高活性硫化鉛電極的制備及在量子點(diǎn)敏化太陽(yáng)能電池中的應(yīng)用
張敬波 李 盼 楊 輝 趙飛燕 唐光詩(shī) 孫麗娜 林 原
(1天津師范大學(xué)化學(xué)學(xué)院,天津市功能分子結(jié)構(gòu)與性能重點(diǎn)實(shí)驗(yàn)室,無(wú)機(jī)-有機(jī)雜化功能材料化學(xué)省部共建教育部重點(diǎn)實(shí)驗(yàn)室,天津 300387;2北京化工大學(xué),化工資源有效利用國(guó)家重點(diǎn)實(shí)驗(yàn)室,北京 100029;3中國(guó)科學(xué)院化學(xué)研究所光化學(xué)重點(diǎn)實(shí)驗(yàn)室,北京分子科學(xué)國(guó)家實(shí)驗(yàn)室,北京 100190)
1 Introduction
Semiconductor quantum dots(QDs)have attracted a great deal of interest owing to their unique optical,electrical,and magnetic properties,which are different from those of bulk semiconductors completely.Therefore,QDs show some great potential applications in the opto-electronic field.1
Lead sulfide(PbS)is an important IV-VI direct gap semiconductor with a bulk band gap of 0.41 eV.Compared with other semiconductors,it has a larger exciton Bohr radius of 18 nm,which leads to a strong quantum confinement.2In addition,PbS QD has received widespread attention because the luminescence that it emits can cover the whole visible and near-infrared range.It has some significant potential applications in infrared detector,3solar absorber,4optical switch5and so on.Most importantly,on the one hand,the optical absorption band of PbS QD can be easily tuned from 0.4 to 1.5 eV by changing its size to achieve the near-infrared absorption.2On the other hand,multiple exciton generation of PbS QDs has been detected recently,that is to say,two or more charge carriers can be generated by a single photon having energy greater than the band gap.6,7These advantages make PbS be a promising QD sensitizer for the optimization of the quantum dots-sensitized solar cells(QDSSCs)to achieve a high conversion efficiency.
Not only can PbS nanoparticle be used as the superior sensitizer,but also presents several advantages as a counter electrode in QDSSCs.As we all know,organicredox electrolyte exhibiting the highest performance in dye-sensitized solar cells,cannot be suitable for QDSSCs due to the corrosion and photodegradation of QDs when they are in contact with theelectrolyte.8As a result,polysulfide redox couples()are often applied in QDSSCs to increase the stability of the photoanode.9In addition,Pt,showing a low charge transfer resistance(Rct)at the counter electrode/electrolyte interface,was widely used for the reduction ofto I-in dye-sensitized solar cells.But it exhibits a great overpotential for the polysulfide electrolyte regeneration,leading to a lower conversion efficiency of QDSSCs.10Until now,several kinds of counter electrode materials for the polysulfide electrolyte,such as CuS/CoS,11Cu2S,12-14WS2,15NiS,16Co3S4,17PbS,18,19and carbonaceous materials,20,21have been studied to substitute the traditional Pt.These studies have shown that CoS and Cu2S electrodes with an inferior long-term stability may contaminate electrolyte and photoanode.11-14Therefore,PbS is considered as the best alternative to the counter electrode(CE)because of its superior chemical stabilities and high performance to the polysulfide reaction.Zaban et al.18have developed a method to prepare a PbS counter electrode by dipping a Pb foil into a sulfuric acid solution and subsequently a polysulfide solution,which significantly increased the photocurrent and photovoltage of QDSSCs.
In this work,the method developed by Zaban et al.18was modified to improve catalytic activity of the prepared PbS electrode.The most important modification is that the treatment time in acid and polysulfide solution was markedly shortened but meanwhile the catalytic performance of the PbS electrode was enhanced.The electrochemical impedance spectroscopy(EIS)of the prepared PbS counter electrode and the photocurrent density-voltage(J-V)curves of the assembled CdSe QDSSCs based on the PbS CE were measured.The results demonstrated that the PbS CE prepared by the modified method provides better catalytic performance and electrochemical stability for the polysulfide electrolyte,which can greatly increase both the photocurrent density and the photovoltage of QDSSCs based on the CdSe QDs-sensitized TiO2nanocrystalline thin films,and thus leading to a higher photoelectric conversion efficiency.The reasons for better electrocatalytic activity of the PbS electrode prepared by the modified method were discussed.
2 Experimental
2.1 Chemicals
Lead foil with thickness of 0.5 mm was purchased from Alfa Aesar(99.9%metal basis)and used for preparing a PbS electrode.Cadmium sulfate(CdSO4),selenium(Se)powder,nitrilotriacetic acid(N(CH2COOH)3,NTA),sodium sulfide(Na2S),sulfur(S)powder,sulfuric acid(H2SO4),zinc nitrate(Zn(NO3)2),triton X-100,acetylacetone,sodium hydroxide(NaOH),and potassium chloride(KCl)were purchased from Alfa Aesar Inc.,China.All chemicals were analytical reagent without further purification.TiO2nanoparticles(P25)with the mean size of 25 nm were purchased from Aladdin reagents.
2.2 Preparation and characterization of the PbS electrodes
Firstly,a Pb metal foil was polished to show the shiny color with a fine sandpaper,and washed with deionized water for several times.The PbS electrodes were prepared according to the method developed by Zaban et al.18and the modified method,respectively.The typical process for the modified method is as follows:a Pb foil was immersed into a concentrated H2SO4solution(volume ratio of water and acid,1:1)at different temperatures(40,60,and 80°C)for different time(0.5,1,and 1.5 h),and then the foil was washed with deionized water thoroughly.The acid treatment changed the foil surface from shiny to gray.Subsequently,the foil was dipped into an aqueous solution of 1 mol·L-1Na2S,1 mol·L-1S,and 1 mol·L-1NaOH.The foil surface was changed from gray to black.Finally,it was washed with deionized water and dried in air.Compared with the reported method,in the modified method,the treatment temperature was increased but the treatment time was shortened markedly.
The surface morphologies and the crystalline structure during the formation process of the PbS electrode were observed by scanning electron microscopy(SEM,Hitachi S-4800,15 kV)and powder X-ray diffraction meter(XRD,Rigaku D/max-2500,Cu Kαradiation).The interfacial charge transfer resistance of the prepared electrode contacted with electrolyte was characterized under darkness by measuring the electrochemical impedance spectroscopy(EIS)of a symmetric thin-layer cell composed of two identical PbS electrodes using a surlyn film with the thickness of 40 μm as the spacer.The active area of the electrode was 0.20 cm2.The EIS measurement was performed using Solartron 1255B frequency response analyzer and Solartron SI 1287 electrochemical interface system at the zero bias with the frequency range from 1 MHz to 0.01 Hz.
2.3 Preparation of CdSe QDs-sensitized nanocrystalline thin film
TiO2paste was prepared by grinding TiO2nanoparticle powder and a few drops of triton X-100 with acetylacetone as a solvent for 6 h.The solid content of the paste was approximately 12%(mass fraction).Nanocrystalline thin film electrode was prepared by coating fluorine-doped tin oxide(FTO)substrate with the prepared TiO2paste using the doctor-blade method,followed by sintering it at 450°C for 30 min.22The film thickness could be increased by repeating the above operation.The thickness of the nanocrystalline thin film in this study is approximately 5 μm.Chemical bath deposition(CBD)of CdSe QDs was carried out on the nanocrystalline TiO2thin film electrode according to the reported method.22Finally,a thin ZnS layer was coated by dipping the electrode into a 0.5 mol·L-1Zn(NO3)2solution for 1 min,rinsing with deionized water,then dipping it into 0.5 mol·L-1Na2S solution for 3 min and rinsing it again.This procedure was repeated for five times.
2.4 Fabrication and characterization of QDSSCs
QDSSC with a sandwich cell configuration was assembled using a CdSe QDs-sensitized photoanode,a PbS CE,and polysulfide electrolyte.The polysulfide electrolyte consisted of 2 mol·L-1Na2S,0.5 mol·L-1S,and 0.2 mol·L-1KCl in an ethanol aqueous solution(volume ratio of ethanol to water,3:7).The photocurrent density-voltage(J-V)curve of the assembled CdSe QDs-sensitized solar cells was measured with Potentiostat/Galvanostat Model 273(EG&G)under light intensity of 100 mW·cm-2at AM 1.5 supplied by a solar simulator(Oriel,91160-1000).The active area was 0.20 cm2.
3 Results and discussion
3.1 Process optimization to prepare PbS electrode
Porous PbS electrode was reported to be prepared by immersing a Pb foil in an acid solution and a polysulfide solution,successively.15In the reported method,it took 24 h to treat the Pb foil at room temperature.We tried to modify the reported method to make the method easier,meanwhile the catalytic activity of PbS as a counter electrode in QDSSCs can be improved.EIS technology was extensively adopted to investigate the carrier dynamics and microstructure of electrode materials.23,24The electrocatalytic properties of the electrode toward the polysulfide reduction can be evaluated by the charge transfer resistance,which can be roughly estimated from the semicircle diameter in impedance spectroscopy or accurately determined by fitting the impedance curve with a reasonable equivalent circuit.Fig.1 presents the impedance spectroscopy measured on a symmetrical cell based on two identical PbS electrodes that were prepared by treating a Pb foil in the acid solution at different temperatures for different times.According to the semicircle diameter in the impedance spectroscopy,it is clear that the PbS electrode prepared by successively treating the Pb foil in the acid solution and the polysulfide solution at 60°C for 1 h displayed superior catalytic activity than the electrodes prepared at other treatment conditions.The temperature higher or lower than 60°C and the treating time longer or shorter than 1 h lead to a larger Rct.On the one hand,the short time or the low temperature is insufficient to treat the Pb foil thoroughly,the Pb foil cannot be adequately oxidized or the oxidized Pb2+cannot react with the polysulfide solution completely.On the other hand,the long treatment time and the high temperature may destroy the structures that were formed on the Pb foil surface after the acid and polysulfide solution treatment in a sense.
3.2 Electrocatalytic enhancement of the prepared PbS electrode
To further assess the catalytic performance of the PbS electrode prepared at the optimized conditions(treated at 60°C for 1 h,hereafter named as the modified method),the electrochemical impedance spectroscopy of the PbS electrode prepared according to the reported method was also measured.The Nyquist plots and the equivalent circuit for two electrodes were shown in Fig.2.It includes the series resistance(Rs)in the highfrequency region,the RC processes at electrode/electrolyte interface consisted of the charge transfer resistance and the electrical double-layer capacitance(Cdl)in the medium-frequency region.The electrocatalytic activity of the electrode to polysulfide reduction can be evaluated according to Rctvalue,which is determined by fitting the Nyquist curve with the equivalent circuit.The fitted values of Rs,Rct,and C to the Nyquist curves in Fig.2 were summarized in Table 1.The smaller semicircle for the PbS electrode prepared by the modified method corresponds to less Rct(2.54 Ω·cm2).It is less than that(55.35 Ω·cm2)of the PbS electrode prepared by the reported method.The small Rctimplies an acceleration of the Sn2-reduction,which indicates that the PbS electrode prepared by the modified method has higher catalytic activity.For comparison,the corresponding data of Pt electrode prepared according to the reported method25were also measured and collected in Table 1.Rctvalues for two PbS electrodes are both less than that of the Pt electrode.And the C values of both PbS electrodes are two orders magnitude larger than that of the Pt electrode.The larger C value indicates the PbS electrodes have a roughly surface with larger surface area,which will benefit the catalytic properties.
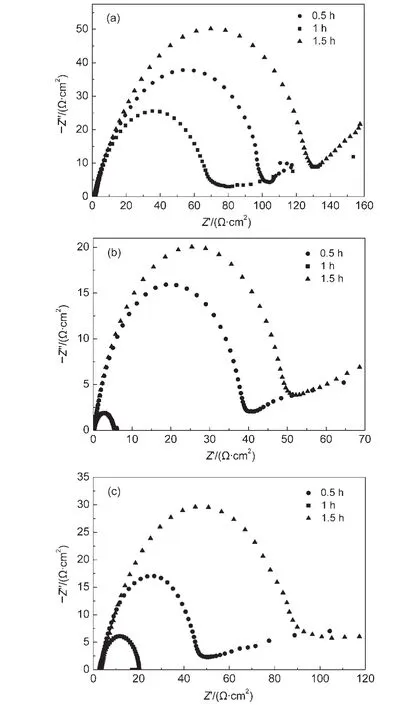
Fig.1 Electrochemical impedance spectroscopies of a symmetrical cell fabricated by two identical PbS electrodes prepared by treating the Pb foils in the acid solution and the polysulfide solution at 40 °C(a),60 °C(b),80 °C(c)for 0.5,1,and 1.5 h
3.3 Performance of the prepared PbS as a counter electrode
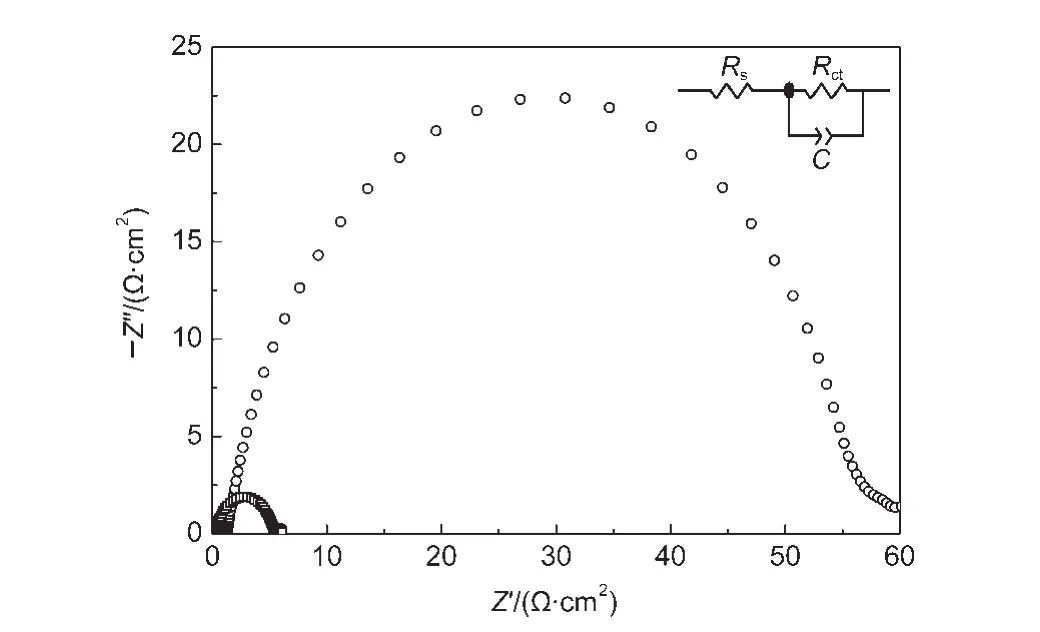
Fig.2 Electrochemical impedance spectroscopies of a symmetrical cell fabricated by two identical PbS electrodes prepared by the modified method(open square)and the reported method(open circle)
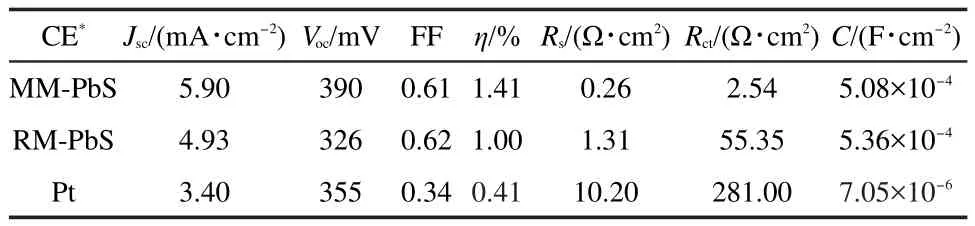
Table 1 Photovoltaic parameters of CdSe QDs-sensitized solar cells based on different counter electrodes and the fitted parameters to EIS in Fig.2
In order to study the photovoltaic performance of the PbS electrode prepared by the modified method,it was used as a counter electrode to fabricate QDSSC based on the CdSe QDssensitized nanocrystalline TiO2thin film photoelectrode.For comparison,the PbS electrode prepared according to the reported method18was also used to fabricate a QDSSC.Fig.3 showed the J-V curves for two QDSSCs measured under the illumination of 100 mW·cm-2(AM 1.5).The short-current density(Jsc),open-circuit voltage(Voc),fill factor(FF),and power conversion efficiency(η)of CdSe QDs-sensitized solar cells based on the prepared PbS CE were summarized in Table 1.For CdSe QDs-sensitized TiO2thin film solar cells,we can see that the use of the PbS CE prepared by the modified method can significantly improve both Jscand Vocsuch that η was increased from 1.00%to 1.41%.The photocurrent density-voltage curves agree well with the EIS results.Therefore,the photovoltaic parameter enrichment in QDSSCs is mainly attributed to the superior electrocatalytic activity of the PbS CE,which significantly reduces charge transfer resistance at the CE/electrolyte interface.
3.4 Performance enhancement mechanism of the prepared PbS electrode
To have a better understanding of the reasons for superior electrocatalytic activity of the PbS electrode prepared by the modified method,SEM accompanied with XRD was employed to analyze the microcosmic morphologies of the prepared electrode.
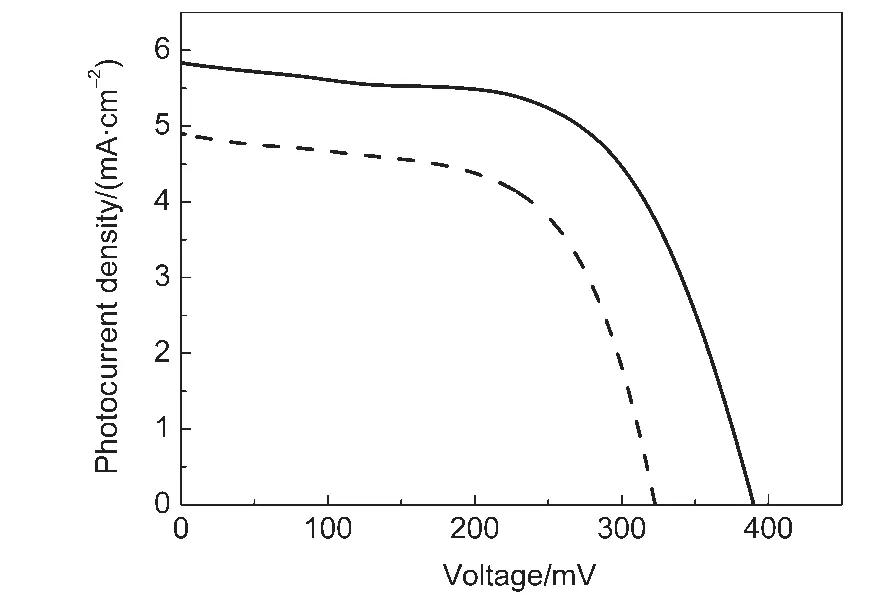
Fig.3 Photocurrent density-voltage curves for QDSSCs based on the CdSe QDs-sensitized TiO2thin film and the PbS CE fabricated by the modified method(solid line)and the reported method(dot line)
XRD patterns of the Pb foil treated in different solutions as shown in Fig.4 indicated the formation process of PbS on the Pb foil surface.The diffraction spectrum of the Pb metal foil dipped into the H2SO4solution at 60°C for 1 h showed several peaks,which are consistent with the standard spectroscopy of the PbSO4crystals(JCPDS No.36-1461),demonstrating the formation of PbSO4on the Pb metal foil surface firstly.And these peaks disappear as the acid-treated Pb foil was immersed in the polysulfide solution.Instead,several new peaks appear at 26.2°,30.4°,43.1°,51.3°,53.6°,and 62.4°.They correspond to the(111),(200),(220),(311),(222),and(400)crystal planes of PbS(JCPDS No.65-9496),respectively,which indicates the formation of cubic crystal structure PbS.The peaks due to Pb remain,because only a thin layer on the Pb foil surface could be oxidized in the concentrated H2SO4solution.The peaks corresponding to PbS are much broader than these of PbSO4illustrating that the size of crystals becomes small as the PbSO4crystals were transformed to the PbS crystals.Because the peaks at 28.8°and 36.5°corresponding to the orthorhombic structure of α-PbO2are very weak,the preparation method developed in this study can effectively suppress the formation of PbO2at the electrode surface.PbO2cannot catalyze the redox reaction of polysulfide electrolyte,therefore,this method can make sure the catalytic properties of the prepared PbS electrode toward the polysulfide electrolyte.

Fig.4 X-ray diffraction patterns of the Pb foil treated in the H2SO4solution(a)and subsequently treated in the polysulfide solution(b)
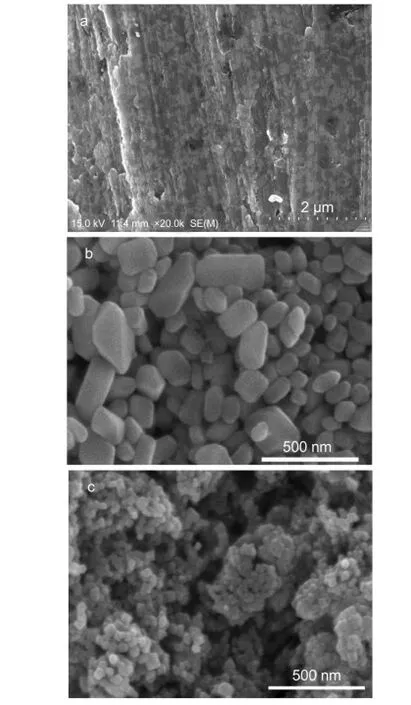
Fig.5 Surface SEM images of the Pb foil before any treatment(a),after dipped into the H2SO4solution at 60°C for 1 h(b),and subsequently treated in the polysulfide solution(c)
As the Pb foil was treated in different solutions,the surface evolution was observed using SEM as shown in Fig.5.There are some protrusions on the Pb foil surface before any treatment(Fig.5a).These protrusions are due to the sanding with the fine sandpaper.After the Pb foil was treated in the concentrated H2SO4solution,its surface image showed many aggregations due to some small particles with the size of 50-300 nm(Fig.5b).These aggregations were characterized as the PbSO4crystals by the XRD measurement(Fig.4).After the subsequent treatment in the polysulfide solution,the PbSO4crystals were converted into smaller PbS particles(also certificated by the XRD result)with diameter of approximate 20 nm(Fig.5c).Obviously,the treatment conditions used by the modified method can provide a larger homogeneous surface area and more catalytic sites than the reported method.18Therefore,the charge transfer kinetics at the PbS CE/polysulfide electrolyte interface in QDSSCs can be significantly enhanced.This is the radical reason why the PbS CE prepared by the modified method presents superior catalytic activity.
4 Conclusions
A facile method was developed to prepare the porous PbS electrode by successively treating a Pb foil in the acid solution and the polysulfide solution.The prepared PbS electrode presented a lower charge transfer resistance at the electrode/electrolyte interface as a result of its superior electrocatalytic activity.Furthermore,the structure and morphology during the formation process of the PbS electrode illustrated that the small and homogeneous PbS nanocrystals were formed on the Pb foil.These can provide a larger active surface area and more catalytic sites,contributing to the improvement of electrocatalytic activity for the redox reaction in the polysulfide electrolyte.Due to simplicity of the preparation processes and superior catalytic activity of the prepared PbS CE,it is a promising alternative to other CE materials widely used in QDSSCs based on the polysulfide electrolyte.
(2)Yue,D.;Zhang,J.W.;Zhang,J.B.;Lin,Y.Acta Phys.-Chim.Sin.2011,27(5),1239.[岳 棟,張建文,張敬波,林 原.物理化學(xué)學(xué)報(bào),2011,27(5),1239.]doi:10.3866/PKU.WHXB20110513
(3)Nair,P.K.;Gomezdaza,O.;Nair,M.T.S.Adv.Mater.Opt.Electron.1992,1,139.
(4)Gadenne,P.;Yagil,Y.;Deutscher,G.J.Appl.Phys.1989,66,3019.doi:10.1063/1.344187
(5)Chaudhuri,T.K.;Chatterjes,S.Proc.Int.Conf.Thermoelectr.1992,11,40.
(6)Kane,R.S.;Cohen,R.E.;Silbey,R.J.J.Phys.Chem.1996,100,7928.doi:10.1021/jp952869n
(7)Sambur,J.B.;Novet,T.;Parkinson,B.A.Science 2010,330,63.doi:10.1126/science.1191462
(8)Jovanovski,V.;González-Pedro,V.;Giménez,S.;Azaceta,E.;Caba?ero,G.;Grande,H.;Tena-Zaera,R.;Mora-Seró,I.;Bisquert,J.J.Am.Chem.Soc.2011,133,20156.doi:10.1021/ja2096865
(9)Chakrapani,V.;Baker,D.;Kamat,P.V.J.Am.Chem.Soc.2011,133,9607.doi:10.1021/ja203131b
(10)Fu,N.Q.;Xiao,X.R.;Zhou,X.W.;Zhang,J.B.;Lin,Y.J.Phys.Chem.C 2012,116,2850.
(11)Yang,Z.;Chen,C.Y.;Liu,C.W.;Li,C.L.;Chang,H.T.Adv.Energy Mater.2011,1,259.doi:10.1002/aenm.201000029
(12)González-Pedro,V.;Xu,X.;Mora-Seró,I.;Bisquert,J.ACS Nano 2010,4,5783.doi:10.1021/nn101534y
(13)Hossain,M.A.;Jennings,J.R.;Koh,Z.Y.;Wang,Q.ACS Nano 2011,5,3172.doi:10.1021/nn200315b
(14)Zhu,J.;Yu,X.C.;Wang,S.M.;Dong,W.W.;Hu,L.H.;Fang,X.D.;Dai,S.Y.Acta Phys.-Chim.Sin.2013,29(3),533.[朱 俊,余學(xué)超,王時(shí)茂,董偉偉,胡林華,方曉東,戴松元.物理化學(xué)學(xué)報(bào),2013,29(3),533.]doi:10.3866/PKU.WHXB201212124
(15)Li,S.J.;Chen,Z.;Zhang,W.F.Mater.Lett.2012,72,22.doi:10.1016/j.matlet.2011.12.052
(16)Yang,Z.;Chen,C.Y.;Liu,C.W.;Chang,H.T.Chem.Commun.2010,46,5485.doi:10.1039/c0cc00642d
(17)Yang,Z.;Chen,C.Y.;Chang,H.T.Sol.Energy Mater.Sol.Cells 2011,95,2867.doi:10.1016/j.solmat.2011.06.002
(18)Tachan,Z.;Shalom,M.;Hod,I.;Rühle,S.;Tirosh,S.;Zaban,A.J.Phys.Chem.C 2011,115,6162.doi:10.1021/jp112010m
(19)Zhang,J.B.;Zhao,F.Y.;Tang,G.S.;Lin,Y.J.Solid State Electrochem.2013,17,2909.doi:10.1007/s10008-013-2210-4
(20)Joshi,P.;Zhang,L.;Chen,Q.;Galipeau,D.;Fong,H.;Qiao,Q.ACS Appl.Mater.Interfaces 2010,2,3572.doi:10.1021/am100742s
(21)Kang,D.Y.;Lee,Y.;Cho,C.Y.;Moon,J.H.Langmuir 2012,28,7033.doi:10.1021/la300644j
(22)Zhao,F.Y.;Tang,G.S.;Zhang,J.B.;Lin,Y.Electrochim.Acta 2012,62,39.
(23)Fan,S.Q.;Fang,B.;Kim,J.H.;Jeong,B.;Kim,C.;Yu,J.S.;Ko,J.Langmuir 2010,26,13644.doi:10.1021/la1019873
(24)Hauch,A.;Georg,A.Electrochim.Acta 2001,46,3457.doi:10.1016/S0013-4686(01)00540-0
(25)Li,X.N.;Bai,S.L.;Yang,W.S.;Chen,A.F.;Sun,L.N.;Lin,Y.;Zhang,J.B.Acta Phys.-Chim.Sin.2012,28(7),1797.[李曉寧,白守禮,楊文勝,陳靄璠,孫麗娜,林 原,張敬波.物理化學(xué)學(xué)報(bào),2012,28(7),1797.]doi:10.3866/PKU.WHXB201205081

