Efficient C N Formation for Preparing α-Branched Primary Amines by Recycled Intramolecular Reactions of 1,8-Naphthosultone Using Ammonia as Nitrogen Source*
Efficient C N Formation for Preparing α-Branched Primary Amines by Recycled Intramolecular Reactions of 1,8-Naphthosultone Using Ammonia as Nitrogen Source*
ZHOU Xinrui (周新銳)1,**, CHEN Jie (陳潔)1, ZENG Xiaoping (曾小萍)1, LIU Jihong (劉季紅)1and Istvan E. Markó1,2,**1State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, China
2Laboratory of Organic and Medicinal Chemistry, Université catholique de Louvain, Place Louis Pasteur 1, B-1348 Louvain-la-Neuve, Belgium
Amination of tertiary and secondary alcohols using aqueous ammonia as nitrogen source was carried out by a process with recyclable intramolecular reaction of 1,8-naphthosultone, which lead to α-branched primary amines. Sulfonic resin serves as the heterogeneous catalyst for C N bond formation and protects the neighboring hydroxyl group until the required hydrolysis starts in the alkaline solution. The process can be conducted under mild conditions, no additional solvent is needed and no overreaction to secondary or tertiary amines occurs.
α-branched primary amine, 1,8-naphthosultone, ammonia
1 INTRODUCTION
α-Branched primary amines, especially tert-alkyl amines containing azo-quaternary carbon atom, as irreplaceable intermediates, provide basically synthetic blocks for manufacture of pharmaceuticals [1-3], agrochemicals [4-6] and various material additives [7]. Hence, the synthesis of tert-alkyl amines in bulk is needed. There are a few synthetic strategies toward tert-alkyl amines, but so far no reaction has been well adapted to the kilo-scale production. Various tert-alkyl primary amines have been prepared in micro-scale by addition of organometals to imines or imine derivatives [8-10], but the low yield with weak electrophilicity of azomethine carbon and instability of organometal are still a problem for industrial applications [11, 12]. Hydroamination of ketones or aldehydes using ammonia is an atom-economy process to produce primary and secondary amines, but tertiary amine is excluded. Nucleophilic substitution is rarely adequate for the synthesis of tert-alkyl amine, since it is difficult for tertiary carbon to undergo SN2 in neutral or basic media, while ammonia protonized in acidic media is too weak a nucleophile to undergo SN1 reaction. To enforce ammonia for electrophilic addition, caustic conditions have to be adopted [13-15]. Some sulfonamides such as p-toluenesulfonamides are used instead of ammonia, but it is practically difficult to obtain amines via desulfonation. It is hardly accessible with N-tert-alkyl sulfonamide, since the conditions are too harsh for selectively keeping tert-alkyl amine [16, 17]. Sulfonamide hydrolysis assisted by neighboring participation has been investigated, but the special sulfonamides needed are not available commercially and difficult to be synthesized [18, 19]. Therefore, a process with lower cost and higher selectivity, which proceeds under mild conditions, is highly desirable.
This work presents an efficient process (Fig. 1), recycled and catalyzed by strong acid resin as a heterogeneous catalyst, for preparing α-branched primary amines, especially tert-alkyl primary amines. Ammonia and tertiary alcohols are adopted as the starting materials, and commercial 1,8-naphthosultone 1 is chosen as a platform for ammonia transformation. Only a few reactions with ammonia as nitrogen source and alcohols as alkyl agents for preparation of tert-alkyl amines have been reported, in which high reaction temperature or expensive catalyst are required [20, 21]. As far as we know, this is the first example of catalytic amination for tert-alkyl amines using alcohol and ammonia as starting materials, under mild conditions, without generating polluting by-products.
2 EXPERIMENTAL
2.1 Materials
2.2 Instruments
The structure of all products was determined by nuclear magnetic resonance (NMR, Bruker AvanceⅡ400 M) and confirmed by infrared spectroscopy (IR, Nicolet-20DXB). The products of amides were identified
Received 2012-09-03, accepted 2013-02-18.
* Supported by the National Natural Science Foundation of China (21076036).
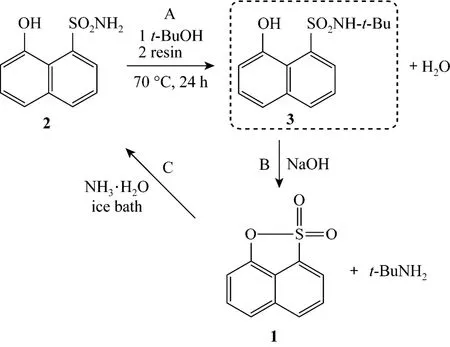
Figure 1 Recyclable process for preparing α-branched primary amines by intramolecular reactions of 1,8-naphthosultone
** To whom correspondence should be addressed. E-mail: xinrui@dlut.edu.cn; istvan.marko@uclouvain.be and quantified by high performance liquid chromatography (HPLC, HP-1100) fitted with a column (4.6 mm× 250 mm, 5 μm) and a UV-detector. A linear gradient elution was performed with 40% (by volume) methanol in water to 100% methanol during 40 min and continuously with pure methanol for additional 10 min. The eluting rate was kept at 1 ml·min?1through the entire program.
The products of amines were identified and quantified by gas chromatography (GC, HP-6890) equipped with flame ionization detector (FID) and compared with authentic samples. The temperature of HP-5 column was held at 35 °C for 4 min and then increased to 180 °C by 20 °C·min?1.
2.3 Ammonolysis of sultones
In a typical reaction, 20 ml concentrated aqueous ammonia (30%) was dripped into sultones (0.05 mol) with magnetically stirring. The reaction flask was placed in an ice bath to keep the temperature below 25 °C. A sample was taken every 5 min to monitor the process, until the sample dissolved completely in the cold 5% Na2CO3solution. Then, 70 ml of water was added into this reaction solution, with precipitate formed immediately. The precipitate was filtered with a Buchner funnel, washed with ice water for 3 times and then dried in a vacuum rotary evaporator below 40 °C.
8-hydroxy-naphthalene-1-sulfonamide 21H-NMR: δ=6.71 (2H, s), δ=7.22 (1H, s), δ=7.49 (1H, t), δ=7.55 (1H, t), δ=7.60 (1H, d), δ=8.10 (1H, d), δ=8.36 (1H, d), δ=10.02 (1H, s); 3-aminopropane-1-sulfonic acid 51H-NMR: δ=2.18 (2H, m), δ=3.08 (2H, t), δ=3.22 (2H, t); 3-hydroxyl-1-sulfonamide 61H-NMR: δ=2.26 (2H, m), δ=3.29 (2H, t), δ=3.47 (2H, t).
2.4 Typical process for sulfonamide alkylation and hydrolysis of N-alkyl sulfonamides
A mixture of 8-hydroxy-naphthalene-1-sulfonamide (0.5 mmol), activated SR732 (0.4 g) [22] and alcohol (0.1 mmol) were stirred in a three-necked flask equipped with a reflux condenser at 70 °C for 24 h. Removing the ethanol by vacuum distillation, the residue was transferred to a sodium hydroxide (0.2 g) ethanol (5 ml) solution. Stirring this solution for 5 min, the sulfonic resin was filtrated using a Buchner funnel. The liquid phase was examined by GC-FID according to the authentic samples.
N-(tert-butyl)-8-hydroxy-naphthalene-1-sulfonamide 31H-NMR: δ=1.19 (9H, s), δ=7.23 (1H, d), δ=7.48 (1H, t), δ=7.56 (1H, t), δ=7.61 (1H, d), δ=8.11 (1H, d), δ=8.41 (1H, d).
3 RESULTS AND DISCUSSION
3.1 Ammonolysis of sultones in aqueous ammonia
Two commercial sultones, easily obtained, were tested to get a proper sulfonamide with aqueous ammonia. When aqueous ammonia was dripped under the ambient conditions, the temperature of sultone 1 solution increased continuously, suggesting that some exothermic reactions happen rapidly. Thin layer chromatography (TLC) examination showed that sultone 1 disappeared in 4 h and new products appeared. After leaving the reaction solution in the flask for one night, a large quantity of crystals formed and was filtrated in 90% yield.1H-NMR spectra (Fig. 2) confirm that the crystal is the desired product, compound 2 (corresponding data in section 2.3).

Figure 2 1H-NMR spectrum of 8-hydroxy-naphthalene-1-sulfonamide

Figure 3 Aminolysis of 1,3-propane sultone
Ammonolysis of sultone 4 generates two products 5 and 6 (Fig. 3), formed by C O cleavage and S O cleavage, respectively, under the same conditions as mentioned above. Unfortunately the dominant product is highly regioselective to the undesired compound 5 with the molar ratio of 100/14 of 5/6, determined by1H-NMR spectroscopic analysis of the crude crystal (Fig. 4 and section 2.3). In the NMR spectrum, the complete integral curve is contributed to 5, while the incomplete integral one is distributed to 6. The result suggests that C O is much easier to obtain than S O cleavage in this reaction system. Based on the principle of hardness or softness of acid or base, the alkyl carbon is a good deal softer than sulfonamide nitrogen. With the addition of a harder nucleophile, sodium amide (NaNH2), instead of ammonia, the selectivity in favour of compound 6 is slightly increased, but compound 5 is still dominant. Alternatively, sultone 1, with phenolic carbon retarded to undergo the substitution, is accepted as an optimized reactant for the process using ammonia as nitrogen source.
3.2 Choosing effective catalyst for selective N-alkylation
Strong solid acids with bulky anions were specially considered, since they are easily recovered and their anions will join the nucleophilic substitution less except contributing a proton to assist the reaction. PWA was first assessed as an acidic catalyst for N-alkylation of tert-butanol at 70 °C and ambient pressure, but most of sulfonamide 2 reversely reacted to form the stating material, sultone 1 (Fig. 1). This indicates that the neighboring OH group at position 8 of naphthalene is so active that it obstructs N-alkylation. Formic acid, which can be partly acylate OH groups, was added in the reaction solution. This strategy successfully pushed N-alkylation forward, with the yield of N-butylation increasing to 27.1 % (Table 1, entry 2) and the selectivity rising to 40%. The alkyl reactant for N-alkylation was not limited to tert-alcohol, and secondary alcohols also formed the substituted N-alkyl sulfonamide in 17.8% yield (Table 1, entry 3). However, formic acid brought in a serious problem, since it dissolves PWA, raw materials and product amine to form a paste as the concentration of tert-butanol decreases, so that amine purification and catalyst recovery are quite difficult.
Next, SR732 was used as the catalyst for N-alkylation. Interestingly, the selectivity of sulfonamide 2 with tert-butanol was increased remarkably to 85% even in the absence of formic acid, which is needed for PWA-catalyzed alkylation to resist the early neighbor participation. Analogous reactions also appeared using 1-phenylethanol instead of tert-butanol. Agreeable results were obtained and the selectivity of N-alkylation was increased from 25% (PWA/HCOOH)to 85% (SR732), probably because the sulfonic acid groups not only served as the acid catalyst but also the protecting agent for the OH group. In general, one shortcoming for ammonia to be used in the preparation of primary amines is that the product is more reactive than ammonia and overreaction occurs, while this procedure selectively produces primary amines without the overreaction.
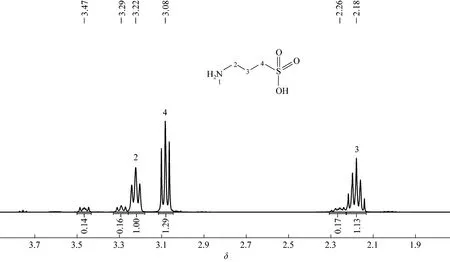
Figure 4 1H-NMR spectrum of 3-aminopropane-1-sulfonic acid 5 (major) and 3-hydroxyl-1-sulfonamide 6 (minor)

Table 1 N-alkylation of 8-hydroxy-naphthalene-1-sulfonamide 2 using tertiary or secondary alcohols
3.3 Base-catalyzed hydrolysis of N-alkyl sulfonamide 3 for regeneration of sultone 1 and release of α-branched primary amines
Pure N-tert-butyl sulfonamide 3 was prepared by the silica chromatographic column and confirmed by NMR (Fig. 5) and IR, to obtain the basic data about this elementary reaction C (Fig. 1). Under the simple conditions as described in section 2.4, sulfonamide 3 reacted nearly completely in 5 min in the presence of sodium hydroxide, as shown by TLC. In the temperature range and wave length of UV-detector, the samples of the crude products were checked by GC-FID and HPLC (High Performance Liquid Chromatography). As expected, the target primary amine and recycled sultone 1 were found, with the yields of tert- butylamine and sultone 1 being 90.5% and 98.5%, respectively, according to external standard of authentic samples. Since N-tert-butyl-p-toluenesulfonamides does not react under the conditions, these results indicate thatthe neighboring participation of 8OH group greatly facilitates the hydrolysis of sulfonamide 3.
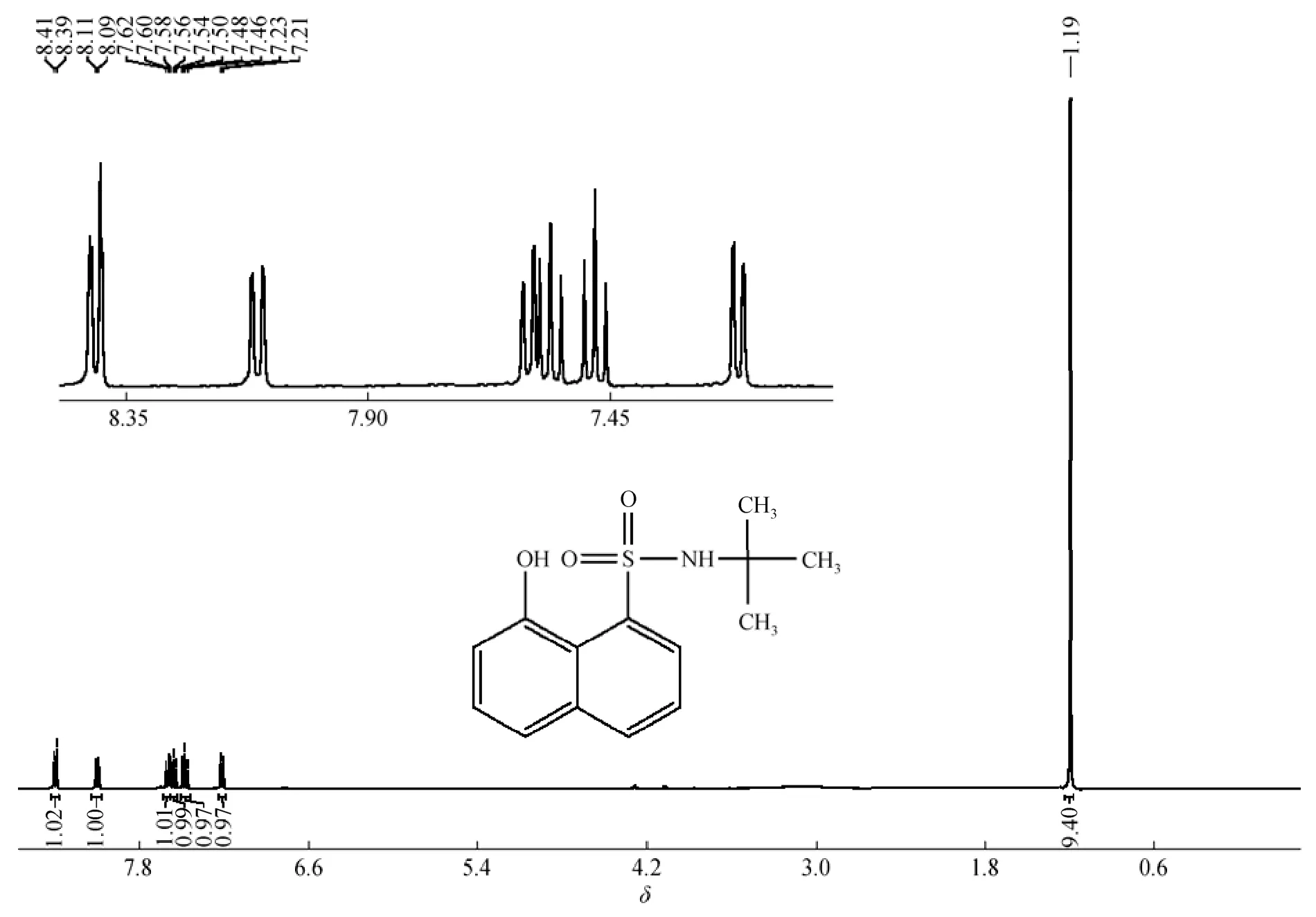
Figure 5 1H-NMR of N-(tert-butyl)-8-hydroxy-naphthalene-1-sulfonamide 3
As mentioned in section 3.2, the hydroxide group of amides 2 and 3 can bind to the sulfonic acid group of the sulfonic resin. Separation of them is difficult and may result in the loss of product. Hence, the crude product obtained with a simple distillation to remove tert-alcohol was used in the alkaline hydrolysis. This simplified process combined steps B and C into a one pot reaction. This way, tert-amylamine, tert-butylamine and 1-phenylethanamine were generated, the yields of which examined by GC-FID according to the authentic samples are given in Table 2. The yield of tert-butylamine, 15.7%, is comparatively higher than those obtained under caustic conditions [13-15].

Table 2 The yield of amines obtained by hydrolysis of N-alkyl sulfonamides
3.4 Main factors influencing the process
To accelerate the reactions and decrease the cost of process, the reaction conditions related with by-products were investigated. As shown in Fig. 6, the reaction did not proceed at room temperature, but increased abruptly as the temperature increased from 60 °C to 70 °C. At the temperature higher than 70 °C, a large amount of white sediment like plastic aggregated atthe bottom of the flask, suggesting that the intermediate tert-carbocation underwent another competitive way, elimination and polymerization, to form polyisobutene. Therefore, the optimum temperature for this reaction is 70 °C.
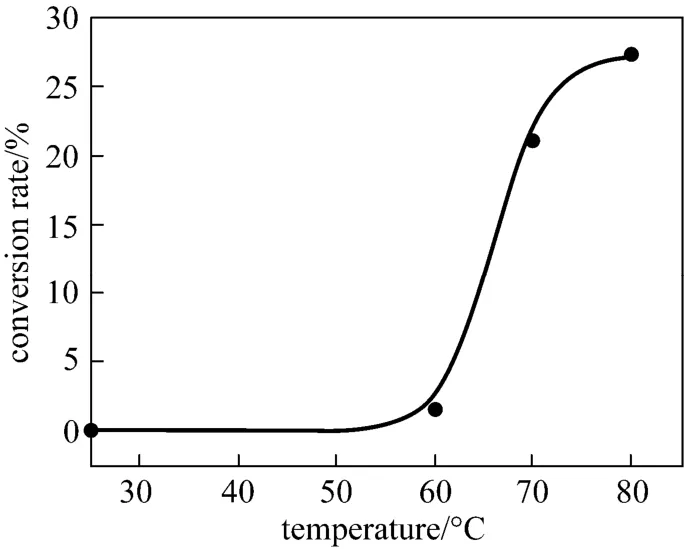
Figure 6 Influences of temperature on the conversion of sulfonamide 2 in N-alkylation using 732 resin as catalystin the absence of formic acid

Figure 7 Ammonolysis of 1,8-naphthosultone
The ammonolysis of sultone is an exothermal reaction, while the yield of sulfonamide 2 is affected by temperature more significantly. When stoichiometric amounts of ammonia were added quickly, the reaction temperature increased rapidly, so that abundant black tar appeared and the yield of sulfonamide 2 was less than 40%. The phenomenon is due to the formation of quinones (Fig. 7) by the auto-oxidation of phenols, which was confirmed by adding the radical scavenger to reduce the black tar. Another way is to control the temperature. With an ice bath and adding ammonia slowly to keep the temperature below 25 °C, the black tar can be avoided and the yield is 90%.
4 CONCLUSIONS
A resin-catalyzed amination using aqueous ammonia for preparation of α-branched primary amines is developed under mild conditions. The desired sulfonamide can be obtained from commercially available sultone 1, and the amine group is further transferred to tertiary or secondary alcohols by the delicate assistance of neighboring participation. Various factors influencing the process are investigated. In the optimized process, the yield of tert-amylamine, tertbutylamine and 1-phenylethanamine is 10.3%, 15.7% and 20.2%, respectively.
REFERENCES
1 Gonzalez-Antuna, A., Lavandera, I., Rodriguez-Gonzalez, P., Rodriguez, J., Alonso, J.I.G., Gotor, V., “A straightforward route to obtain13C1-labeled clenbuterol”, Tetrahedron, 67 (31), 5577-5581 (2011).
2 Joshi, R.A., Gurjar, M.K., Tripathy, N.K., “A new and improved process for celiprolol hydrochloride”, Org. Process. Res. Dev., 5 (2), 176-178 (2001).
3 Bujnowski, K., Synoradzki, L., Dinjus, E., Zevaco, D., Augustynowicz-Kope, E., Zwolska, C., “Rifamycin antibiotics-new compounds and synthetic methods. Part 1: Study of the reaction of 3-formylrifamycin SV with primary alkylamines or ammonia”, Tetrahedron, 59 (11), 1885-1893 (2003).
4 Esser, T., Karu, A.E., Toia, R.F., Casida, J.E., “Recognition of tetramethylenedisulfotetramine and related sulfamides by the brain GABA-gated chloride channel and a cyclodiene-sensitive monoclonal antibody”, Chem. Res. Toχicol., 4 (2), 162-167 (1991).
5 Jabusch, T.W., Tjeerdema, R.S., “Partitioning of penoxsulam, a new sulfonamide herbicide”, J. Agric. Food. Chem., 53 (18), 7179-7183 (2005).
6 Gonzalez, M.A., Gorman, D.B., Hamilton, C.T., Roth, G.A., “Process development for the sulfonamide herbicide pyroxsulam”, Org. Process. Res. Dev., 12 (2), 301-303 (2008).
7 Xu, W.P., Deng, F.X., Zhang, Z.L., “The synthesis of the vulcanization accelerator-NS”, Fine Chemicals (China), 17 (5), 277-279 (2000). (in Chinese)
8 Ellman, J.A., Owens, T.D., Tang, T.P., “N-tert-butanesulfinyl imines: Versatile intermediates for the asymmetric synthesis of amines”, Acc. Chem. Res., 35 (11), 984-995 (2002).
9 Enders, D., Reinhold, U., “Asymmetric synthesis of amines by nucleophilic 1,2-addition of organometallic reagents to the CN-double bond”, Tetrahedron: Asymmetry, 8 (12), 1895-1946 (1997).
10 Cogan, D.A., Liu, G., Ellman, J., “Asymmetric synthesis of chiral amines by highly diastereoselective 1,2-additions of organometallic reagents to N-tert-butanesulfinyl imines”, Tetrahedron, 55 (29), 8883-8904 (1999).
11 Bloch, R., “Additions of organometallic reagents to C N bonds: reactivity and selectivity”, Chem. Rev., 98 (4), 1407-1438 (1998).
12 Buey, J., Espinet, P., “Monomeric and dimeric ortho-metalated palladium (II)-imine systems as liquid crystals”, J. Organomet. Chem., 507, 137-145 (1996).
13 Yan, J.X., Jin, Y.F., Liu, Z.X., Wang, Y.F., “Environment-friendly preparation of tert-butylamine”, Chemical Industry and Engineering Progress (China), 31 (9), 2107-2109 (2012). (in Chinese)
14 Josef, P., Josef, K., Stanislav, S., Ivan, D., Julius, K., Pavol, S.,“Process of preparation of aliphatic amines”, European Pat., 1289925 B1, (2000).
15 Ma, H., Huo, W.Z., “Synthesis of tert-butylamine by direct amination of iso-butene”, Petrochemical Technology (China), 34 (8), 766-769 (2005). (in Chinese)
16 Searles, S., Nukina, S., “Cleavage and rearrangement of sulfonamides”, Chem. Rev., 59 (6), 1077-1103 (1959).
17 Neumann, R., Sasson, Y., “Mechanism of base-catalyzed reactions in phase-transfer systems with poly(ethylene glycols) as catalysts. The isomerization of allylanisole”, J. Org. Chem., 49 (19), 3448-3451 (1984).
18 Wagenaar, A., Engberts, J.B.F.N., “Intramolecular nucleophilic catalysis by the neighboring hydroxyl group in acid-and base-catalyzed hydrolysis of aromatic sulfonamides and sultones. Mechanism of intramolecular nucleophilic substitution at sulfonyl sulfur”, J. Org. Chem., 53 (4), 768-772 (1988).
19 Graafland, T., Wagenaar, A., Kirby, A.J., Engberts, J.B.F.N., “Structure and reactivity in intramolecular catalysis. Catalysis of sulfonamide hydrolysis by the neighboring carboxyl group”, J. Am. Chem. Soc., 101 (23), 6981-6991 (1979).
20 Yamaguchi, R., Kawagoe, S., Asai, C., Fujita, K., “Selective synthesis of secondary and tertiary amines by Cp*iridium-catalyzed multi alkylation of ammonium salts with alcohols”, Org. Lett., 10 (2), 181-184 (2008).
21 Kawahara, R., Fujita, K., Yamaguchi, R., “Multialkylation of aqueous ammonia with alcohols catalyzed by water-soluble Cp*Ir-ammine complexes”, J. Am. Chem. Soc., 132 (43), 15108-15111 (2010).
22 Huo, W.Z., Gou, L.K., Yu, Z.M., “Study on pretreatment of strong acidity canion exchange resin catalyst”, Industrial Catalysis (China), 7 (6), 13-18 (1999). (in Chinese)
cals were used as
unless otherwise noted. 1,8-Naphthosultone (98%) was purchased from Vcare Pharmatech Co., and 1,3-propanesultone (analytical-grade reagent, AR) was obtained from Sun Chemicals Co. Tert-butanol, tert-pentanol, phosphotungstic acid hydrate (PWA) and 732 sulfonic resin (SR732, Ф0.3-1.2 mm) were purchased from Sinopharm Chemical Reagent Co., Ltd.
 Chinese Journal of Chemical Engineering2014年4期
Chinese Journal of Chemical Engineering2014年4期
- Chinese Journal of Chemical Engineering的其它文章
- PVC Membrane Selective Electrode for Determination of Cadmium(II) Ion in Chocolate Samples
- Determination and Correlation of Solubilities of Four Novel Benzothiazolium Ionic Liquids with6PF?in Six Alcohols*
- An Approach to Formulation of FNLP with Complex Piecewise Linear Membership Functions
- One Step Preparation of Sulfonated Solid Catalyst and Its Effect in Esterification Reaction*
- Impacts of Power Density on Heavy Metal Release During Ultrasonic Sludge Treatment Process*
- Determination and Correlation of Solubility for D-Xylose in Volatile Fatty Acid Solvents*
