C@CdS/埃洛石納米管復合光催化劑一步熱解法的制備及光降解性能
邢偉男 倪 良 顏學升 劉馨琳羅瑩瑩 逯子揚 閆永勝 霍鵬偉,*
(1江蘇大學化學化工學院,江蘇鎮(zhèn)江 212013;2江蘇貝斯特環(huán)保科技工程有限公司,江蘇鎮(zhèn)江 212013;3江蘇大學材料科學與工程學院,江蘇鎮(zhèn)江 212013)
1 Introduction
Tetracycline(TC)is a kind of drug antibiotics,which is widely used for human and animal therapies,and also in the agricultural antimicrobials.However,the overuse and misuse of TC had aroused worldwide concern as its biological impacts and potential environmental impacts,as well as to public health.Due to its antibacterial nature,biochemical approach can not effectively remove it from the environment.1-3Therefore,it is necessary to find a new method to remove the residues of antibiotics in the waste water.In recent years,photocatalytic oxidation technologies had been demonstrated to be an inexpensive and effective technology for the treatment of antibiotics.4,5
Semiconductors have attracted extensive attention because of their novel structures,unique optical,electrical,and catalytic properties.One important application was employed as photocatalyst to eliminate the environmental organic pollutant.6CdS,as one kind of photocatalysts,has been extensively studied because of its suitable band gap(2.42 eV),which can effectively absorb solar-light.However,photocorrosion of CdS is easy to occur in photocatalytic reaction where CdS itself is oxidized by the photogenerated holes,which is a major obstacle to its potential use in photocatalysis.In order to improve the stability and the photocatalytic activity of CdS photocatalyst,more and more researchers have focused on coupling CdS with other wide-band-gap semiconductor compound,7,8supporting CdS with big mesoporous material,9,10embedding CdS particles in a polymer matrix11etc.But few of the methods mentioned above have successfully increased both the photochemical stability and the photocatalytic activity of CdS.
Recently,surface coating or surface modification has been recognized as one of the most interesting methods to build complex nanostructures,which can alter the charge,functionality,and reactivity of the substrate.Designing core-shell structure is regarded as an effective surface coating method.As we all know,the design of core-shell structured composites have received much more attention as a measure to improve the stability and surface chemistry of the core materials.Carbon-coated nanomaterials are of great interest due to their chemical stability,biocompatibility,and adsorbability.12,13Many important applications had been found for use as an inter-component to be deposited on the surfaces of polymers,ceramics,metals,and oxides.This prompts us to encapsulate our CdS nanocrystals in a carbon layer.Some intrinsic defects of CdS will be overcome,if the surface of CdS coated by carbon.First,carbon is an inert material and is less affected in physiological conditions.Thus,the coating of carbon on CdS will make CdS more environmentally safe.Second,the existence of carbon shell can largely avoid the photocorrosion of CdS.Finally,combining carbon with CdS will modify the optical and electronic properties of CdS.
Some methods,such as the solvo-thermal technique,the laser technique,the sol-gel method etc.,have been applied to fabricate carbon-coated material.Among them,pyrolysis method is a simple,economical,and environmental method.However,the conventional pyrolysis method usually leads to sintered products with aggregation,which would have great effect on the unique properties of the nanomaterial.Supported technology can be effectively used to block the aggregation of nanoparticles,such as carbon nanotubes supported composites,14magnetic supported composites,15and graphene supported composites.16But the high cost and complex preparation of process had limited to the applications.Thus,the great priority may be given to the development of a substitute for carbon nanotubes.Halloysite nanotubes(HNTs)are a two-layered aluminosilicate clay mineral.They have hollow nanotube structure and large specific surface area.HNTs possess stabile property,resistible against organic solvents,and ease of disposal or reusability.More importantly,compared with other supported composites,HNTs are readily obtainable and much cheaper.17-22HNTs have adequate hydroxyl groups on the surface of HNTs,which are potential anchoring sites for catalyst particles.Therefore,the combination of C@CdS and HNTs is promising to simultaneously improve the photochemical stability and the photocatalytic activity in photocatalytic degradation of pollutants.
In this study,a one-step pyrolysis process was applied to prepare C@CdS/HNTs nanophotocatalyst.Dodecanethiol was selected as both carbon source and sulfur source.The choice not only promoted the formation of the nanoparticles,but also avoided other impurities into C@CdS/HNTs nanophotocatalyst.The existence of HNTs can effectively solve the agglomeration problem of the conventional pyrolysis method.What′s more,this simple approach is desirable for synthesis of other carbon-coated core-shell nanomaterials or other sulfides.The as-prepared photocatalysts have been characterized by scanning electron microscopy(SEM),transmission electron microscopy(TEM),X-ray energy dispersive spectroscopy(EDS),X-ray diffraction(XRD),UV-Vis diffuse reflectance spectra(UVVis DRS),Fourier transform infrared(FT-IR)spectroscoopy,specific surface area,and Raman spectrum(RS).The photodegradation results showed that the photocatalyst had high photocatalytic activity.Then,we used mass spectra(MS)to identify the intermediates of TC.Finally the mechanism of photodegradation TC was discussed in detail.
2 Experimental
2.1 Materials
HNTs were purchased from Zhengzhou Jinyang Guang Chinaware Co.Ltd.,Henan,China.Hydrochloric acid(AR),ethanol(AR),cadmium chloride(AR),and dodecanethiol(>97.0%)were all purchased from Shanghai Chemical Reagent Co.,Ltd.,and used as received.Tetracycline was purchased by Shanghai Shunbo Biological Engineering Co.,Ltd.Deionized and doubly distilled water were used throughout this work.
2.2 Preparation of C@CdS/HNTs nanophotocatalyst
Preparation of C@CdS/HNTs was partly according to the literature of Zhang and Liu.23The specific preparation process of C@CdS/HNTs was as follows.Briefly,HNTs(0.5 g)were added into 40.0 mL ethanol solution by ultrasonication for 10 min at room temperature to obtain HNT suspension.0.7878 g cadmium chloride in 20 mL ethanol solution was dropped into 40.0 mL HNT suspension.The mixture was agitated for 3 h on a magnetic stirrer,and added into dodecanethiol solution.The white precipitate of the cadmium complex was allowed to stir for 3 h.Then it was filtered off,washed with ethanol and H2O,dried at 80°C.The white solid powder was heated in a tube furnace at a heating rate of 2.5 °C·min-1,maintained at 300,400,500,and 600°C for 4 h in N2.In addition,for simplicity,photocatalysts at different pyrolysis temperatures were denoted as C@CdS/HNTs-1,C@CdS/HNTs-2,C@CdS/HNTs-3,C@CdS/HNTs-4.For contrast,the C@CdS was obtained in the absence of HNTs during the process of prepartion of C@CdS/HNTs.The CdS was obtained in a simple chemical deposition method.
It should be noted that,in this paper a chemical method was introduced to characterize the core-shell structure of the as-prepared photocatalyst.This method can adequately show the effect of carbon-coated CdS.It was evaluated by acid treatment.23The method was as follows:appropriate photocatalyst was immerging in hydrochloric acid aqueous solution(HCl,volume fraction:12%)at room temperature for 60 min.After filtering,the precipitates were washed with deionized water several times to neutrality and dried at 80°C.Then the sample was named as“acid treatment”.
2.3 Material characterization
The SEM images were examined by JSM-7001F scanning electron microscopy(JEOL Ltd.,Japan).Elemental mapping over the selected regions of the photocatalytst was conducted by X-ray energy dispersive spectroscopy(EDS).The transmission electron microscopice(TEM)images were taken on a JEOL IEM-200CX TEM(Japan).The X-ray diffractometer(XRD)patterns were obtained with a D8 ADVANCE X-ray diffractometer(Bruker AXS Company,Germany)equipped with Ni-filtrated Cu Kαradiation(40 kV,200 mA).The UV-Vis diffuse reflectance spectra(UV-Vis DRS)were obtained for the dry-pressed disk samples using Specord 2450 spectrometer(Shimazu,Japan)equipped with the integrated sphere accessory for diffuse reflectance spectra,using BaSO4as the reflectance sample.The Fourier transform infrared(FT-IR)absorption spectra were obtained with a Nicolet Nexus 470 FT-IR(America thermo-electricity Company),using KBr pellets.The specific surface area(BET)was estimated from the N2adsorption/desorption isotherms,measured by a Quantachrome NOVA4000 surface area apparatus.Raman spectra(RS)were obtained at ambient temperature using a WITEC Spectra Pro 2300I spectrometer equipped with an Ar-ion laser(LabRam inva),which provided a laser beam of 532 nm wavelength.
2.4 Measurement of photocatalytic activity
The photodegradation reaction of TC was carried out at 500 W in GHX-2 photocatalytic reactor under xenon lamp.In this part,light filter(wavelength range:360-760 nm)was used in the photocatalytic reactor and less than 420 nm was removed by light filter.The photochemical reactor contained 0.1 g catalyst and 20 mg·L-1of 100 mL TC aqueous solution.After 30 min in the dark,it reached absorption balance,its initial absorbency was determined.The temperature of the reactant solution was maintained below 298 K by a flow of cooling water during the reaction.The reaction continued for 1 h and conducted in 10 min interval.It was determined at λmax=357 nm.The photocatalytic degradation rate(DR)was calculated by this equation:DR=[(1-Ai/A0)]×100%,where A0is the initial absorbency of TC solution which reached absorption equilibrium and Aiis the absorbency of reaction solution.
3 Results and discussion
3.1 SEM,EDS and TEM analyses
The morphologies of the as-prepared photocatalysts were characterized by SEM,EDS,and TEM.Fig.1(a)shows the SEM image of the as-prepared photocatalyst.As can be seen,the nanoparticles were irregularly dispersed on the surface of HNTs.Fig.1(b)displays the elemental EDS images of C,O,Si,Al,Cd,and S.The peaks of Al,Si,and O were mainly generated by HNTs.EDS and SEM images clearly reveal that C,CdS,and HNTs have been well-mixed in the photocatalyst.Unfortunately,the carbon shell could not be testified by the above analysis.However,the TEM images in Fig.1(c,d)clearly show the core-shell morphology of the photocatalyst.Fig.1(d,e)shows the magnified image and HRTEM image of photocatalyst.Obviously,the CdS core was encapsulated into a thin gray shell,which is expected to be carbon.EDS clearly indicates that there is a uniform dispersion of the carbon element on the surface of CdS.Consequently,above characterizations provide direct evidence that hydrocarbons can be converted to amorphous carbon in situ on the surface of CdS via the present route.Simultaneously,C@CdS was well loaded on the surface of HNTs.
3.2 XRD analysis
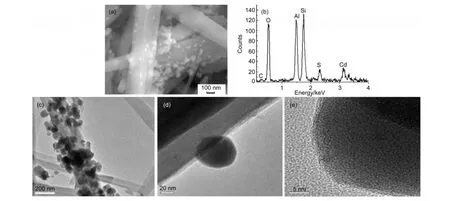
Fig.1 (a)SEM image,(b)EDS spectrum,(c)TEM image,(d)magnified TEM image,and(e)HRTEM image of the photocatalyst
The XRD patterns of photocatalysts were shown in Fig.2.The recorded diffraction peaks were assigned to the characteristic peaks of CdS and HNTs,which had been reported before.24,25However,some diffraction peaks of HNTs were no longer observed with the increasing pyrolysis temperature,which was probably due to the dehydroxylation of HNTs during the reduction process conducted at 450°C.19All of the diffraction peaks of CdS except pure CdS(Fig.2(a))can be indexed to CdS in a hexagonal phase structure(JCPDC No.06-0314).Their strong and sharp features suggested that CdS coated with carbon was still well crystalline.It can be clearly seen that the peak intensities were increased along with the increasing pyrolysis temperature.Their strong and sharp features manifest that highly crystalline CdS was obtained.It is suggested that the photocatalyst could retain a stable structure even after being heated at 600°C.Carbon XRD peaks were not observed,which might be due to the amorphous or semicrystalline nature of the encapsulating shell.26
3.3 BET analysis

Fig.2 XRD patterns of(a)CdS,(b)C@CdS,(c)C@CdS/HNTs-1,(d)C@CdS/HNTs-2,(e)C@CdS/HNTs-3,(f)C@CdS/HNTs-4
The specific surface area values were calculated using data of liquid nitrogen physisorption experiments(BET method).The BET analyses of the surface area,pore volume,and pore size of the photocatalyst were shown in Table 1.The results showed that C@CdS/HNTs-2 has the larger surface area(13.67 m2·g-1),while the surface area of C@CdS was only 0.645 m2·g-1,this may be because the introduction of HNTs can effectively block the aggregation of nanoparticles and increase the surface area.
3.4 FT-IR and RS analyses
Fig.3(A)shows the FT-IR spectra of the as-prepared photocatalyst.As can be seen from Fig.3(A),the double peaks at 3690 and 3621 cm-1were due to the stretching vibrations of hydroxyl groups on the surface of HNTs.The deformations of Al―O―Si,Si―O―Si,and O―H groups of the inner hydroxyl groups were at 537,468,and 912 cm-1.The peaks near 1000 cm-1were assigned to Si―O groups in HNTs.17,22However,some diffraction peaks of HNTs were disappeared with the increasing pyrolysis temperature.It was corresponding to the result of XRD,which was probably due to the dehydroxylation of HNTs during the reduction process conducted at 450°C.The characteristic peaks of other nanoparticles could not be seen in the FT-IR spectra.This may be that the bond was too weak or be overlapped by HNTs.Raman spectroscopy measurement was used to further confirm the carbonization of carbon shell and CdS.Raman peaks at 296 and 592 cm-1(Fig.3(B)),which were attributed to the first harmonic(1LO)and second harmonic(2LO)longitudinal optic phonon modes of CdS,respectively.27The distinct peak at 1326 cm-1corresponded to the D bands of the carbon shell,which was attributed to the Raman-inactive A1gvibration mode assigned to the vibrations ofcarbon atoms with dangling bonds in planar terminations of disordered graphite.The distinct peak at 1596 cm-1corresponded to the G bands of the carbon shell,which was attributed to the Raman active optical mode,E2g,of two-dimensional graphite,and which was closely related to the vibrations in sp2-bonded carbon atoms.28These results completely prove that the as-obtained products were carbon shell encapsulating the CdS core and the core-shell structure was well loaded on the surface of HNTs,in good agreement with the TEM results.
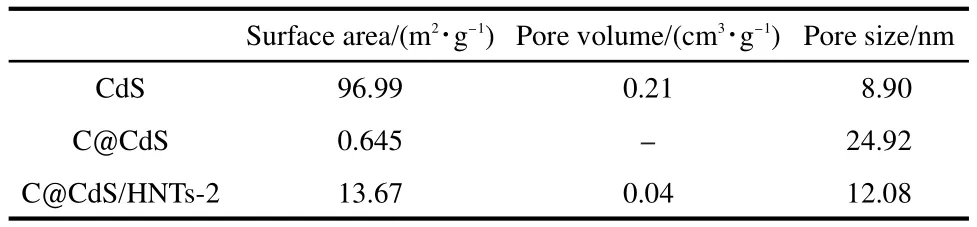
Table 1 BET results and pore analysis
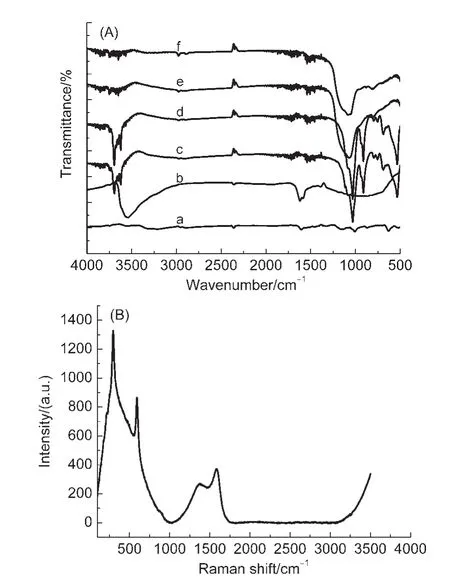
Fig.3 (A)FT-IR spectra of(a)CdS,(b)C@CdS,(c)C@CdS/HNTs-1,(d)C@CdS/HNTs-2,(e)C@CdS/HNTs-3,(f)C@CdS/HNTs-4;(B)RS image of photocatalyst at pyrolysis temperature 400°C
3.5 UV-Vis DRS analysis

Fig.4 UV-Vis DRS spectra of(a)CdS,(b)C@CdS,(c)C@CdS/HNTs-1,(d)C@CdS/HNTs-2,(e)C@CdS/HNTs-3,(f)C@CdS/HNTs-4
To evaluate the effect of the photocatalyst on the optical property,the UV-Vis diffuse reflectance spectra are presented in Fig.4.From Fig.4,the samples have obvious absorbency in the visible light range.The absorption spectra of the samples(C@CdS/HNTs-1 to C@CdS/HNTs-4)all had a strong absorption edge at ca 525 nm,indicating that the pyrolytic temperature had no distinct effects on the absorption edge.Compared with pure CdS,a blue shift was observed clearly.The reason may be due to the size quantization effects.According to the formula:λg=1240/Eg(where λgis absorption threshold and Egis the energy gap),it was estimated that the Egof photocatalyst was 2.36 eV,whereas the band gap of photocatalyst showed a significant rise than pure CdS(2.21 eV).
3.6 Growth mechanism of C@CdS/HNTs
The possible growth mechanism of the photocatalyst was shown in Scheme 1.In the first step,the surface of HNTs was modified with Cd2+by interacting with cadmium chloride.The large surface area,large pore volume and adequate hydroxyl groups of HNTs made Cd2+adsorb on the surface of HNTs.Then,Cd(C12H25S)2molecules were grown on the surface of HNTs through the reaction between Cd2+and dodecanethiol.23Finally,HNTs modified by Cd(C12H25S)2were thermally decomposed in N2atmosphere.As the temperature increased,all the C―S bonds were broken,forming CdS nanoparticles and the ligand fragments.Further pyrolysis process resulted in the carbonization of the ligand fragments to form carbon shell on the surface of CdS.
3.7 Photocatalytic activity

Scheme 1 Aschematic diagram of the formation process of C@CdS/HNTs photocatalyst
From Fig.5A,it can be clearly seen that the photo-degradation rate of TC was increased along with the increasing pyrolysis temperature during the process of preparing C@CdS/HNTs at the beginning,and the photo-degradation rate was decreased at a higher temperature at 400°C.This may be the reason that at lower temperature,the crystallinity of the product was decreased,amplifying the crystal defects and promoting the charge recombination,which could lead to the decrease of photocatalytic activity.On the other hand,it may be that the pyrolysis of the precursor was not completely at low temperature.When the temperature was higher than 400°C,the crystallinity of products increased,coupled with crystal grain growth,the big crystal grain made specific surface area smaller,decreased the surface activity content contacted with pollutants,then the photocatalytic activity decreased.
At last,we compared the degradation rates of CdS,C@CdS,and C@CdS/HNTs-2 in Fig.5(B).It can be seen that C@CdS/HNTs-2 had much higher degradation efficiency than CdS and C@CdS.The degradation rate of C@CdS increased along with the photocatalytic time,while CdS was only increased till 10 min and remained approximately constant during the following 50 min,this may be the role of carbon shell.Meanwhile,two control experiments were performed under different conditions:(a)with visible light irradiation but in the absence of the photocatalysts,(b)in the presence of the photocatalysts but in the dark.It was clearly seen that TC molecules contacting with photocatalyst did not cause a sharp decline of the TC degradation rate in the dark reaction condition.And,there was no significant degradation of TC after 60 min in the absence of photocatalysts.

Fig.5 (A)Photocatalytic degradation rate of TC in the presence of(a)C@CdS/HNTs-1,(b)C@CdS/HNTs-2,(c)C@CdS/HNTs-3,(d)C@CdS/HNTs-4;(B)photodegradation of TC(a)with visible light irradiation but in the absence of the photocatalysts,(b)in the presence of the photocatalysts but in the dark,(c)CdS,(d)C@CdS,(e)C@CdS/HNTs-2
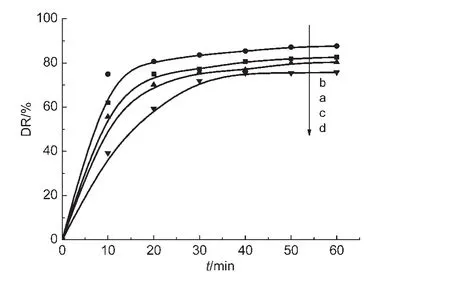
Fig.6 Photocatalytic degradation rate of acid-treated samples(a)C@CdS/HNTs-1,(b)C@CdS/HNTs-2,(c)C@CdS/HNTs-3,(d)C@CdS/HNTs-4
The acid treatment sample was used as a photocatalyst to degrade TC.Then,we compared the photocatalytic activity of acid-treated samples with untreated ones.From Fig.6,we can clearly see that the photo-degradation rate of TC did not have a significant change.It indicated that the surface of CdS core was completely coated by the carbon shell,almost no CdS particles bared outside.This will make the photocatalyst more environmentally safe and more stabile.It will not suffer from the corrosion even in an acid environment.This point endowed this material with a further bright perspective in practical applications.
3.8 Mechanism of photo-degradation TC
In order to investigate the changes of molecular and structural characteristic of TC as a result of photo-degradation,a typical UV-Vis spectra changes of the TC solution were shown in Fig.7.The UV-Vis spectra of the solution of TC were plotted from 200 to 800 nm.As could be seen from these spectra,before the photo-degradation,the spectrum of TC characterized by one main band was located at the visible region with the maximum absorption at 357 nm and another band in the ultraviolet region situated at 275 nm.The peak at 275 nm was the characteristic peak of acylamino and hydroxyl,which could associated with aromatic ring 1 structure.The peak at 357 nm comprised the extended chromophores,which originated from aromatic rings 2-4.The disappearance of two bands decreased continuously in time,which was owing to the fragmentation of enolic groups connected to aromatic ring 2 and acylamino and enolic groups connected to the aromatic ring 1 by the·OH radical attack.29

Fig.7 UV-Vis spectral changes of TC with reaction time

Fig.8 Degradation products and proposed reaction pathways of TC
In order to further identify the intermediate products during the photo-degradation,mass spectrum(MS)analysis was employed,the results are shown in Fig.S1(in Supporting Information).Fig.S1a was the spectrum of 20 mg·L-1TC,Fig.S1b was the 60 min degradation spectrum of TC.Compared with Fig.S1a,many new peaks appeared in Fig.S1b,the peak at 445(which was the tetracycline peak)became smaller and smaller,which suggested that TC had been decomposed gradually.At last,the intermediate products could be further degraded to CO2,H2O,and other small inorganic molecular material.30
In addition,the proposed degradation products and reaction pathways of TC are shown in Fig.8.It can be found that the degradation of TC was mainly separated by two processes.At first,TC was attacked by active species,which left a group of―CONH2to form product C.Then C was further attacked to form products D,E.The formation of product F might be due to the break of δ bond and rearrangement reaction(E to E2).31F to G,G to H,and I to J were similar with E to F.D to K was protonation process.The next break of δ bond was similar with E to I.The other reaction pathways may be attributed to TC attacked by·OH and the H of TC can be substituted by OH,it can obtain B.Then B was further attacked by active species to form products N,O.This process was similar with D to M.At last,the intermediate products could be further degraded to,CO2,H2O,etc.small inorganic molecular materials.
3.9 Stability and reusability of C@CdS/HNT nanophotocatalyst

Fig.9 Photocatalytic degradation rate of photocatalysts for the first time and after being laid aside for one year(1 year)
The most important factor for the practical utility of a photocatalyst is its longevity,reusability,and stability during the course of illumination.As shown in Fig.9,no appreciable decay of DR was found for nanophotocatalyst after being laid aside for one year(one year1).A slight decrease of DR was found after they were repeatedly performed and recycled in TC photo-degradation for three times,indicating that C@CdS/HNT nanophotocatalyst had excellent photochemical stability and reusability.The good recycling performance and longevity maintained on the photocatalyst make it promising candidate in practical applications.Meanwhile,the XRD patterns of the photocatalyst after three times photo-degradation were shown in Fig.S2,indicating that there were no structural changes in the nanophotocatalyst.Thus,we may conclude that the existing of carbon shell can prevent the photocorrosion of CdS.
4 Conclusions
The C@CdS/HNTs photocatalyst was well prepared by a one-step pyrolytic method.The photocatalyst exhibited high photocatalytic activity,environmentally safe,and good chemical stability.The optimal experimental conditions were discussed.When the pyrolysis temperature was at 400°C,the photocatalyst possessed the highest photocatalytic activity.The photo-degradation rate could reach 86%in 60 min under visible light irradiation.For the coating of carbon,some intrinsic defects of CdS were overcome,which made this material more stable and environmentally safe.For the loading of HNTs,the agglomeration problem of nanoparticles was solved.Therefore,the combination of carbon,CdS,and HNTs endowed this material with a bright perspective in purification of waste water.What deserves to be mentioned the most is that the photocatalyst laid aside for one year still kept high photocatalytic activity.In addition,the photocatalyst successfully retained its high catalytic activity after three catalytic cycles.On the other hand,the synthetic method gives a new avenue for fabricating carbonbased materials.
Supporting Information: Mass spectra of degraded TC and XRD patterns of photocatalyst after three-time photodegradation have been included.This information is available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1)Marshall,B.M.;Levy,S.B.Clin.Microbiol.Rev.October 2011,24,718.doi:10.1128/CMR.00002-11
(2)Baquero,F.;Martínez,J.L.;Canton,R.Curr.Opin.Biotechnol.2008,19,260.doi:10.1016/j.copbio.2008.05.006
(3)Jiao,S.J.;Zheng,S.R.;Yin,D.Q.;Wang,L.H.;Chen,L.Y.Chemosphere 2008,73,377.
(4)Zhao,C.;Deng,H.P.;Li,Y.;Liu,Z.Z.J.Hazard.Mater.2010,176,884.doi:10.1016/j.jhazmat.2009.11.119
(5)Pereira,J.H.O.S.;Vilar,V.J.P.;Borges,M.T.;González,O.;Esplugas,S.;Boaventura,R.A.R.Sol.Energy 2011,85,2732.doi:10.1016/j.solener.2011.08.012
(6)Lin,X.;Yu,L.L.;Yan,L,N.;Guan,Q.F.;Yan,Y.S.;Zhao,H.Acta Phys.-Chim.Sin.2013,29,1771.[林 雪,于麗麗,閆麗娜,關慶豐,閆永勝,趙 晗.物理化學學報,2013,29,1771.]doi:10.3866/PKU.WHXB201305131
(7)Nayak,J.;Sahu,S.N.;Kasuya,J.;Nozaki,S.Appl.Surf.Sci.2008,254,7215.doi:10.1016/j.apsusc.2008.05.268
(8)Shi,J.W.;Yan,X.;Cui,H.J.;Zong,X.;Fu,M.L.;Chen,S.H.;Wang,L.Z.J.Mol.Catal.A:Chem.2012,356,53.doi:10.1016/j.molcata.2012.01.001
(9)Barpuzary,D.;Khan,Z.;Vinothkumar,N.;De,M.;Qureshi,M.J.Phys.Chem.C 2012,116,150.
(10)Ryu,S.Y.;Balcerski,W.;Lee,T.K.;Hoffmann,M.R.J.Phys.Chem.C 2007,111,18195.doi:10.1021/jp074860e
(11)Zhang,H.;Zhu,Y.F.J.Phys.Chem.C 2010,114,5822.doi:10.1021/jp910930t
(12)Guo,Y.;Wang,H.S.;He,C.L.Langmuir 2009,25,4678.doi:10.1021/la803530h
(13)Zhong,J.;Chen,F.;Zhan,J.L.J.Phys.Chem.C 2010,114,933.doi:10.1021/jp909835m
(14)Yang,H.P.;Zhang,Y.C.;Fu,X.F.;Song,S.S.;Wu,J.M.Acta Phys.-Chim.Sin.2013,29,1327.[楊漢培,張穎超,傅小飛,宋雙雙,吳俊明.物理化學學報,2013,29,1327.]doi:10.3866/PKU.WHXB201303212
(15)Li,S.K.;Huang,F.Z.;Wang,Y.;Shen,Y.H.;Qiu,L.G.;Xie,A.J.;Xu,S.J.J.Mater.Chem.2011,21,7459.doi:10.1039/c0jm04569a
(16)Gao,Z.Y.;Liu,N.;Wu,D.P.;Tao,W.G.;Xu,F.;Jiang,K.Appl.Surf.Sci.2012,258,2473.doi:10.1016/j.apsusc.2011.10.075
(17)Papoulis,D.;Komarneni,S.;Nikolopoulou,A.;Tsolis-Katagas,P.;Panagiotaras,D.,Kacandes,H.G.;Zhang,P.;Yin,S.;Satoe,T.;Katsuk,H.Appl.Clay Sci.2010,50,118.doi:10.1016/j.clay.2010.07.013
(18)Pan,J.M.;Yao,H.;Xu,L.C.;Ou,H.X.;Huo,P.W.;Li,X.X.;Yan,Y.S.J.Phys.Chem.C 2011,115,5440.doi:10.1021/jp111120x
(19)Wang,L.;Chen,J.L.;Ge,L.;Zhu,Z.H.;Rudolph,V.Energy Fuels 2011,25,3408.doi:10.1021/ef200719v
(20)Vergaro,V.;Abdullayev,E.;Lvov,Y.M.;Zeitoun,A.;Cingolani,R.;Rinaldi,R.;Leporatti,S.Biomacromolecules 2010,11,820.doi:10.1021/bm9014446
(21)Reshmi,R.;Sanjay,G.;Sugunan,S.Catal.Commun.2006,7,460.doi:10.1016/j.catcom.2006.01.001
(22)Zhao,M.F.;Liu,P.Microporous Mesoporous Mat.2008,112,419.doi:10.1016/j.micromeso.2007.10.018
(23)Zhang,K.J.;Liu,X.H.J.Solid State Chem.2011,184,2701.doi:10.1016/j.jssc.2011.08.011
(24)Wang,Q.Q.;Zhao,G.L.;Han,G.R.Mater.Lett.2005,59,2625.doi:10.1016/j.matlet.2005.04.004
(25)Wang,R.J.;Jiang,G.H.;Ding,Y.W.;Wang,Y.;Sun,X.K.;Wang,X.H.;Chen,W.X.Appl.Mater.Interfaces 2011,3,4154.doi:10.1021/am201020q
(26)Bhattacharyya,S.Y.;Zitoun,D.;Gedanken,A.J.Phys.Chem.C 2008,112,7624.doi:10.1021/jp801353w
(27)Bhattacharyya,S.Y.;Estrin,Y.;Rich,D.H.;Zitoun,D.;Gedanken,A.J.Phys.Chem.C 2010,114,22002.doi:10.1021/jp107083f
(28)Mahendirana,C.;Maiyalaganb,T.;Scottb,K.;Gedanken,A.Mater.Chem.Phys.2011,128,341.doi:10.1016/j.matchemphys.2011.02.067
(29)Yang,H.;An,T.C.;Li,G.Y.;Song,W.H.;Cooper,W.J.;Luo,H.Y.;Guo,X.D.J.Hazard.Mater.2010,179,834.doi:10.1016/j.jhazmat.2010.03.079
(30)Wu,J.;Zhang,H.;Oturan,N.;Wang,Y.;Chen,L.;Oturan,M.A.Chemosphere 2012,87,614.doi:10.1016/j.chemosphere.2012.01.036
(31)Lu,Z.Y.;Huo,P.W.;Luo,Y.Y.;Liu,X.L.;Wu,D.;Gao,X.;Li,C.X.;Yan,Y.S.J.Mol.Catal.A:Chem.2013,378,91.doi:10.1016/j.molcata.2013.06.001

