to maintain body temperature at 37—38°C. A postauricular approach was utilized to expose the tympanic bulla following the deafening procedure and a stereomicroscope (Carl Zeiss, Jena, Germany) was used to visualize the cochlea. A 6-μL volume of HBSS or disaggregated NI-hADSCs, at a concentration of 1 × 104cells/μL in HBSS, was delivered at the speed of 2 μL/ min through the hole in the scala tympani using a Hamilton syringe and infusion pump. The needle of the Hamilton syringe was kept in the inner ear for 10 seconds to prevent the NI-hADSCs from flowing out. The hole was plugged with connective tissue, the round window was sealed with adherent agents, and the incision was approximated with sutures.
Immunohistochemistry and hematoxylin-eosin staining
Immunohistochemistry and hematoxylin-eosin staining were performed 8 weeks after transplantation. The animals were given an overdose of pentobarbital (200 mg/kg of body weight, intraperitoneal injection, JW Pharmaceutical) and perfused intracardially with PBS followed by 10% neutral buffered formalin (NBF; Merck, Australia). The injured cochleae were carefully removed, trimmed, fixed in 10% neutral-buffered formalin (NBF) overnight, and decalcified for 16 hours in 10% EDTA in NBF according to the previously described procedure (Jang et al., 2015b). The decalcified cochleae were then embedded in paraffin wax and subsequently sliced into 3-μm-thick serial paramodiolar sections. The sections were mounted on glass slides, and the morphology of the surviving cells was observed using hematoxylin and eosin (Sigma-Aldrich) staining. Representative sections were used for fluorescent immunohistochemistry.
Immunohistochemistry was then conducted to detect the transplanted cells in the spiral ganglion. After blocking in 0.5% Triton X-100 containing 10% normal goat serum in PBS, the sections were incubated with mouse anti-human nuclei monoclonal antibody (1:300), rabbit anti-microtubule-associated protein 2 (MAP2) polyclonal antibody (1:300), and rabbit anti-NFH polyclonal antibody (1:300) at 4°C overnight. Then, the sections were incubated with secondary antibodies at room temperature for 1 hour which were used to visualize primary antibodies. Secondary antibodies used throughout this procedure were: FITC-conjugated goat anti-mouse IgG antibody (1:300), FITC-conjugated goat anti-rabbit IgG antibody (1:300), Alexa Fluor 546-conjugated goat anti-rabbit IgG antibody (1:300), and Alexa Fluor 546-conjugated goat anti-mouse IgG antibody (1:300). Primary antibody was omitted from the reaction series as a means of negative control. All antibodies were purchased from Millipore (Billerica, MA, USA) and all images were acquired using a Zeiss LSM 700 confocal microscope (Carl Zeiss).
ABR threshold recording
ABR thresholds were recorded as described in our previously published study (Jeong et al., 2014). ABR testing was conducted in a sound-attenuated, electrically shielded booth under anesthesia. The stimulus presentation, ABR acquisition, equipment control, and data management were coordinated using a computerized Navigator SE evoked potential system (Bio-logic Systems Corp., Mundelein, IL, USA). ABRs were recorded using three silver needle electrodes placed subdermally over the vertex (active) and the bilateral retroauricular region (ground and reference) of each guinea pig. For clickevoked ABRs, alternative click sounds (150—3,000 kHz, stimulation rate 11/s, stimulation frequency 1,024) were presented to evoke an ABR, and the intensity of the stimulus varied in 10 dB stepwise decrements from 90 dB. An evoked potential system was used to measure the threshold of the ABR. To measure the 8 kHz pure tone-burst ABRs, the intensity of the stimulus varied in 5 dB stepwise decrements from 80 dB. The hearing status of all guinea pigs was assessed by measurement of click-evoked and 8 kHz pure tone-burst ABRs. ABR thresholds were examined prior to neomycin exposure and at 2, 4, 6, and 8 weeks following transplantation. Prior to deafening, all animals exhibited responses at thresholds equivalent to less than 10 dB of peak sound pressure level, representing hearing in the normal range. The deafening process was considered to be successful if no ABR was detected at an intensity of 80 dB.
Cell counting and statistics
For quantification of the number of SGNs, midmodiolar sections were stained with hematoxylin and eosin. Transplanted cells identified by positive immunoreactivity to human nuclei antibody were also counted in the inner ear in which the NI-hADSCs had been engrafted. The fraction of SGNs that were immunopositive was examined in double-blind fashion by superimposing a 100 × 100-μm2box on the spiral ganglion image and counting the number of cells within each box. One-way analysis of variance (Bonferroni post hoc comparison) was used to analyze the differences between groups, with P < 0.05 being considered significant. All values are expressed as the mean ± SEM.
Results
Human ADSCs underwent neurogenic differentiation in vitro
Human ADSCs were differentiated into the neurogenic lineage using our established protocol (Jang et al., 2010), and then assessed for the expression of neuronal markers. The majority of NI-hADSCs showed the morphological characteristics of neurons following neurogenic induction with bFGF and forskolin. The cytoplasm of the flat ADSCs retracted toward the nucleus, resembling a neuron-like soma, and the cells exhibited bipolar or multipolar morphology with branched processes. NI-hADSCs expressed positive immnuoreactivities to mature neuronal markers such as NFL, NFM, and NFH (Figure 1).
In addition, the expression of neuronal transcription factors was evaluated using RT-PCR. Neurogenin 1 (Ngn1), Math1, and Hash1 are known to be expressed in neuronal precursors during development of the nervous system and promote neuronal differentiation (Kageyama et al., 1997). The mRNA expressions of Ngn1, Math1, and Hash1 are significantly increased in NI-hADSCs when compared with those observed in primary hADSCs (Figure 2).
Survival and localization of NI-hADSCs after transplantation
We then assessed the potential for regeneration of SGNs by grafting NI-hADSCs into the animal model. To destroy the SGNs, neomycin was injected into the scala tympani in the most basal turn of the cochlea. Seven days after this injection, NI-hADSCs were grafted into the same area of the damaged cochlea.
In HBSS-injected control animals, neuronal degeneration and a decreased number of cell bodies were observed in the spiral ganglia (Figure 3). In NI-hADSC-engrafted animals, however, a significant increase (P < 0.01) was observed in the number of cell bodies in the spiral ganglia 8 weeks after NI-hADSC transplantation (Figure 3). The engrafted NI-hADSCs were found in the guinea pig cochleae up to 8 weeks after stem cell transplantation. At 8 weeks after transplantation, cells that were immunoreactive for human-specific anti-nuclei antibody were found in the spiral ganglia (Figure 4). To further validate the fate of the transplanted cells, double staining was conducted using anti-human nuclei antibody to specifically identify NI-hADSCs and tissue type-specific markers (NFH and MAP2) in the guinea pig cochlea. The transplanted human nuclei-positive cells located in the SG were observed to express NFH and MAP2 (Figure 4), which is a neuronal cell-specific marker, suggesting differentiation of the transplanted cells into neuronal cells.
Hearing improvement after NI-hADSCs transplantation
In the present study, we investigated the potential therapeutic effect of NI-hADSCs in an experimental model of neomycin-induced hearing loss. To evaluate the effects of NI-hADSC transplantation on the restoration of hearing in animals, we tested ABR thresholds, which were evoked by either click or 8 kHz pure tone-burst stimuli. Assessments were conducted prior to hearing loss and every 2 weeks after transplantation. All experimental animals in this study exhibited normal hearing with ABR thresholds of 0 ± 10 dB prior to hearing loss. After deafening procedure, the guinea pigs were shown to be profoundly hearing impaired, displaying almost no response to 80 dB of clicks or tone burst. This condition was maintained for up to 8 weeks in HBSS-injected control group (Figure 5A). Following transplantation of NI-hADSCs, progressive improvements in hearing as measured by ABR thresholds were observed for 8 weeks (Figure 5). Eight weeks after stem cell administration into damaged cochleae, the ABR thresholds were decreased from 76.11 ± 3.80 dB to 45 ± 5.04 dB to click evoked stimuli or from 77.10 ± 2.12 dB to 56.66 ± 9.27 dB to 8 kHz pure tone-burst stimuli in NI-hADSC transplantation group (Figure 5B).
Discussion
In the present study, hADSCs induced toward neurogenic differentiation were transplanted into an animal model of hearing impairment and the following results were obtained: (1) The number of cells in the damaged SG increased following transplantation of NI-hADSCs compared with the number observed in HBSS-injected control group; (2) human nuclei-positive cells were localized in the SG region and expressed neuronal markers; (3) improvements in hearing ability were observed in the NI-hADSCs-injected group over the 8-week period after transplantation.
Stem cell therapy is one of the most promising ways of treating hearing impairment; it aims to replace damaged or degenerated cells with healthy functional ones. Earlier studies have shown that transplantation of undifferentiated MSCs into the inner ear serves to repair injured cochlear fibrocytes in animal models of mitochondrial toxin-induced acute sensorineural hearing loss (Kamiya et al., 2007) and improve cell survival in the modiolus of ouabain-treated cochleae (Matsuoka et al., 2007). NSCs transplanted into the sound-damaged scala tympani of animal models have been reported to survive and express markers of hair cells and SGNs (Parker et al., 2007). More recently, transplantation of otic progenitor cells differentiated from human ESCs has been observed to improve auditory-evoked response thresholds in a model of auditory neuropathy (Chen et al., 2012).
ADSCs are of particular clinical interest among the various types of stem cells because they can be easily isolated from the patient's own adipose tissue. We previously reported on the ability of bFGF and forskolin to induce neurogenic differentiation of hADSCs (Jang et al., 2010). Ngn1, Math1, and Hash1 are known as basic helix-loop-helix transcription factors that promote neuronal differentiation (Kageyama et al., 1997) and Ngn1 is reported to be initially expressed in developing SGNs (Lu et al., 2011). In this study, the mRNA expressions of these neuronal transcription factors were significantly increased following neurogenic induction.
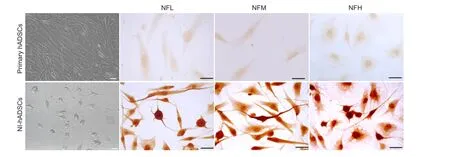
Figure 1 In vitro neurogenic differentiation of human adipose tissue-derived stem cells (hADSCs).
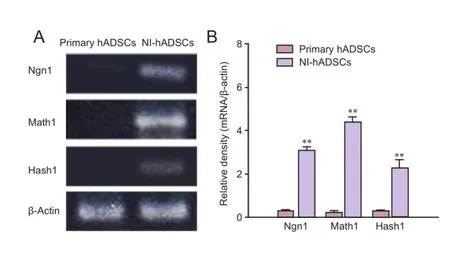
Figure 2 Reverse transcription-polymerase chain reaction (RT-PCR) for neuronal transcription factors in human adipose tissue-derived stem cells (hADSCs).
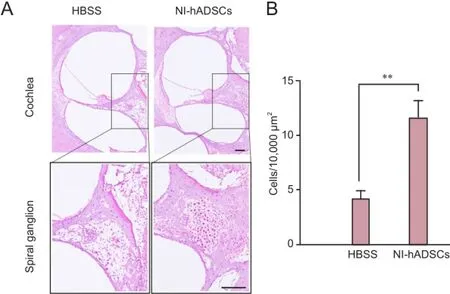
Figure 3 Hematoxylin-eosin staining of the injured cochlea after transplantation of neural induced-human adipose tissue-derived stem cells (NI-hADSCs).
The objective of this study is to demonstrate the scientific basis of NI-hADSC transplantation as a therapeutic approach that may lead to recovery of hearing in deaf mammals. The application of neomycin to the inner ear is a well-known method of inducing the death of or reducing the number of hair cells or spiral ganglion cells (Jang et al., 2015b). In this study, the number of SGNs in NI-hADSC transplantation group increased than that in the HBSS-injected control group. Furthermore, the injected NI-hADSCs, which were positive to human-specific anti-nuclei antibody, were found in the SG area and observed to express neuronal markers including NFH and MAP2. These results indicate that a number of NI-hADSCs migrate to the SG region and differentiate into neuronal cells, replacing lost or damaged neural cells in the SG. Previous studies suggested that SGNin the injured cochleae provide a more permissible environment than do normal cochleae for stem cells to survive and migrate to the injured tissue (Sekiya et al., 2006) and that neuronal differentiation is enhanced in injured cochleae when compared with normal cochleae (Hu et al., 2005). Our results were consistent with the results of these studies. This suggests that the NI-hADSCs can adapt to the cochlear environment and gives hope for treatment of damaged cochlea and sensorineural hearing loss.
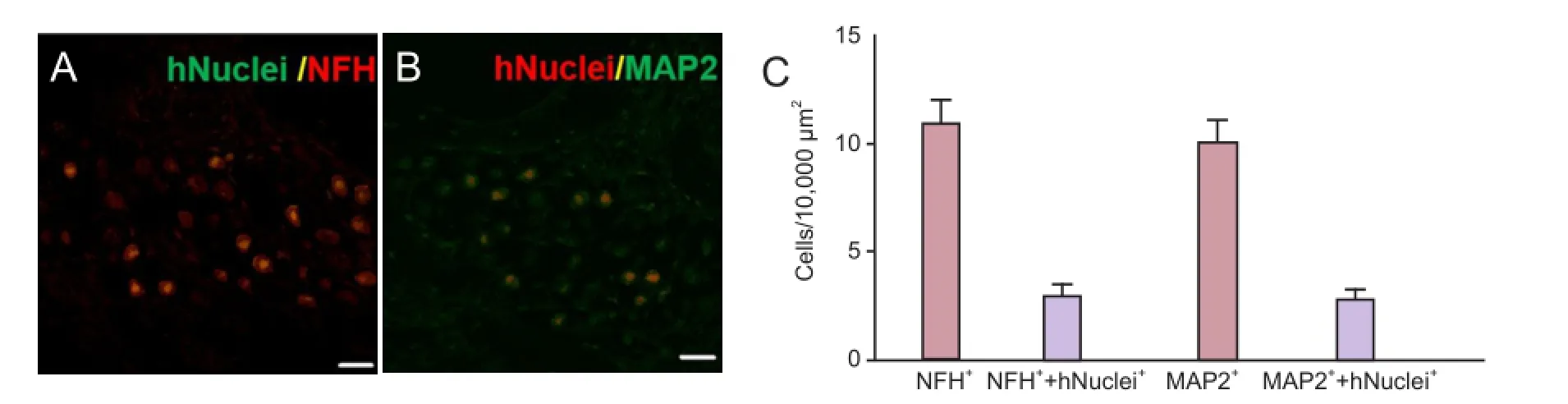
Figure 4 Localization and differentiation of transplanted neural induced-human adipose tissue-derived stem cells (NI-hADSCs).
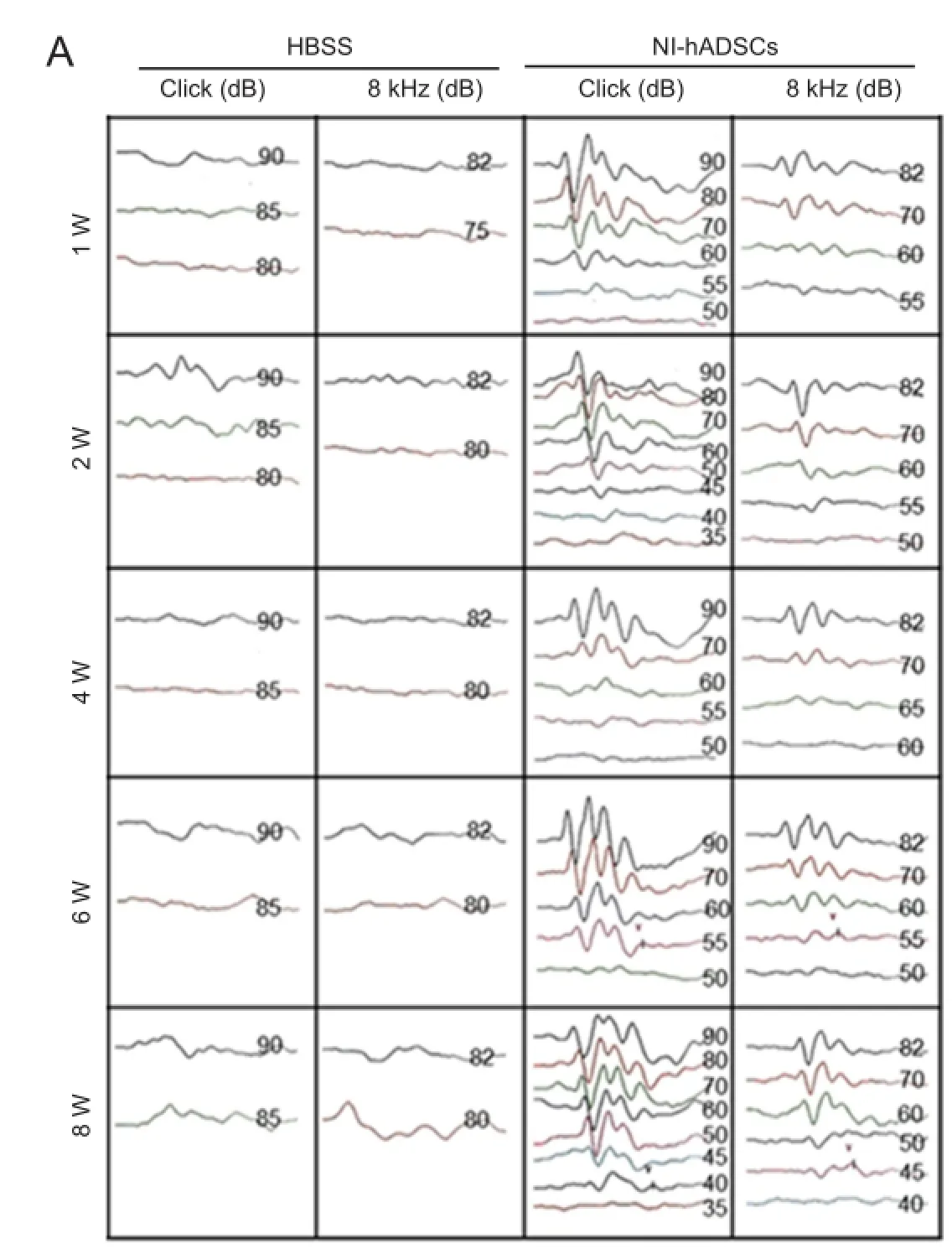
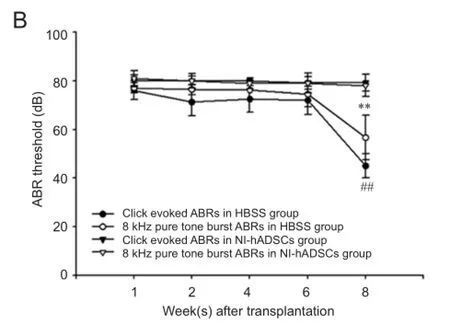
Figure 5 Auditory brainstem response (ABR) threshold change in deaf guinea pigs after transplantation of neural inducedhuman adipose tissue-derived stem cells (NI-hADSCs).
The ultimate goal of the type of stem cell therapy presented in the study is not only replacement of SGNs, but also restoration of hearing ability following damage to the area. In the present study, the decreased average ABR thresholds for click and pure tone stimuli indicate that NI-hADSCs transplantation is an effective therapeutic approach for sensorineural hearing loss. This significant recovery demonstrated in animalmodels might make such stem cell therapies more attractive to hearing-impaired patients. Since the presence of some SGNs is recognized to enhance cochlear implant function, supporting or replacing the SGNs using NI-hADSCs would also improve the therapeutic effect of cochlear implantation (Coleman et al., 2007; Boulet et al., 2016).
In conclusion, this study demonstrates that NI-hADSCs have the ability to replace the damaged SGNs and promote restoration of hearing function in an animal model of hearing impairment. In addition, these results could enhance further research regarding the development of stem cellbased therapies for hearing impairment.
Author contributions: SJ performed the main experimental work, was responsible for data collection, assembly, analysis and interpretation, and wrote the paper. HHC and YBC provided human tissue, discussed and commented about the data. SHK and KHL performed the immunohistochemistry. JSP discussed and commented about the data. HSJ contributed to financial support, conceived and designed the study, and wrote the paper. All authors approved the final version of this paper. Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Boulet J, White M, Bruce IC (2016) Temporal considerations for stimulating spiral ganglion neurons with cochlear implants. J Assoc Res Otolaryngol 17:1-17
Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, Kuhn S, Milo M, Thurlow JK, Andrews PW, Marcotti W, Moore HD, Rivolta MN (2012) Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature 490:278-282.
Coleman B, de Silva MG, Shepherd RK (2007) Concise review: the potential of stem cells for auditory neuron generation and replacement. Stem Cells 25:2685-2694.
Corrales CE, Pan L, Li H, Liberman MC, Heller S, Edge AS (2006) Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: growth of processes into the organ of Corti. J Neurobiol 66:1489-1500.
Geleoc GS, Holt JR (2014) Sound strategies for hearing restoration. Science 344:1241062.
Gimble JM, Katz AJ, Bunnell BA (2007) Adipose-derived stem cells for regenerative medicine. Circ Res 100:1249-1260.
He Y, Zhang PZ, Sun D, Mi WJ, Zhang XY, Cui Y, Jiang XW, Mao XB, Qiu JH (2014) Wnt1 from cochlear schwann cells enhances neuronal differentiation of transplanted neural stem cells in a rat spiral ganglion neuron degeneration model. Cell Transplant 23:747-760.
Holley MC (2005) Keynote review: The auditory system, hearing loss and potential targets for drug development. Drug Discov Today 10:1269-1282.
Hu Z, Wei D, Johansson CB, Holmstrom N, Duan M, Frisen J, Ulfendahl M (2005) Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res 302:40-47.
Incesulu A, Nadol JB Jr. (1998) Correlation of acoustic threshold measures and spiral ganglion cell survival in severe to profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol 107:906-911.
Ishikawa M, Ohnishi H, Skerleva D, Sakamoto T, Yamamoto N, Hotta A, Ito J, Nakagawa T (2015) Transplantation of neurons derived from human iPS cells cultured on collagen matrix into guinea-pig cochleae. J Tissue Eng Regen Med doi: 10.1002/term.2072
Jang S, Park JS, Jeong HS (2015a) Neural differentiation of human adipose tissue-derived stem cells involves activation of the Wnt5a/JNK signalling. Stem Cells Int 2015:178618.
Jang S, Cho HH, Cho YB, Park JS, Jeong HS (2010) Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol 11:25.
Jang S, Cho HH, Kim SH, Lee KH, Jun JY, Park JS, Jeong HS, Cho YB (2015b) Neural-induced human mesenchymal stem cells promote cochlear cell regeneration in deaf Guinea pigs. Clin Exp Otorhinolaryngol 8:83-91.
Jeong HS, Kim CM, Jang S, Park JS, Lee S, Cho YB, Cho HH (2014) Valproic acid increases formation of ROS and interferes with hearing recovery after noise trauma in guinea pigs. J Int Adv Otol 10:211-216.
Kageyama R, Ishibashi M, Takebayashi K, Tomita K (1997) bHLH transcription factors and mammalian neuronal differentiation. Int J Biochem Cell Biol 29:1389-1399.
Kamiya K, Fujinami Y, Hoya N, Okamoto Y, Kouike H, Komatsuzaki R, Kusano R, Nakagawa S, Satoh H, Fujii M, Matsunaga T (2007) Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol 171:214-226.
Li H, Liu H, Heller S (2003) Pluripotent stem cells from the adult mouse inner ear. Nat Med 9:1293-1299.
Lu CC, Appler JM, Houseman EA, Goodrich LV (2011) Developmental profiling of spiral ganglion neurons reveals insights into auditory circuit assembly. J Neurosci 31:10903-10918.
Matsuoka AJ, Kondo T, Miyamoto RT, Hashino E (2007) Enhanced survival of bone-marrow-derived pluripotent stem cells in an animal model of auditory neuropathy. Laryngoscope 117:1629-1635.
Nagase T, Matsumoto D, Nagase M, Yoshimura K, Shigeura T, Inoue M, Hasegawa M, Yamagishi M, Machida M (2007) Neurospheres from human adipose tissue transplanted into cultured mouse embryos can contribute to craniofacial morphogenesis: a preliminary report. J Craniofac Surg 18:49-53.
Okano T, Nakagawa T, Endo T, Kim TS, Kita T, Tamura T, Matsumoto M, Ohno T, Sakamoto T, Iguchi F, Ito J (2005) Engraftment of embryonic stem cell-derived neurons into the cochlear modiolus. Neuroreport 16:1919-1922.
Parker MA, Corliss DA, Gray B, Anderson JK, Bobbin RP, Snyder EY, Cotanche DA (2007) Neural stem cells injected into the sound-damaged cochlea migrate throughout the cochlea and express markers of hair cells, supporting cells, and spiral ganglion cells. Hear Res 232:29-43.
Schettino AE, Lauer AM (2013) The efficiency of design-based stereology in estimating spiral ganglion populations in mice. Hear Res 304:153-158.
Sekiya T, Kojima K, Matsumoto M, Kim TS, Tamura T, Ito J (2006) Cell transplantation to the auditory nerve and cochlear duct. Exp Neurol 198:12-24.
Sergeyenko Y, Lall K, Liberman MC, Kujawa SG (2013) Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci 33:13686-13694.
Sharif S, Nakagawa T, Ohno T, Matsumoto M, Kita T, Riazuddin S, Ito J (2007) The potential use of bone marrow stromal cells for cochlear cell therapy. Neuroreport 18:351-354.
Wong AC, Ryan AF (2015) Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front Aging Neurosci 7:58.
Zhang Y, Liu N, Tang Y, Yang E, Dong S, Huang M, Pan C, Zhang Y, Zhang P, Chen H, Tang Z (2014) Efficient generation of neural stem cell-like cells from rat adipose derived stem cells after lentiviral transduction with green fluorescent protein. Mol Neurobiol 50:647-654.
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279-4295.
Copyedited by Dubovy P, Godfrey DA, Li CH, Song LP, Zhao M
10.4103/1673-5374.184503
How to cite this article: Jang S, Cho HH, Kim SH, Lee KH, Cho YB, Park JS, Jeong HS (2016) Transplantation of human adipose tissue-derived stem cells for repair of injured spiral ganglion neurons in deaf guinea pigs. Neural Regen Res 11(6)∶994-1000.
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by Ministry of Education, Science and Technology, No. 2010-0025501; a grant from Chonnam National University Hospital Biomedical Research Institute, No. CRI11048-1 and a grant from the Chonnam National University, No. 2012-2894.
*Correspondence to: Han-Seong Jeong, M.D., Ph.D. or Sujeong Jang, Ph.D., jhsjeong@hanmail.net or sujeong.jjang@gmail.com.
 中國(guó)神經(jīng)再生研究(英文版)2016年6期
中國(guó)神經(jīng)再生研究(英文版)2016年6期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Bone marrow mesenchymal stem cell therapy in ischemic stroke: mechanisms of action and treatment optimization strategies
- Optic radiation injury in a patient with intraventricular hemorrhage: a diffusion tensor tractography study
- Synergetic effects of ciliary neurotrophic factor and olfactory ensheathing cells on optic nerve reparation (complete translation)
- miR-148b-3p promotes migration of Schwann cells by targeting cullin-associated and neddylationdissociated 1
- Indirubin-3′-monoxime suppresses amyloid-betainduced apoptosis by inhibiting tau hyperphosphorylation
- ROCK inhibition enhances neurite outgrowth in neural stem cells by upregulating YAP expression in vitro
