Analysis of H2S Tolerance of Pd-Cu Alloy Hydrogen Separation Membranes*
GAO Huiyuan (高會元)** and WANG Ling (王嶺)College of Chemical Engineering, Hebei United University, Tangshan 063009, China
Analysis of H2S Tolerance of Pd-Cu Alloy Hydrogen Separation Membranes*
GAO Huiyuan (高會元)** and WANG Ling (王嶺)
College of Chemical Engineering, Hebei United University, Tangshan 063009, China
The presence of a limited amount of H2S in H2-rich feed adversely affects the Pd-Cu membrane permeation performance due to the sulphidization of the membrane surface. A theoretical model was proposed to predict the S-tolerant performance of the Pd-Cu membranes in presence of H2S under the industrial water-gas-shift (WGS) reaction conditions. The ideas of surface coverage and competitive adsorption thermodynamics of H2S and H2on Pd-Cu surface were introduced in the model. The surface sulphidization of the Pd-Cu membranes mainly depended on the pressure ratio of H2S to H2, temperature and S-adsorbed surface coverage, i.e., the occurrence of sulphidization on the surface was not directly related with the bulk compositions and structures [body centered cubic and face centered cubic (bcc or fcc)] of Pd-Cu alloy membranes because of the surface segregation phenomena. The resulting equilibrium equations for the H2S adsorption/sulphidization reactions were solved to calculate the pressure ratio of H2S to H2over a wide range of temperatures. A validation of the model was performed through a comparison between lots of literature data and the model calculations over a rather broad range of operating conditions. An extremely good agreement was obtained in the different cases, and thus, the model can serve to guide the development of S-resistant Pd alloy membrane materials for hydrogen separation.
surface coverage, Pd-Cu alloy membranes, H2S tolerance, theoretical model, hydrogen separation
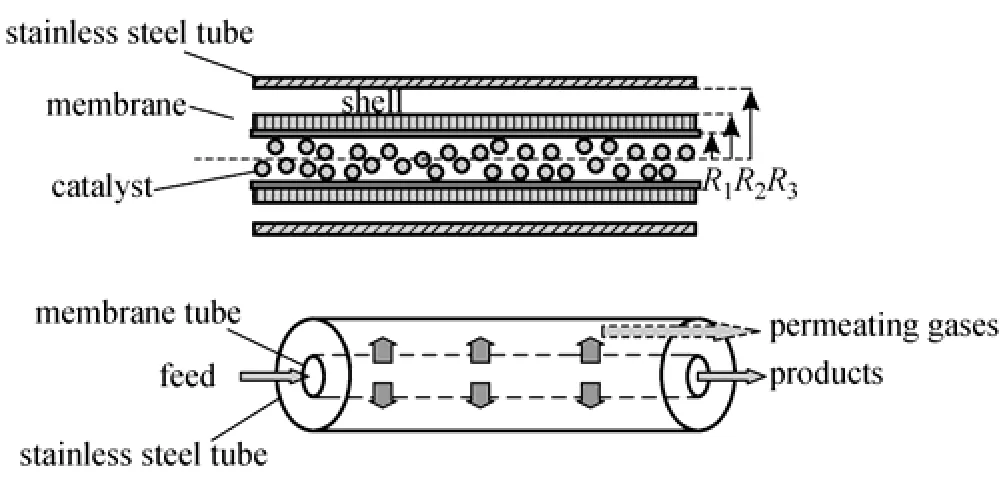
Figure 1 Schematic illustration of the catalytic membrane reactorR1—inside radius of membrane tube; R2—outside radius of membrane tube; R3—inside radius of stainless steel tube
1 INTRODUCTION
Hydrogen is not only the important chemical raw materials, but also an important clean energy. A variety of technologies have been and continue to be developed for the production of hydrogen [1] including coal/biomass gasification, oil and natural gas reforming, electrolysis of conductive water and so on. As a matter of fact, the gasification of coal is an effective method of thermal hydrogen production and is considered to be a key technology in the transition to a hydrogen economy. In the gasification process, carbon-based feedstocks are converted in the gasifier in the presence of steam and oxygen at high temperatures and moderate pressure to synthesis gas, a mixture H2, CO, CO2, CH4and trace components (H2S, HCN, etc.). The synthesis gas containing these minor and trace components must be cleaned to predetermined levels as consistent with further downstream processing.
Membranes that can efficiently and economically remove hydrogen produced from the gasification processes have the potential to increase the overall efficiency of the gasification process. Recently, one study has focused on using a membrane reactor to remove H2produced, thus improving the overall conversion of the water-gas-shift (WGS) reaction. The membrane reaction is depicted in the schematic diagram in Fig. 1. A significant technical barrier impeding hydrogen separation membrane development is resistance to impurities such as H2S. Palladium-based membranes have been considered as the good candidates for membrane reactors used in the clean coal conversion processes because of the excellent selectivity and the good permeability. Especially, Pd-Cu alloy membranes [2] with bcc/fcc structures have been of interest in recent years due to the high permeability, the costadvantage relative to Pd [3, 4], and suppression of the hydride-phase transition and resistance to poisoning performance.
Some groups [5-9] conducted research on the effect of H2S on the performance of Pd-Cu alloy membranes, and believed that membrane tolerance to H2S was strongly dependent on operating temperature, alloy composition, crystalline structure and exposure time, others [10] argued that failure of the Pd-Cu alloy membranes did not depend on H2S exposure time, only the H2S concentrations. Obviously, the statements of the observed changes in the Pd-Cu membrane performance in the presence of S-containing gases were varied considerably between studies because of the different experimental conditions and the different cognizance of poisioning mechanism.
The effect of H2S on the membrane performance is the deposition of sulfur on the surface and the formation of sulfides, which causes the decrease in hydrogen permeation through the membrane or membrane breakage. Therefore, H2S adsorption, decomposition and the formation of palladium sulfide on the metallic surface are the important steps in the poisoning process. From the thermodynamic equilibrium point of view, it is well known that the formation of noble metal sulphides depends on the ratio of H2S to H2in the atmosphere, and to accurately predict the formation of metal sulfides is possible. However, to the best of our knowledge, so far there has not been a theoretical model based on this type of mechanism to quantitatively predict the performance of H2S tolerance of Pd-Cu alloy membranes.
The objective of this paper is to set up a quantitative theoretical model and use it to predict the behavior of H2S tolerance of Pd-Cu alloy membranes based a surface kinetic mechanism. The rationality of the model is tested using the data derived from the literature. It is hoped that the model can guide the development of S-resistant Pd alloy membrane materials.
2 THEORETICAL MODEL
The elementary reactions of abstraction of H from H2S to form a surface SH intermediate and abstraction of H from SH to form a surface S intermediate as the dissociation pathway of H2S were examined by using the atomic models by several researchers [11-14]. The results suggest that dissociation of hydrogen sulfide has relatively low activation barriers on Pd alloy surfaces, and that the pathway to the resulting S adsorption is very favorable. The S adsorption strength does not change significantly with Pd alloy composition, and increases with increasing alloy d-band center energies [12] and lattice size [13].
The S adsorption strength decreases with increasing coverage on the metallic surface, which was attributed to the change in the adsorption sites and the repulsive interactions between the S adatoms as the S coverage increases. With increasing exposure to S-bearing contaminants, atomic S has been shown to irreversibly absorb into susceptible Pd alloys, especially Pd and Pd-Ag alloys, to form a non-limiting corrosion product layer [15, 16]. This layer often predominantly contains Pd4S, which has a significantly lower H2permeability [16]. The absorption of the relatively large S atoms significantly distorts the lattice to facilitate a high degree of S-metal coordination, serving as a precursor structure for formation of a Pd4S surface layer.
On the basis of above analysis, a kinetic mechanism of the palladium-based membranes of hydrogen sulfide poisoning is proposed consisting of the following elementary steps:
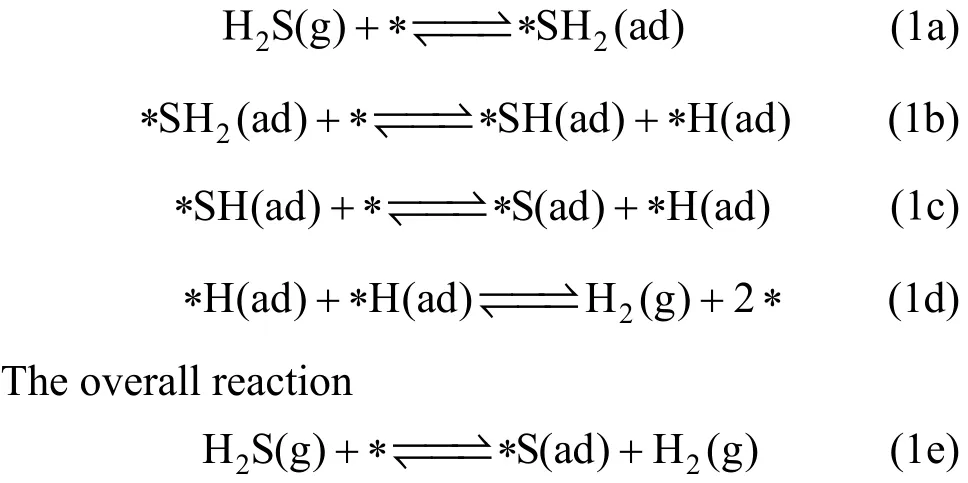
where g, ? and ad stand for gas, surface site and a different adsorbed intermediate, respectively.
The transition between a sulfur chemisorbed phase and the appearance of a sulfide scale [Eq. (2)] may be expected at the threshold coverage with chemisorbed sulfur, which is thermodynamically, not kinetically, determined. Similar facts also exist in the oxidation processes of the metallic surfaces [17].

where MxS represents the products of sulphidation.
Sulphidation reaction Eq (3) can be derived from coupling Eqs. (1e) and (2), regardless of the microscopic processes underlying the phase transition from the two-dimensional sulfur adsorbate layer to the three-dimensional sulfide.
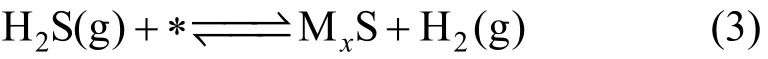
As mentioned above, at elevated temperatures H2S is easily decomposed on Pd surface, suggesting that there is only S and H adsorbed on the surface [18]. It is reasonable to further assume the competitive adsorption only involved S and H adsorbates on the membrane surfaces for our thermodynamic model under the condition of WGS reaction, i.e.,2SH0θ=,SH0θ=.
So,

hydrogen sulfide to hydrogen ratio,
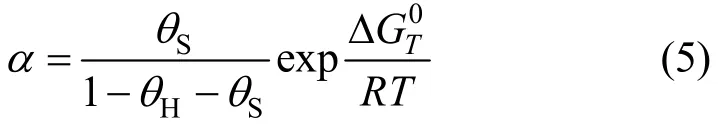
Since a linear relation exists between the heats of formation of the two-dimensional sulfur layers and the corresponding bulk metal sulfides [19, 20], thethermodynamic relationship Eq. (5) should be applicable to0sulphidation reaction Eq. (3) as long as one uses ΔGTof sulphidation reaction instead of the sulfur chemisorption.
According to the free energies of formation of Pd- and Cu-sulfides, Pd4S and Cu2S [21] will mainly be concerned in the PdxCu1?x-H2-H2S system. It is noted that other reactions forming tertiary compounds such as Pd13Cu3S7are possible, however, these sulfides are observed to be less common at the conditions of interest, and hence they will not be considered further in this model. Furthermore, in order to predict H2S tolerance performance of the pure Pd and PdxCu1?xalloy membranes at the different temperatures by using our theoretical model, on the basis of the research results [22-27], the surface atomic coveragesSθ andHθ of Pd membrane sulphidization in this study are chosen as 0.25 and 0.125, respectively. Actually, the value ofSθ(S/Pd)=1︰4 corresponds with the atomic ratio of Pd4S.
As for alloying Pd with Cu membranes, it is considered that Cu does not adsorb significant amounts of hydrogen at the studied temperatures, while Pd adsorbs hydrogen with the stoichiometry H/Pd≈1.
Meanwhile, palladium-copper alloy membranes require three times higher H2S feed concentrations to achieve the same inhibition of H2permeation as the pure Pd membranes [10]. Thus, it is assigned the H and S surface coverage (θH, θS) to 0.125 monolayer (ML) and 0.75 ML, respectively. In fact, some experimental and theoretical researches [10] have revealed that the incorporation of sulfur into the metal becomes favorable at a critical coverage of about 0.75 ML at elevated temperatures [28]. The threshold coverages, sulphidization reactions and the standard Gibbs free energies for Pd and Pd-Cu membranes are shown in Table 1.
3 RESULTS AND DISCUSSION
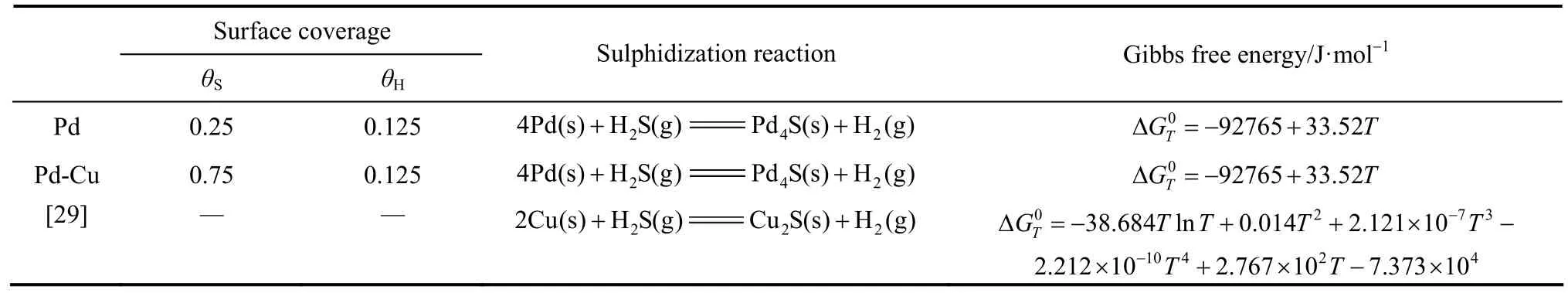
Table 1 Surface coverages, sulfidization reactions and the standard Gibbs free energies for Pd and Pd-Cu membranes
Figure 2 represents the variation of H2S tolerance performance (H2S-to-H2partial pressure ratio) with increasing temperature predicted by our thermodynamic model for pure Pd and PdxCu1?xmembranes. The limited values of H2S-to-H2ratios require formation Pd4S for the pure Pd membrane poisoning, and formation Pd4S or/and Cu2S for Pd-Cu membrane poisoning are clearly shown as the three lines, respectively. H2S-to-H2partial pressure ratio obviously increases with increasing temperature for the pure Pd and Pd-Cu alloy membranes H2S tolerance, i.e., the sulfur tolerance performance of Pd-based membranes increases with increasing temperature due to the exothermic nature of the sulphidization reactions. On the other hand, the sulphidization of the membrane alloyed Pd and Cu requires much higher H2S/H2ratios (at least more than 1.5 orders of magnitude) as compared with the pure Pd membrane, indicating that the PdxCu1?xalloy membrane has better sulfur resistance than the pure Pd membrane.
The composition of the formed sulfide depends on the H2S-to-H2partial pressure ratio rather than H2S concetration. When H2S-to-H2ratios are greater than or equal to the equilibrium values predicted for Pd4S and Cu2S formations, a stable Pd4S scale and a stable Pd4S+Cu2S scale should be formed on the PdxCu1?xalloy membrane surface at the corresponding temperature of interest, respectively. From thermodynamic considerations the ΔG0values of the sulphides of Pd indicate greater stability of these compounds ascompared to those of Cu. The experimental studys have pointed out that as a comparison of these two metals, Pd is less resistant to attack by H2S. Therefore, both thermodynamics and chemical kinetics predict a predominant formation of palladium sulphide over copper sulphide when an alloy of them is sulphidized in H2S.
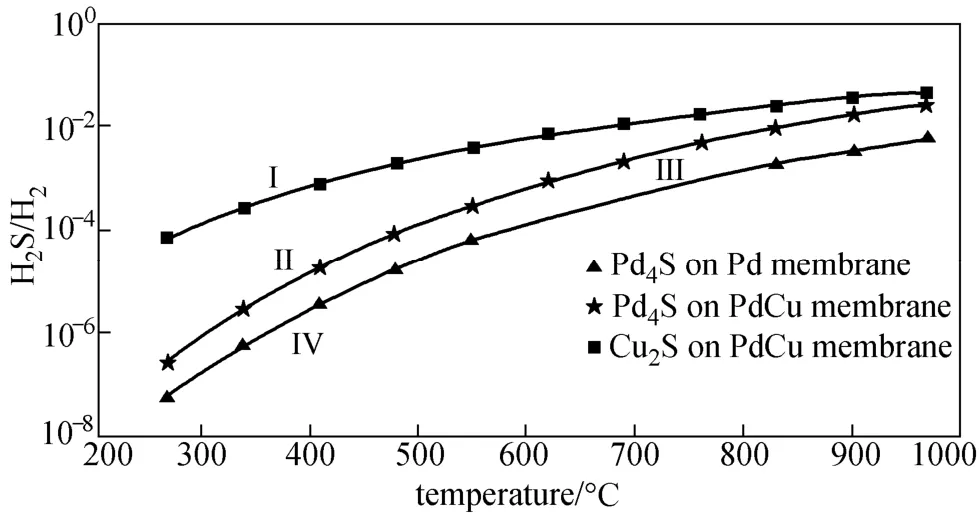
Figure 2 Predicting H2S tolerance for Pd and Pd-Cu membranes with increasing temperature Zone I: Pd4S+Cu2S; Zone II: Pd4S; Zone III: no sulfide for Pd-Cu membranes; Zone IV: no sulfide for Pd membranes
Furthermore, the comparison of the experimental results with the predicted outcomes for the PdxCu1?xalloy membranes at the respective H2S-to-H2feed ratios and temperatures is tabulated in Table 2. The agreement between the experimental and the model predicted results is excellent. Table 3 compares the experimental results with the predicted outcomes for the pure Pd membranes, illustrating a completely accurate prediction.
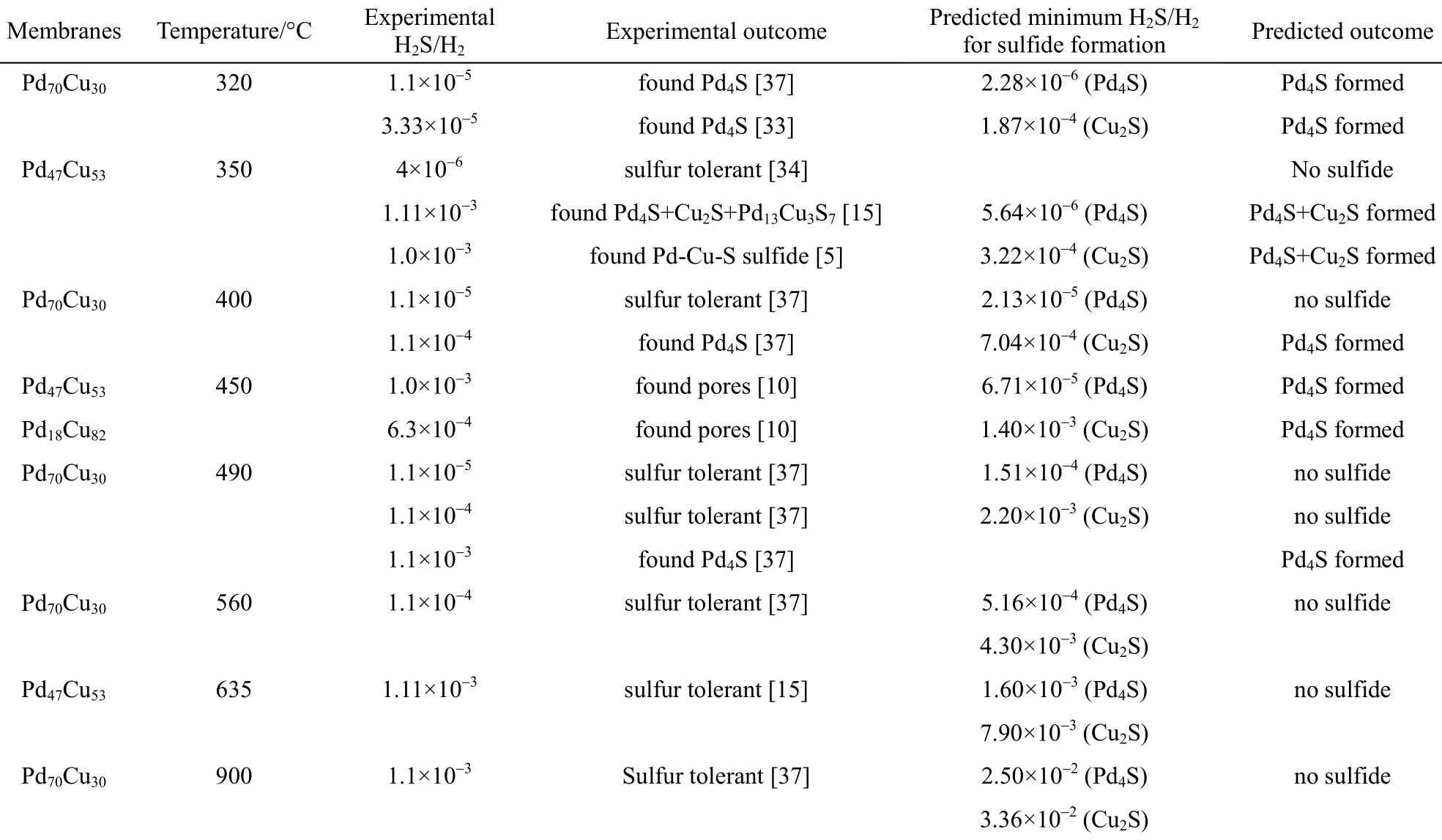
Table 2 Comparing predicted outcome to experimental results of PdxCu1?xalloy membranes (%, by atom)
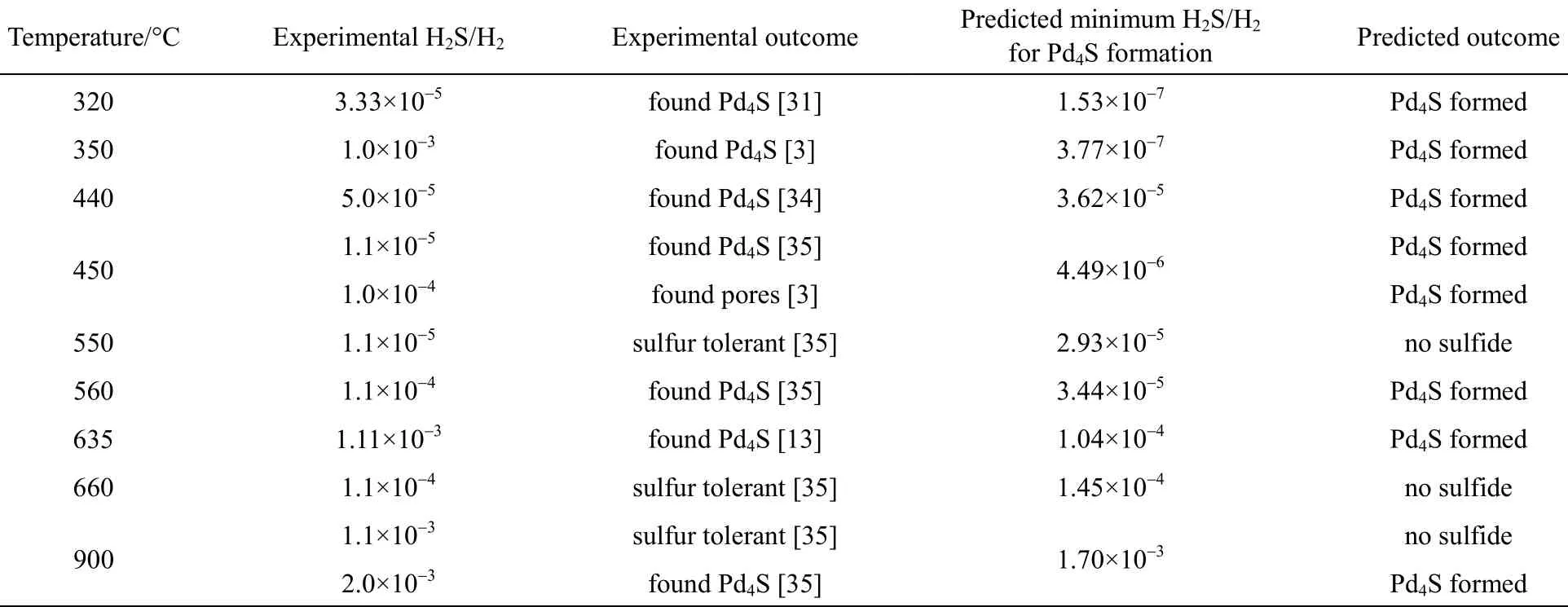
Table 3 Comparing predicted outcome to experimental results of Pd membrane
Why can the PdxCu1?xalloy membrane resist H2S poisoning? As a matter of fact, the higher sulfur tolerance of PdxCu1?xalloy compared with pure Pd metal should be attributed to both ligand (surface reactivity) and ensemble (spatial distribution of atom) effects due to alloying [30]. Fig. 3 shows the high-symmetry sites including top, bridge, fcc and hcp sites, which adsorb the reactants.
The preferred adsorption sites for S on Pd(111), PdCu3(1 1 1) and Pd3Cu(1 1 1) surfaces are the threefoldface centered cubic/hexagonal close-packed (fcc/hcp) hollow sites [12], the comparison of the relative concentration of various Pd sites for pure Pd and PdxCu1?xalloys shows that Pd0.7Cu0.3(111) has a lower relative concentration of Pd threefold sites, similar bridge sites, and higher Pd top sites than the Pd(111) surface [31]. Therefore, from the viewpoint of the ensemble effects, a Pd-Cu alloy membrane is conducive to decrease the poisoning effect of sulfur for the permeation of H2. What should be pointed out is, that the real Pd alloy surfaces are highly complex. PdCu alloys, for example, exhibit random distributions of the alloying metal. The effective adsorption energy on a polycrystalline surface examined in experiments is expected to be somewhat different from that on a single facet of a perfect crystal, but the difference does not affect the above conclusion. A detailed interpretation is beyond the scope of this paper, but it is noted that the possibilities are interesting.
On the other hand, in view of the ligand effects, the interaction between Pd and Cu leads to a decrease in the charge in the electronic bands of Cu 4s4p and, to a smaller extent, of Pd 4d and an increase in the population of the Pd 5s5p bands. The interaction does not modify the occupancy of the Cu 3d band. As a result, the S-Pd bonding interactions are reduced. In other words, the PdxCu1?xmembranes have acquired tolerance for sulfure poisoning due to alloying.
It is noted that the surface segregation, except the ratio of H2S to H2and temperature, plays an important role in the sulphidization of the Pd-Cu alloy. Clean surface studies have shown that the top atomic layer of PdxCu1?xalloys is rich in Cu, whereas the nearsurface region (~7 atomic layers) is rich in Pd. The surface compositions varied smoothly with bulk composition [35], suggesting that segregation is insensitive to bulk structure (bcc or fcc) of the alloy. However, if there are strongly interacting adsorbates present such as sulfur, Pd is brought to the surface. Segregation reversal may be driven by the formation of thermodynamically favored Pd S bonds at the terminating surface of the alloy. That is to say, the occurrence of sulphidization on the surface is not directly related with the bulk compositions and structures (bcc or fcc) of Pd-Cu alloy membranes because of the surface segregation phenomena.
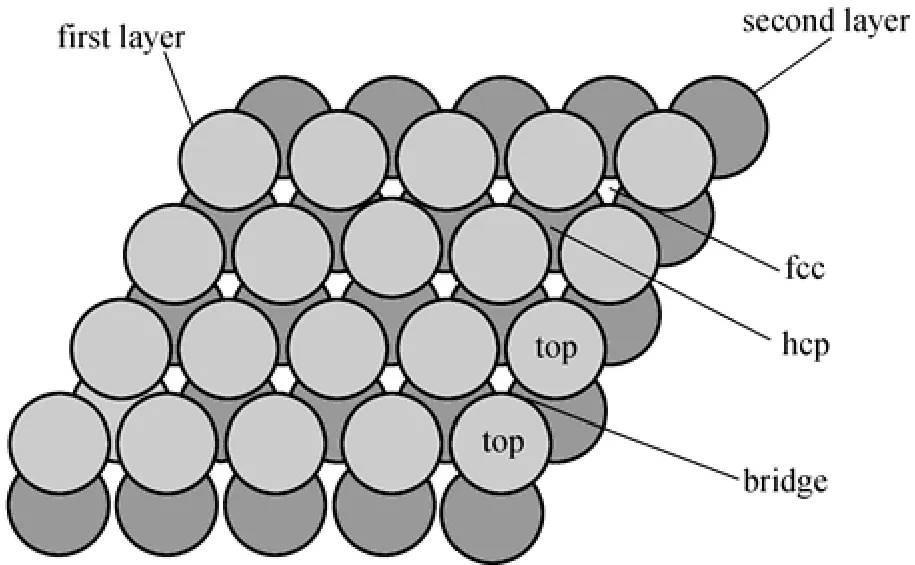
Figure 3 Schematic illustration of the high symmetry sites of low index surface
4 CONCLUSIONS
This study reports a theoretical model for the calculation of the pressure ratio of H2S to H2, which can be used in the prediction of the S-tolerant performance for Pd-Cu membranes in coal-derived syngas streams containing a limited H2S. The model explicitly considers the interaction of both H2and H2S with a alloy surface including surface coverage and segregation effects. Unlike previous equilibrium models in the literature, this model based on the surface reaction theory introduces the idea of S-adsorbed surface coverage rather than the activity of the solid solutions in the Pd-Cu system because the sulphidization reactions occur on the surface, not in the bulk. In other words, the occurrence of sulphidization on the surface involving S-adsorbed surface coverage and surface segregation is not directly related with the bulk compositions and structures (bcc or fcc) of Pd-Cu alloy membranes.
The thermodynamic predictions are in exact agreement with the reported studies in the literature involving Pd and Pd-Cu membranes exposed to varying H2-H2S mixtures at various temperatures. The demonstration of the predictive capability of our proposed approach is not only limited to the Pd-Cu alloy membranes, but also readily extended to other Pd-based membranes such as PdCuAu, PdCuAg ect. In this procedure the only fitting parameter used is the sulfure surface coverage, which is allowed to vary in the range of values experimentally observed for different Pd-based membranes.
ACKNOWLEDGEMENTS
The Excellent Going Abroad Experts’ Training Program in Hebei Province is gratefully acknowledged. Furthermore, the authors would like to thank Professor Jerry Lin from Arizona State University for helpful discussions and modifying the paper.
NOMENCLATURE

REFERENCES
1 Stiegel, G. J., Ramezan, M., “Hydrogen from coal gasification: An economical pathway to a sustainable energy future”, Int. J. Coal Geology, 65, 173-190 (2006).
2 Gao, H.Y., Lin, Y.S., Li, Y.D., Zhang, B.Q., “Electroless plating synthesis, characterization and permeation properties of Pd-Cu membranes supported on ZrO2modified porous stainless steel”, J. Membr. Sci., 265 (1-2), 142-152 (2005).
3 Zhang, K., Gao, H.Y., Rui, Z.B., Lin, Y.S., Li, Y.D., “Preparation of thin palladium composite membranes and application to hydrogen/nitrogen separation”, Chin. J. Chem. Eng., 15 (5), 643-647 (2007).
4 Fan, J., Hu, X.Y., Ohya, H., Ueda, Y., Yamawaki, M., Aihara, M., Takeuchi, T., Negishi, Y., “Preparation of palladium-silica conjugated membrane for selective hydrogen permeation”, Chin. J. Chem. Eng., 10 (5), 580-586 (2002).
5 O’Brien, C.P., Howard, B.H., Miller, J.B., Morreale, Br.D., Gellman, A.J., “Inhibition of hydrogen transport through Pd and Pd47Cu53membranes by H2S at 350 °C”, J. Membr. Sci., 349, 380-384 (2010).
6 Morreale, B.D., Ciocco, M.V., Howard, B.H., Killmeyer, R.P., Cugini, A.V., Enick, R.M., “Effect of hydrogen-sulfide on the hydrogen permeance of palladium-copper alloys at elevated temperatures”, J. Membr. Sci., 241, 219-224 (2004).
7 Yang, J.Y., Nishimura, C., Komaki, M., “Hydrogen permeation of Pd60Cu40alloy covered V-15Ni composite membrane in mixed gases containing H2S”, J. Membr. Sci., 309, 246-250 (2008).
8 Opalka, S.M., Huang, W., Wang, D., Flanagan, T.B., L?vvik, O.M., Emerson, S.C., She, Y., Vanderspurt, T.H., “Hydrogen interactions with the PdCu ordered B2 alloy”, J. Alloys Compd., 446-447 , 583-587 (2007).
9 Pomerantz, N., Ma, Y.H., “Effect of H2S on the performance and long-term stability of Pd/Cu membranes”, Ind. Eng. Chem. Res., 48, 4030-4039 (2009).
10 Kulprathipanja, A., Alptekin, G.O., Falconer, J.L., Way, J.D., “Pd and Pd-Cu membranes: inhibition of H2permeation by H2S”, J. Membr. Sci., 254, 49-62 (2005).
11 Alfonso, D.R., Cugini, A.V., Sorescu, D.C., “Adsorption and decomposition of H2S on Pd(1 1 1) surface: A first-principles study”, Catal. Today, 99, 315-322 (2005).
12 Alfonso, D.R., Cugini, A.V., Sholl, D.S., “Density functional theory studies of sulfur binding on Pd, Cu and Ag and their alloys”, Surf. Sci., 546, 12-26 (2003).
13 Hyman, M.P., Loveless, B.T., Medlin, J.W., “A density functional theory study of H2S decomposition on the (1 1 1) surfaces of model Pd-alloys”, Surf. Sci., 601 (23), 5382-5393 (2007).
14 Ozdogan, E., Wilcox, J., “Investigation of H2and H2S adsorption on niobium- and copper-doped palladium surfaces”, J. Phys. Chem. B, 114, 12851-12858 (2010).
15 Morreale, B.D., “The influence of hydrogen sulfide on palladium and palladium-copper alloy membranes”, Ph.D. Thesis, The University of Pittsburgh, USA (2006).
16 Morreale, B.D., Howard, B.H., Iyoha, O., Enick, R.M., Ling, C., Sholl, D.S., “Experimental and computational prediction of the hydrogen transport properties of Pd4S”, Ind. Eng. Chem. Res., 46 (19), 6313-6319 (2007).
17 Todorova, M., Li, W.X., Ganduglia-Pirovano, M.V., Stampfl, C., Reuter, K., Scheffler, M., “Role of subsurface oxygen in oxide formation at transition metal surfaces”, Phys. Rev. Lett., 89 (9), 096103-1-096103-4 (2002).
18 Miller, J.B., Morreale, B.D., Gellman, A.J., “The effect of adsorbed sulfur on surface segregation in a polycrystalline Pd70Cu30alloy”, Surf. Sci., 602, 1819-1825 (2008).
19 Benard, J., Oudar, J., Barbouth, N., Margot, E., Berthier, Y., “The thermodynamics of some metallic 2D sulphides”, Surf. Sci., 88, L35-L41 (1979).
20 Oudar, J., “Sulphur-metal interactions”, Mater. Sci. Eng., 42, 101-109 (1980).
21 Chattopadhyay, B., Sadigh-Esfandiary, S., “Sulphidation of Cu and Cu-Ni alloys in H2S/argon mixture”, Corrosion Sci., 13, 747-757 (1973).
22 Burke, M.L., Madix, R.J., “Hydrogen on Pd(100)-S: The effect of sulfur on precursor mediated adsorption and desorption”, Surf. Sci., 237 (1-3), 1-19 (1990).
23 Kiskinova, M., Goodman, D.W., “Modification of chemisorption properties by electronegative adatoms: H2and CO on chlorided sulfided, and phosphided Ni( 100)”, Surf. Sci., 108, 64-76 (1981).
24 Mccarty, J.G., Sancier, K.M., Wise, H., “Thermodynamics of sulfur chemisorption on metals IV. Alumina-supported platinum”, J. Catal., 82, 92-97 (1983).
25 Castro, F.J., Meyer, G., Zampieri,G., “Effects of sulfur poisoning on hydrogen desorption from palladium”, J. Alloys Compd., 330-332, 612-616 (2002).
26 Gravil, P.A., Toulhoat, H., “Hydrogen, sulphur and chlorine coadsorption on Pd(111): A theoretical study of poisoning and promotion”, Surf. Sci., 430 (1-3), 176-191(1999).
27 Wilke, S., Scheffler, M., “Poisoning of Pd(100) for the dssociations of H2: A theoretical study of co-adsorption of hydrogen and sulphur”, Surf. Sci., 329, L605-L610 (1995).
28 Alfonso, D.R., “Initial incorporation of sulfur into the Pd(111) surface: A theroretical study”, Surf. Sci., 600, 4508-4516 (2006).
29 Brooks, A.A., “A thermodynamic study of the equilibrium 2Cu(s) + H2S(g)=Cu2S(r) + H2(g)”, J. Am. Chem. Soc., 75, 2464-2467 (1953). 30 Gao, H.Y., Lin, Y.S., Li, Y.D., Zhang, B.Q., “Chemical stability and its improvement of palladium-based metallic membranes”, Ind. Eng. Chem. Res., 43, 6920-6930 (2004).
31 Noordermeer, A., Kok, G.A., Nieuwenhuys, B.E., “Comparison between the adsorption properties of Pd (111) and PdCu (111) surfaces for carbon monoxide and hydrogen”, Surf. Sci., 172 (2), 349-362 (1986).
32 Coq, B., Figueras, F., “Bimetallic palladium catalysts: influence of the co-metal on the catalyst performance”, J. Mol. Catal. A: Chemical, 173, 117-134 (2001).
33 Mundschau, M., Xie, X., Evenson, C.R., Sammells, A.F., “Dense inorganic membranes for production of hydrogen from methane and coal with carbon dioxide sequestration”, Catal. Today, 118 (1-2), 12-23 (2006).
34 McKinley, D.L., “Metal alloy for hydrogen separation and purification”, U. S. Pat., 3350845 (1967).
35 Priyadarshini, D., Kondratyuk, P., Picard, Y.N., Morreale, B.D., Gellman, A.J., Miller, J.B., “High-throughput characterization of surface segregation in CuxPd1?xalloys”, J. Phys. Chem. C, 115, 10155-10163 (2011).
36 Mundschau, M., Xie, X., Sammells, A., “Advances in hydrogen separation membrane technology for the separation of CO2and the purification of hydrogen produced from coal”, In: Proceedings of the 30th International Technical Conference on Coal Utilization & Fuel Systems, Clearwater, USA (2005).
37 Iyoha, O., Enick, R., Killmeyer, R., Morreale, B., “The influence of hydrogen sulfide-to-hydrogen partial pressure ratio on the sulfidization of Pd and 70 mol% Pd-Cu membranes”, J. Membr. Sci., 305, 77-92 (2007).
2012-07-17, accepted 2012-10-05.
* Supported by the National Natural Science Foundation of China (50972038), the National Natural Science Foundation of Hebei Province (B2009000739, B2014209258), Science and Technology Support Program of Hebei Province (09215142D).
** To whom correspondence should be addressed. E-mail: hygao@heuu.edu.cn
 Chinese Journal of Chemical Engineering2014年5期
Chinese Journal of Chemical Engineering2014年5期
- Chinese Journal of Chemical Engineering的其它文章
- Soft Sensor Model Derived from Wiener Model Structure: Modeling and Identification*
- Kinetics of Forward Extraction of Boric Acid from Salt Lake Brine by 2-Ethyl-1,3-hexanediol in Toluene Using Single Drop Technique*
- Influence of Solvent on Reaction Path to Synthesis of Methyl N-Phenyl Carbamate from Aniline, CO2and Methanol*
- Effect of Adsorbent Diameter on the Performance of Adsorption Refrigeration*
- High-Thermal Conductive Coating Used on Metal Heat Exchanger*
- A Facile Route for Synthesis of LiFePO4/C Cathode Material with Nano-sized Primary Particles*
