C-LiFePO4/聚三苯胺復(fù)合鋰離子電池正極材料的制備與性能
蘇 暢 黃啟飛 徐立環(huán) 張 誠,*
(1浙江工業(yè)大學(xué)綠色化學(xué)合成技術(shù)國家重點(diǎn)實(shí)驗(yàn)室培育基地,杭州 310014;2沈陽化工大學(xué)化學(xué)工程學(xué)院,沈陽 110142)
1 Introduction
Olivine-structured lithium iron phosphate(LiFePO4)is becoming a focus of research in developing the low cost and high performance cathode materials for lithium-ion batteries.However,the low lithium-ion diffusivity(~10-18cm2·s-1)1and the electronic conductivity(~10-9S·cm-1)2of bare LiFePO4cause low rate capacity and low utilization of lithium in the host structure,which become the obstacles for its large-scale application in high power fields.Over the past few years,tremendous attempts have been devoted to overcome those limitations by improvement of purity,control of morphology and size,optimization of particles,adding conductive agent(typically carbon,metals,or polymers,etc.),3-9and doping the foreign atoms/ions(Cr3+,V5+,MO2+,Zn2+).10-13Among various endeavors,nanoarchitecture provided one of the desirable approaches to develop high-performance electrode materials for lithium store due to the high active surface area and shortened pathway for lithium insertion/deinsertion.14-16The solvothermal synthetic approach,17-20which is based on the use of high organic boiling point solvent instead of water as solvent,appears quite attractive for constructing the nanoarchitecture LiFePO4.Like water,organic high boiling point solvent has excellent solvent properties,as well as high thermal stability and negligible volatility,so that the use of autoclave is not mandatory.When alcohol analogues(ethylene glycol,glycerin,and tetraethylene glycol,etc.)are used as solvents to synthesize LiFePO4,the oxidation of the Fe2+ions to Fe3+ions,which often occurs during the hydrothermal synthesis process,21-23can be much avoided due to reduction nature of alcohol analogues,resulting in the improved electrochemical performances of LiFePO4.
Meanwhile,conductive carbon coating was a conventional way to conquer the limited rate capacity because the dispersed carbon conductive agent provides pathway of electron transfer,which results in improvement of the conductivity and electrochemical properties.Recently,application of electrically conductive polymers,such as polythiophene(PTh),polyaniline(PAn),polypyrrole(PPy),and their derivatives,as conductive agents to modify inorganic electrode materials has attracted much attention.Those conducting polymer layers on the surface of inorganic electrode materials should play a role of plastic protecting shell and the collapse of inorganic electrode materials because volume expansion during the charge-discharge process can be prevented effectively.And the investigations on LiFePO4/poly(3,4-ethylenedioxythiophene)(PEDOT),24LiFe-PO4/PAn,25LiFePO4/PPy,26V2O5/PPy,27and LiV3O8/PPy28have also been reported,which exhibited the improved electrochemical properties as the cathode for lithium-ion battery.
Compared with other conducting polymers,polytriphenylamine(PTPAn)and its derivatives,which contain triphenylamine radical units and a highly conductive polyparaphenylene(PPP)back-bone combined with high energy density of electroactive polyaniline unit,belong to family of radical polymer.It exhibited a reversible,rapid,and stable radical redox reaction during charge-discharge processes,29which makes the triphenylamine-based polymer materials be explored recently as the electrode active material applied in the energy storage field,such as super capacitors and lithium-ion battery.Moreover,PTPAn as a cathode material for lithium-ion batteries has relatively smooth charging and discharging voltage platform in 3.5 V place,which is similar to that of LiFePO4.Therefore,we expected to construct a new composite cathode material,which consists of LiFePO4and PTPAn conducting coatings,to improve the electrochemical properties of the LiFePO4-based cathode materials.
In this investigation,we firstly synthesized nano-size carboncoated LiFePO4(C-LiFePO4)by solvothermal method to improve Li+diffusivity in LiFePO4bulk and then C-LiFePO4/PTPAn composites were prepared by coating the PTPAn on the surface of C-LiFePO4particles by solution mixing method.The electrochemical properties of the series of C-LiFePO4/PTPAn as cathode materials were investigated systematically.
2 Experimental
2.1 Material preparation
C-LiFePO4powder was prepared by a low-temperature solvothermal method,and the detail procedure was described as following:LiOH·H2O(95.0%,Aladdin)was firstly dissolved in ethylene glycol,FeSO4·7H2O(99.0%,Aladdin)and H3PO4(85.0%,Aladdin)were dissolved in a small amount of distilled water,and then the above solutions were mixed at ambient temperature in a three-necked round-bottomed flask to realize a Li:Fe:P molar ratio of 3:1:1.The homogeneous and green mixed solution was kept reacting at 220°C for 20 h under magnetic stirring,and the whole course was protected by N2atmosphere with a tube blowing.After the reaction solution being cooled naturally to room temperature,the resultant solution was separated by centrifugation with distilled water several times,and the finally obtained light green-grayish product was dried in vacuum drying oven(DZF-6053,Yiheng Technology Co.)at 80°C for 12 h,then the C-LiFePO4was prepared by using LiFePO4powders as precursor to mix with a certain amount of sucrose(AR,Guangdong Guanghua Chemical Co.)as carbon source,where the sucrose was weighed in stoichiometric amount according to the LiFePO4to carbon mass ratio of 100 to 8,and the as-obtained mixture was dried,followed calcination at 650 °C with pure N2at a flow rate of 60 mL·min-1for 5 h.
The polymer of PTPAn was prepared by chemical oxidative method.The polymerization reaction was carried out in 20 mL chloroform(AR,Tianjin Yongda Chemical Co.)using ferric chloride(96%,Junze Chemical Co.)as oxidant.The solution was stirred over night at room temperature under N2.After completion of the solution polymerization reaction,the reaction mixture was poured into methanol to deposit the polymer product,which was then filtered and washed with methanol several times.Finally,the polymer product was filtered and dried in vacuum at 60°C for 12 h.
In order to prepare the C-LiFePO4/PTPAn composites,the PTPAn was dispersed in chloroform to form a colloidal solution.Then the C-LiFePO4nanocrystals were mixed with the above colloidal solution by ultrasonic dispersion for 20-30 min at ambient temperature to get the organic-inorganic nano hybrid,which was then dried in a vacuum oven at 60°C.The samples with 3%,10%,and 20%(w)PTPAn were prepared,respectively.
2.2 Structural characterization and electrochemical measurement
The crystalline phase of the resulting materials was analyzed by powder X-ray diffraction(XRD)(X1PertPRO,PNAlytical,Holand),which was carried out using a X1PertMPD diffractometer equipped with a X1Celerator detector and Cu Kαradiation(λ=0.1542 nm)operated at 40 kV and 40 mA.The sample morphology was characterized by a field emission scanning electron microscopy(FE-SEM)(S-4700,Hitachi,Japan)and a transmission electron microscopy(TEM)(Tecnai G2 F30 STwin,Philips-FEI,Holand).
The electrochemical performance of the C-LiFePO4/PTPAn composite as cathode was evaluated using a CR2032 coin-type cell.The C-LiFePO4/PTPAn composite electrode and PTPAn electrode were produced by dispersing active materials(70%(w)),carbon black(20%(w)),and poly(tetrafluoroethylene)binder(10%(w))in N-methylpyrrolidone(NMP)solvent to form a homogeneous slurry,respectively.The slurry was then deposited on a current collector consisting of Al foil by blade and then dried at 60°C for 10 h in an oven.The coin-type cell was assembled in a glove box filled with pure Ar.The electuolyte used was 1 mol·L-1LiPF6dissolved in a mixture of ethylene carbonate(EC)and dimethyl carbonate(DMC)(VEC/VDMC=1:1).A Li-foil and a polypropylene micro-porous film(Celgard 2300)were used as the counter electrode and separator,respectively.
The cells were charged and discharged in the range of 2.5-4.2 V at different rates.Electrochemical impedance spectroscopy(EIS)was measured over a frequency range of 100 kHz to 10 mHz at a discharged stage with an applied amplitude of 5 mV on an electrochemical workstation(CHI 660C,Shanghai Chenhua Co.).
3 Results and discussion
3.1 Material characterizations
XRD patterns of the C-LiFePO4and C-LiFePO4/PTPAn are shown in Fig.1(a,b).As can been seen in Fig.1a,the diffraction peaks of C-LiFePO4can be well indexed to pure LiFePO4with an orthorhombic olivine structure(JCPDS card No.83-2092).No impurities such as Li3PO4and others,which often appear in the LiFePO4product synthesized by traditional solid reaction route,are observed.All diffraction peaks are the same as the following standard peaks and all peaks are strong and narrow,indicating that the high crystallinity of the LiFePO4samples can be synthesized by low-temperature solvothermal method and then heat-treatment process.In addition,the diffraction peaks on carbon were not detected because the residual carbon on the surface of LiFePO4is amorphous.With increasing the PTPAn coatings on the C-LiFePO4,we found that the similar characteristic diffraction peaks for LiFePO4are presented(as shown in Fig.1(b-d)),indicating that PTPAn does not affect the crystal structure of the C-LiFePO4.
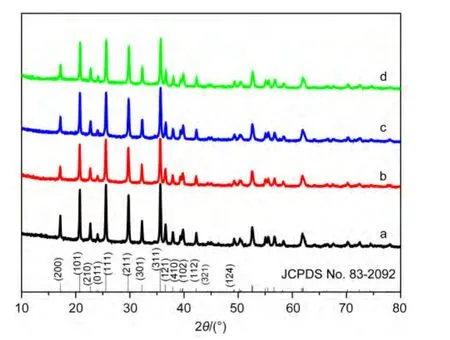
Fig.1 XRD patterns of(a)C-LiFePO4,(b)C-LiFePO4/3%PTPAn,(c)C-LiFePO4/10%PTPAn,and(d)C-LiFePO4/20%PTPAn

Fig.2 SEM images of(a)C-LiFePO4,(b)C-LiFePO4/3%PTPAn,(c)C-LiFePO4/10%PTPAn,and(d)C-LiFePO4/20%PTPAn;(e)TEM images of C-LiFePO4/10%PTPAn;(f)HRTEM images of C-LiFePO4/10%PTPAn
Fig.2 shows the typical SEM and/or TEM images of pure CLiFePO4and the series of C-LiFePO4/PTPAn samples obtained by blending of C-LiFePO4in chloroform solution with the PTPAn contents of 3%,10%,and 20%(w),respectively.From Fig.2a,we can see that the pure C-LiFePO4particles prepared by low-temperature solvothermal method display a spindleshape with a uniform size of about 100 nm in width diameter.The uniform and moderate particle size about C-LiFePO4is expected to benefit to lithium-ion migration in LiFePO4bulk and to enhancement of the electrochemical performance.For the CLiFePO4/PTPAn composites,we can see that the PTPAn polymer is well coated on the surface of the C-LiFePO4particles,which makes the surface of C-LiFePO4particles coarse.With increasing the amount of the PTPAn in the C-LiFePO4/PTPAn composites,most of the particles still keep their good dispersity,and a few of sticky PTPAn polymers among the CLiFePO4particles can benefit to decrease of the particle-to-particle contact resistance and thus to enhancement of the electrical conductivity of the composites.When the PTPAn content is 20%(w),the particles become seriously agglomerated and form larger aggregation(Fig.2d).TEM image(as shown in Fig.2e)further reveals that C-LiFePO4particles are spindleshape with well defined diffraction pattern of olivine phase(as shown in Fig.2e and the SAED pattern of top inset).HRTEM image(Fig.2f)shows that the carbon and both PTPAn coatings have been successfully coated onto the surface of the LiFePO4particles by our solution blending tactics and the thickness of carbon layer and PTPAn coating are about 1-5 nm and 1-3 nm,respectively.Because the LiFePO4particles are firstly coated with carbon and then are covered with the polymer,it is obvious that PTPAn coating is tightly covered on the surface of carbon.
3.2 Charge-discharge performance
The electrochemical properties of pure LiFePO4,C-LiFePO4,and the C-LiFePO4/PTPAn composites with different PTPAn contents are compared.Fig.3 shows cell voltage versus specific capacity for pure LiFePO4,C-LiFePO4,and various C-LiFePO4/PTPAn samples.Therein,the specific capacity is defined as the capacity per gram of the total active cathode material in the electrodes.And the theory discharge specific capacity of CLiFePO4/PTPAn(C0)can be calculated by following relation(Eq.(1)):

where,C1is the theory discharge specific capacity of LiFePO4(170.0 mAh·g-1),C2is the theory discharge specific capacity of PTPAn(109.0 mAh·g-1),w is the mass fraction of PTPAn in the C-LiFePO4/PTPAn composite.Compared to the pure LiFePO4,C-LiFePO4exhibits an increasing initial discharge specific capacity of 146.4 mAh·g-1,indicating that the carbon conductive coating on the surface of LiFePO4can effectively improve the utilization rate of LiFePO4.For the C-LiFePO4/PTPAn composites,according to the theoretical calculation,the theory discharge specific capacities of the C-LiFePO4/PTPAn composites are 168.2,163.9,and 157.8 mAh·g-1when the PTPAn contents are 3%,10%,and 20%(w),respectively.Usually,PTPAn has much lower theoretically specific capacity than that of the CLiFePO4,so an increase of PTPAn content in the C-LiFePO4/PTPAn composite is generally considered to reduce the specific capacity of the composite electrode,as compared with the parent C-LiFePO4cathode.However,as for the PTPAn in which modified C-LiFePO4is applied as the composite,we can clearly see the positively electrochemical contribution from PTPAn,and the measured discharge capacities of the C-LiFePO4,C-LiFePO4/3%PTPAn,C-LiFePO4/10%PTPAn,and C-LiFePO4/20%PTPAn at 0.1C are about 146.4,149.6,154.5,and 142.1 mAh·g-1,respectively.And specially,the C-LiFePO4/10%PTPAn composite cathode delivered the highest specific chargedischarge capacity of 154.5 mAh·g-1.In those four electrodes,the utilization rates of LiFePO4are 86.12%,88.96%,94.26%,and 90.05%,respectively,based on Eq.(1),where supporting that the theory discharge specific capacity of PTPAn is 109.0 mAh·g-1.The enhanced capacity of C-LiFePO4by PTPAn coatings can be explained as follows:the imperfect carbon layer coating30on the surface of LiFePO4can result in the fact that the surface of the LiFePO4is partly exposed and naked,which induces the ineffectively electron/ion transformation on the naked surface part and the poor utilization of LiFePO4during the charge-discharge process.As compared with the conductive PTPAn in solution,a tightly electroactive PTPAn film can form a supplementary conductive coating on the surface of C-LiFe-PO4particles or among the particles,resulting in an improved electrical/ionic conductivity and full utilization of the active materials of C-LiFePO4.Therefore,both the redox behavior of PTPAn and the synergistic effect provided by PTPAn and carbon layer attribute to the improvement of the specific capacity of the cathode.Specially,the degradation specific capacity for C-LiFePO4/20%PTPAn can be attributed to an excess of PTPAn and the serious agglomerated morphology.
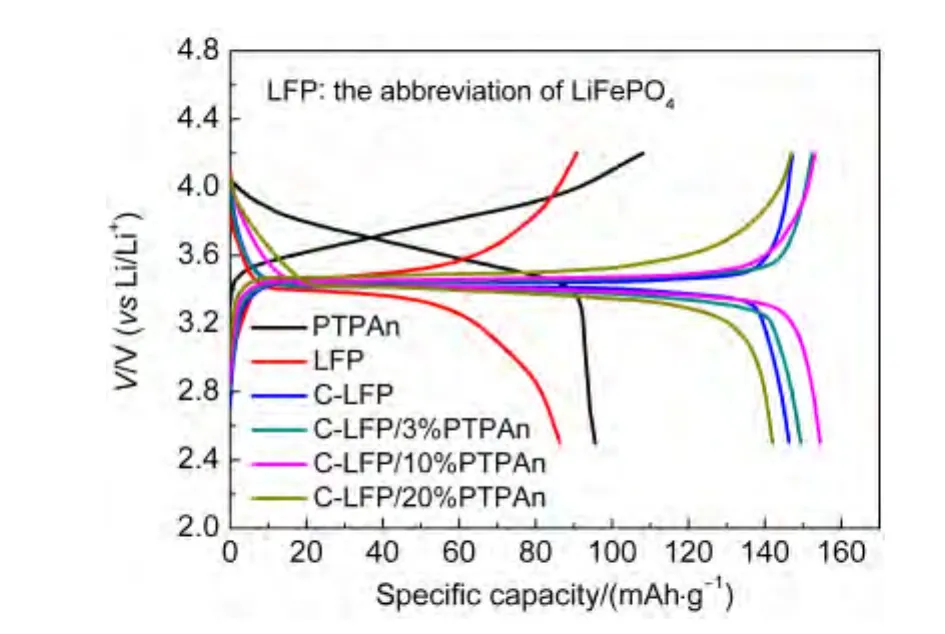
Fig.3 Initial charge and discharge curves of PTPAn,LFP,C-LiFePO4,C-LiFePO4/3%PTPAn,C-LiFePO4/10%PTPAn,and C-LiFePO4/20%PTPAn at 0.1C rate
The cycling performances at 0.1C for C-LiFePO4and various C-LiFePO4/PTPAn composites with different PTPAn contents are examined by cycling testing and the results are shown in Fig.4.It is found that C-LiFePO4,C-LiFePO4/3%PTPAn,and C-LiFePO4/10%PTPAn composite cathodes display the higher specific discharge capacities than C-LiFePO4/20%PTPAn,as well as the improved cycling stability during 50 cycles.This result demonstrates that the structure of the composite is relatively stable and the electrochemical lithium-ion insertion/extraction process is quite reversible at the lower PTPAn content of the composites.However,at high PTPAn content(CLiFePO4/20%PTPAn composite cathode),since the much more PTPAn exists among the C-LiFePO4/PTPAn composites,which connect C-LiFePO4particles,resulting in the serious agglomeration of the C-LiFePO4(as shown in Fig.2d),which tends to cause the seriously re-aggregation of C-LiFePO4particles during the initial charge-discharge process,as well the unstable cycling performance of the composite electrode.
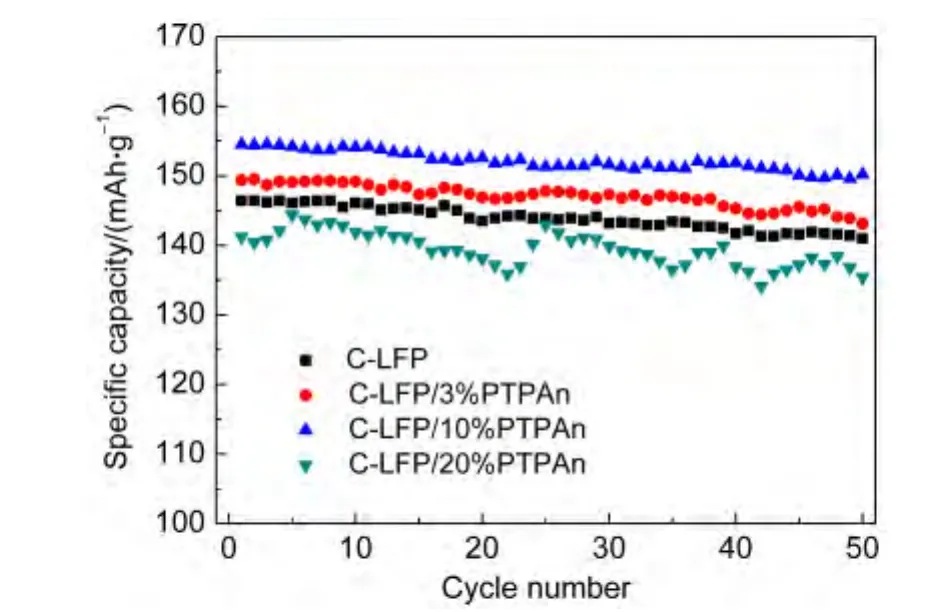
Fig.4 Cycling performances(herein refers to discharge capacities)of C-LiFePO4,C-LiFePO4/3%PTPAn,C-LiFePO4/10%PTPAn,and C-LiFePO4/20%PTPAn at 0.1C rate

Fig.5 Reversible capacities of C-LiFePO4,C-LiFePO4/3%PTPAn,C-LiFePO4/10%PTPAn,and C-LiFePO4/20%PTPAn during continuous cycling at various discharge rates from 0.1C to 10C
We further investigate the discharge properties for the CLiFePO4and C-LiFePO4/PTPAn composites at various rates and the results are illustrated in Fig.5 and Fig.6.Compared with parent C-LiFePO4,the C-LiFePO4/PTPAn composites with different PTPAn contents generally display an improved rate capability and the flat plateaus curve of charge-discharge.As shown in Fig.5,C-LiFePO4/3%PTPAn composite shows slightly improvement of rate capability compared to the parent C-LiFe-PO4,indicating that incorporation of 3%(w)PTPAn is not enough to improve the performance.Further increasing the content of PTPAn to 10%(w),the specific rate capacity of the obtained C-LiFePO4/10%PTPAn composite exhibits the best rate capability,and the discharged capacity can even reach up to 114.2 mAh·g-1at 10C,compared to the 85.5 mAh·g-1of the C-LiFePO4at the same high rate.As the content of PTPAn increases to 20%(w),the discharged rate capacity decreases slightly,but still higher than that of the parent C-LiFePO4at high rate(10C).The improved rate capability can be ascribed to the addition of PTPAn coating on the surface of C-LiFePO4,which possesses the advanced charge migration nature during the charge-discharge process to make it be able to serve as a host for lithium-ion intercalation/extraction.31In addition,the PTPAn coating can form a perfect conducting carbon layer coating on the surface of C-LiFePO4and provides good electronic contact between the particles and the current collector which decreases the internal resistance of the electrode.The electrodes with better lithium-ion charge migration and lower electric resistance should display better capacity retention at the higher discharge rate.
3.3 Electrochemical impedance analysis
Fig.7 further shows electrochemical impedance spectra of cycled cells with C-LiFePO4and C-LiFePO4/PTPAn composites with different PTPAn contents.The impedance spectra can be explained on the basis of an equivalent circuit with the electrolyte resistance(Re),charge transfer resistance(Rct),double layer capacitance(Cd),and Warburg impedance(Zw).31,32In these impedance plots,the initial intercept of the spectrum at the Z?axis in high frequency corresponds to the resistance of the electrolyte(Re).The semicircle at medium frequencies represents the charge-transfer reaction resistance,while the straight lines at low frequencies indicate the Warburg impedance,which displays the diffusion-controlled process.As can be seen in Fig.7,the resistance of the electrolyte is similar for the parent C-LiFePO4and C-LiFePO4/PTPAn electrodes.However,Rctvaries with different cathodes:348.9 Ω for C-LiFePO4electrode,160.9 Ω for C-LiFePO4/3%PTPAn electrode,and 191.1 Ω for C-LiFePO4/20%PTPAn electrode.Specially,the Rctof C-LiFePO4/10%PTPAn is only 140.7 Ω,which is the lowest among the four electrodes.Those results further indicate that the PTPAn coating significantly increases the electrical conductivity between C-LiFePO4particles,resulting in the improved rate performance.
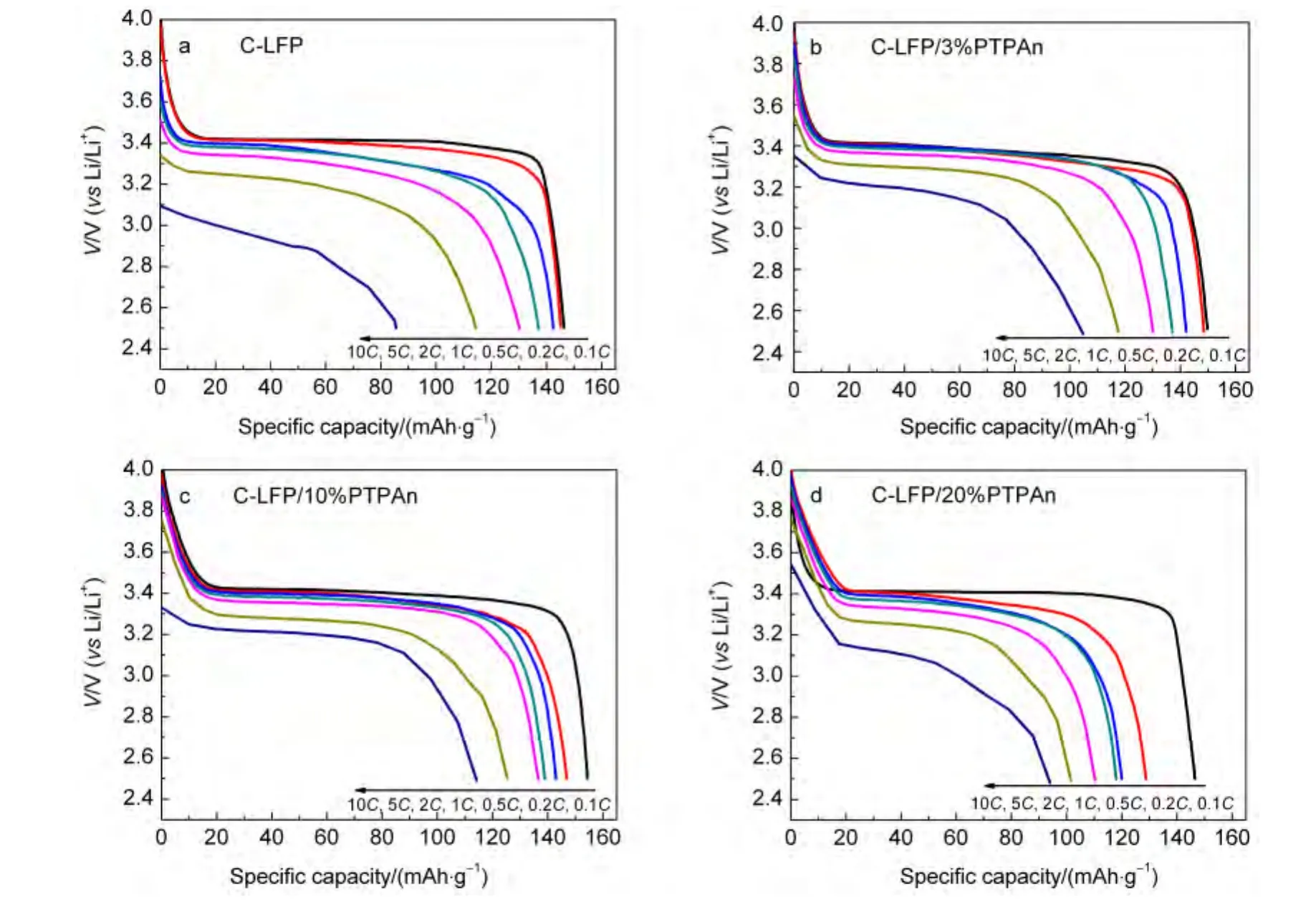
Fig.6 Discharge curves of(a)C-LiFePO4,(b)C-LiFePO4/3%PTPAn,(c)C-LiFePO4/10%PTPAn,and(d)C-LiFePO4/20%PTPAn at various rates from 0.1C to 10C

Fig.7 Electrochemical impedance spectra of C-LiFePO4,C-LiFePO4/3%PTPAn,C-LiFePO4/10%PTPAn,and C-LiFePO4/20%PTPAn
4 Conclusions
The olivine C-LiFePO4was prepared by a low-temperature solvothermal method and a subsequent high temperature postannealing processes.Then,the C-LiFePO4/PTPAn composites with PTPAn as electroactive coatings were prepared by the method of solution blending.The enhancement of the capacity and rate capability of the composite electrode materials can be attributed to both the nano-size effect of LiFePO4particles and the superior electric/ionic and electrochemical characteristics of PTPAn coatings.Specially,the C-LiFePO4/10%PTPAn composite electrode demonstrated the improved initial discharge capacity and the best high-rate capability,which displayed the discharge specific capacity from 0.1C to 10C were 154.5,148.6,143.1,139,136.7,125.4,114.8 mAh·g-1,respectively.The measurements on the electrochemical impedance spectra also demonstrated that PTPAn coating significantly decreased the charge-transfer resistance of C-LiFePO4electrodes.The perfect performances of the C-LiFePO4/PTPAn composites made it a good candidate for the potential applications in lithium-ion batteries.
(1)Srinivasan,V.;Newman,J.J.Electrochem.Soc.2004,151,1517.doi:10.1149/1.1785012
(2)Chung,S.Y.;Chiang,Y.M.Electrochem.Solid State Lett.2003,6,278.doi:10.1149/1.1621289
(3)Xie,H.M.;Wang,R.S.;Ying,J.R.;Zhang,L.Y.;Jalbout,A.F.;Yu,H.Y.;Yang,G.L.;Pan,X.M.;Su,Z.M.Adv.Mater.2006,18,2609.
(4)Kim,D.K.;Park,H.M.;Jung,S.J.;Jeong,Y.U.;Lee,J.H.;Kim,J.J.J.Power Sources 2006,159,237.doi:10.1016/j.jpowsour.2006.04.086
(5)Bewlay,S.L.;Konstantinov,K.;Wang,G.X.;Dou,S.X.;Liu,H.K.Mater.Lett.2004,58,1788.doi:10.1016/j.matlet.2003.11.008
(6)Wu,S.H.;Hsiao,K.M.;Liu,W.R.J.Power Sources 2005,146,550.doi:10.1016/j.jpowsour.2005.03.128
(7)Alvaro,C.;Manuel,C.Y.;Julian,M.;Jesus,S.P.;Enrique,R.C.Eur.J.Inorg.Chem.2006,2006,1758.
(8)Wang,G.X.;Bewlay,S.L.;Konstantinov,K.;Liu,H.K.;Dou,S.X.;Ahn,J.H.Electrochem.Acta 2004,50,443.doi:10.1016/j.electacta.2004.04.047
(9)Barker,J.;Saidi,M.Y.;Swoyer,J.L.Electrochem.Solid State Lett.2003,6,252.doi:10.1149/1.1621288
(10)Ni,J.F.;Zhou,H.H.;Chen,J.T.;Su,G.Y.Acta Phys.-Chim.Sin.2004,20,582.[倪江鋒,周恒輝,陳繼濤,蘇光耀.物理化學(xué)學(xué)報(bào),2004,20,582.]doi:10.3866/PKU.WHXB20040606
(11)Sun,C.S.;Zhou,Z.;Xu,Z.G.;Wei,J.P.;Bian,X.K.;Yan,J.J.Power Sources 2009,193,841.doi:10.1016/j.jpowsour.2009.03.061
(12)Yu,C.Y.;Wang,Z.L.;Chen,Y.;Xia,D.G.;Chu,W.S.;Wu,Z.Y.Rare Metals 2009,28,317.doi:10.1007/s12598-009-0062-y
(13)Liu,H.;Cao,Q.;Fu,L.J.;Wu,Y.P.;Wu,Q.H.Electrochem.Commun.2006,8,1553.doi:10.1016/j.elecom.2006.07.014
(14)Sun,G.;Jin,B.;Sun,G.P.;Jin,E.;Gu,H.B.;Jiang,Q.J.Appl.Electrochem.2011,41,99.doi:10.1007/s10800-010-0213-8
(15)Saravanan,K.;Balaya,P.;Reddy,M.V.;Chowdari,B.V.R.;Vittal,J.J.Energy Environ.Sci.2010,3,457.doi:10.1039/b923576k
(16)Malik,R.;Burch,D.;Bazant,M.;Ceder,G.Nano Lett.2010,10,4123.doi:10.1021/nl1023595
(17)Recham,N.;Dupont,L.;Courty,M.;Djellab,K.;Larcher,D.;Armand,M.;Tarascon,J.M.Chem.Mater.2009,21,1096.doi:10.1021/cm803259x
(18)Yang,H.;Wu,X.L.;Cao,M.H.;Guo,Y.G.J.Phys.Chem.C 2009,113,3345.doi:10.1021/jp808080t
(19)Tarascon,J.M.;Recham,N.;Armand,M.;Chotard,J.N.;Barpanda,P.;Walker,W.;Dupont,L.Chem.Mater.2010,22,724.doi:10.1021/cm9030478
(20)Murugan,A.V.;Muraliganth,T.;Manthiram,A.J.Phys.Chem.C 2008,112,14665.doi:10.1021/jp8053058
(21)Ellis,B.;Kan,W.H.;Makahnouk,W.R.M.;Nazar,L.F.J.Mater.Chem.2007,17,3248.doi:10.1039/b705443m
(22)Dokko,K.;Koizumi,S.;Kanamura,K.Chem.Lett.2006,35,338.doi:10.1246/cl.2006.338
(23)Dokko,K.;Koizumi,S.;Nakano,H.;Kanamura,K.J.Mater.Chem.2007,17,4803.doi:10.1039/b711521k
(24)Murugan,A.V.;Muraliganth,T.;Manthiram,A.Electrochem.Commun.2008,10,903.doi:10.1016/j.elecom.2008.04.004
(25)Lei,G.T.;Yi,X.H.;Wang,L.;Li,Z.H.;Zhou,J.Polym.Adv.Technol.2009,20,576.doi:10.1002/pat.v20:6
(26)Huang,Y.H.;Goodenough,J.B.Chem.Mater.2008,20,7237.doi:10.1021/cm8012304
(27)Zhao,H.B.;Yuan,A.B.;Liu,B.D.;Xing,S.Y.;Wu,X.Y.;Xu,J.Q.J.Appl.Electrochem.2012,42,139.doi:10.1007/s10800-012-0380-x
(28)Liu,L.L.;Wang,X.J.;Zhu,Y.S.;Hu,C.L.;Wu,Y.P.;Holze,R.J.Power Sources 2013,224,290.doi:10.1016/j.jpowsour.2012.09.100
(29)Feng,J.K.;Cao,Y.L.;Ai,X.P.;Yang,H.X.J.Power Sources 2008,177,199.doi:10.1016/j.jpowsour.2007.10.086
(30)Wang,Y.;Wang,Y.;Hosono,E.;Wang,K.;Zhou,H.Angew.Chem.Int.Edit.2008,47,7461.doi:10.1002/anie.v47:39
(31)Nobili,F.;Croce,F.;Scrosat,I.B.;Marassi,R.Chem.Mater.2001,13,1642.doi:10.1021/cm000600x
(32)Rodrigues,S.;Munichandraiah,N.;Shukla,A.K.J.Solid State Electrochem.1999,3,397.doi:10.1007/s100080050173

