Treatment of Sebacic Acid Industrial Wastewater by Extraction Process Using Castor Oil Acid as Extractant*
XU Hang (徐航), ZHOU Quan (周全)and WANG Jinfu (王金福),**
1Chemical Engineering and Pharmaceutics School, Henan University of Science and Technology, Luoyang 471023, China
2Beijing Key Laboratory of Green Chemical Reaction Engineering and Te c hnol o gy, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
Treatment of Sebacic Acid Industrial Wastewater by Extraction Process Using Castor Oil Acid as Extractant*
XU Hang (徐航)1,2, ZHOU Quan (周全)2and WANG Jinfu (王金福)2,**
1Chemical Engineering and Pharmaceutics School, Henan University of Science and Technology, Luoyang 471023, China
2Beijing Key Laboratory of Green Chemical Reaction Engineering and Te c hnol o gy, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
Wastewater containing high concentrations of phenol and sodium sulfate is generated in sebacic acid (SA) industry. Castor oil acid, a raw material for producing SA, can be used to extract phenol from wastewater in order to reduce the amount of phenol used in the process and discharge of phenol. The results show that the extraction mechanism is that hydroxyl group of phenol is linked to carboxyl group of castor oil acid by hydrogen bond. The extraction process approaches equilibrium in 30 min. Extraction ratio increases with the increase of sodium sulfate and castor oil acid, and decreases as phenol increases. When the oil-water ratio is 1︰3, the optimal distribution coefficient of 40 is obtained. Phenol saturation concentration in castor oil acid is 1.03 mol·L?1after extraction for 4 times. The equilibrium constant (Kex) at 25 °C is 8.41 and the endothermic enthalpy (ΔH) is 1.513 kJ·mol?1. The Gibbs free energy (ΔG) is ?5.277 kJ·mol?1and the value of ΔS is calculated to be 22.3 J·mol?1·K?1.
castor oil acid, wastewater, extraction, phenol, sodium sulfate
1INTRODUCTION
The sebacic acid (SA) is an important chemical product and has a wide application in production of plastic material [1], nylon [2], functioned polyester [3], elastomer [4], and medical composition material [5]. There are some methods to produce sebacic acid such as cracking [6], synthesis [7], and hydrolysis [8] in chemical industry. China is the second major producer of castor oil, which can be used to produce castor oil acid by hydrolysis. Domestic factories produce sebacic acid by cracking castor oil acid with NaOH and phenol settings. Fig. 1 shows the flow chart of sebacic acid production using castor oil acid by cracking method. Much NaOH and phenol are added in the cracking section and excessive H2SO4is used in the acidifying stage. A large quantity of water is consumed in the decoloring process of product. As a result, SA industry discharges an acidic wastewater with high concentrations of phenol and Na2SO4. According to the daily detection results of the effluent of the SA factory, the process releases nearly 30 tons of such wastewater containing about 3g·L?1phenol and 50 g·L?1Na2SO4for production of one ton of sebacic acid. Phenol is a common organic matter that is highly toxic and difficult to be degraded and therefore severely harmful to natural environment. The wastewater containing salt is also difficult to be treated. In our preliminary work the process of sebacic acid production was improved so that the amount of wastewater discharged could be reduced to 1/4 of that in the original process. Namely, to produce one ton of sebacic acid, about 8 tons of wastewater will be emitted. Consequently, the concentrations of phenol and sodium sulfate in the wastewater are increased, with Na2SO4concentration of more than 200 g·L?1and phenol concentration of 12 g·L?1(0.1277 mol·L?1). Enrichment of pollutant is advantage to be disposal, especially with extraction and evaporation methods.
There are some ways to treat phenol wastewater, such as biological [9] and chemical methods [10], liquid membrane [11], adsorption [12], advanced oxidation [13], and solvent extraction [14]. High efficient strains are difficult to be cultured in this case of high salinity. High salt concentration reduces the degradation effect of advanced oxidation. Adsorption method consumes a large amount of expensive adsorbent. Thus conventional wastewater treatment methods arenot feasible and economical. As the solvent extraction process is simple and easy to operate with good extraction efficiency, it is often used for the treatment of phenol wastewater [15]. Nowadays, there is no effective way to treat high salt wastewater except evaporation method.
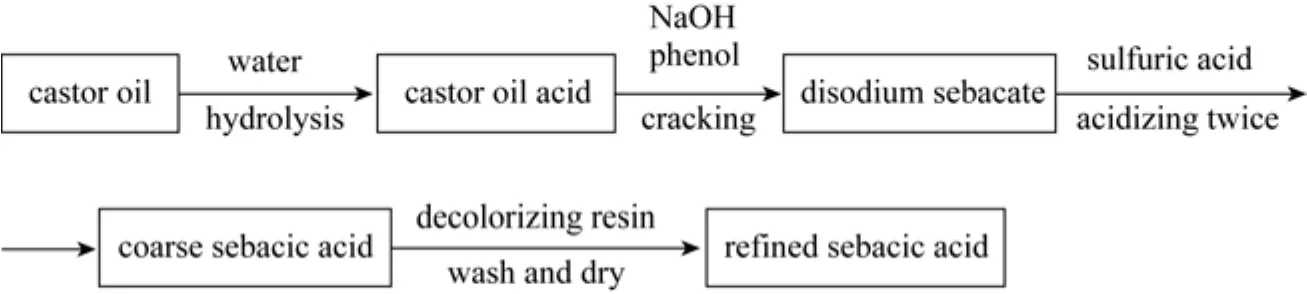
Figure 1Preparation technological process of sebacic acid by cracking method
In this work, castor oil acid is used to extract phenol from the wastewater, and the mixture of castor oil acid and recovered phenol can be used directly in the cracking process. This method can reduce the amount of phenol used and treat phenol wastewater. High Na2SO4concentration in wastewater causes salting-out effect, increasing the extraction ratio of castor oil acid and reduces its dissolvability in water. Therefore, it is necessary to investigate the phenol extraction performance of castor oil acid from wastewater containing phenol and Na2SO4in SA industry [16]. In this paper, factors that affect the extraction process are examined and the thermodynamic parameters are determined, which provides an effective method for treatment of wastewater containing high salt-phenol in SA industry.
2MATERIALS AND METHOD
2.1Materials
Materials including phenol, sodium sulfate, sulfuric acid, sodium hydroxide, and n-hexane were of analytical grade and purchased from the Beijing Modern Eastern Fine Chemicals Co. Ltd. Castor oil acid is a present from the Nanjing Institute of Zhong Zhou Patented Technology Development and its chemical structure is CH3(CH2)5CHOHCH2CH CH(CH2)7COOH, with relative molecular mass of 298 g?mol?1, density of 0.94 g?ml?1and molar concentration of 3.15 mol?L?1.
2.2Analysis of phenol
Figure 2 shows the UV-Vis absorption spectra of phenol and sodium sulfate. There are two characteristic absorption peaks for phenol at 215 and 270 nm, and one for sodium sulfate at 198 nm. There is no overlap of the characteristic peak of sodium sulfate and phenol, so the two absorption peaks of phenol can be used as its characteristic absorption peak. 270 nm is selected as the characteristic absorption peak of phenol, and the standard curve for the absorbance (A) and phenol concentration is A=3908[C6H5OH]+0.0773, with R2=0.9999, where [C6H5OH] is the molar concentration of phenol (mol·L?1).
2.3Experimental method
Extraction was carried out by shaking equal volumes of castor oil acid as extractant and simulated wastewater containing C6H5OH and Na2SO4in a centrifugal tube. After shaking for 30 min, the distribution ratio changed little and the extractant reaction reached the equilibrium. Phase disengagement was obtained by centrifugation for a while at 4000 r·min?1. Sodium hydroxide and sulfuric acid were used to adjust pH value. After extraction the centrifugal stratified water phase was brought out and phenol in water was analyzed by UV-Vis spectrophotometer. The extraction ratio of phenol (E) is calculated as follows [17].
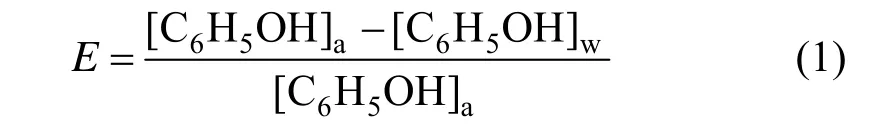
Where [C6H5OH]wis the molar concentration of phenol in water phase after extraction, [C6H5OH]ois the molar concentration of phenol in oil phase afterextraction, and [C6H5OH]ais the initial molar concentration of phenol.

Figure 2UV-Vis spectrum of phenol and typical curve
3RESULTS AND DISCUSSION
3.1The influence of extraction time
To determine the time required for extraction equilibrium, the extraction ratio of castor oil acid at different extraction time is presented in Fig. 3. The castor oil acid has a strong ability to extract phenol. At 1 min the extraction ratio reaches 65%, and after 30 min it reaches extraction equilibrium with the extraction ratio of 78%. In the following experiments, the extraction time is chosen to be 30 min.

Figure 3The influence of extraction time on extraction ratio ([C18H34O3]=3.15 mol·L?1; [C6H5OH]a=0.1277 mol·L?1; Na2SO4=200 g·L?1; T=25 °C; oil/water=1/5; pH=7.18)
3.2The influence of initial phenol concentration
The mechanism for castor oil acid (C18H34O3) to extract phenol is as follows [18]
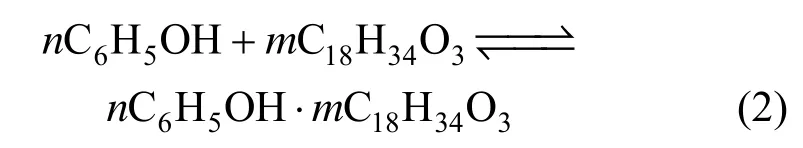
The expression of reaction equilibrium constant Kexis [19, 20]
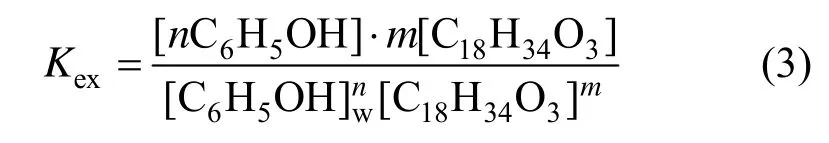
where [C18H34O3] is the molar concentration of castor oil acid, and [n C6H5OH·m C18H34O3] is the molar concentration of phenol in oil phase.
The expression of distribution coefficient D is

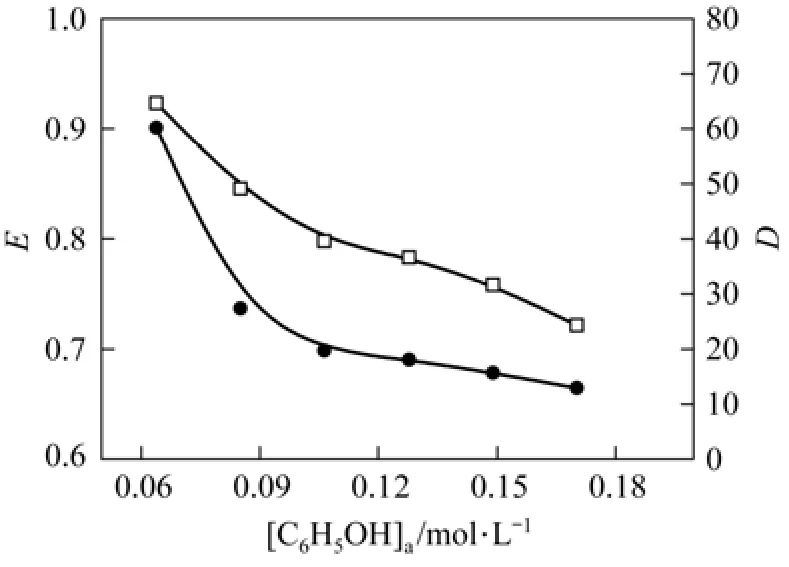
Figure 4The influence of initial phenol concentration on extraction ratio and distribution coefficient ([C18H34O3]= 3.15 mol·L?1; Na2SO4=200 g·L?1; pH=7.18; T=25 °C; t=30 min; oil/water=1/5)
Figure 4 shows the effects of initial phenol concentration on extraction ratio and distribution coefficient. As the phenol concentration in water increases from 0.064 mol·L?1to 0.17 mol·L?1, the extraction ratio decreases from 92.32% to 72.1% and the distribution coefficient changes from 60 to 13, while the corresponding phenol concentration in oil phase is 0.2945 and 0.6139 mol·L?1, respectively. The extraction ratio and distribution coefficient at equilibrium decrease, since the extractant dosage does not change while the initial phenol concentration increases. After extraction equilibrium, phenol concentrations in water and in oil increase with the initial phenol concentration, but the recruitment in water is higher than that in oil. As a result, the extraction ratio and distribution coefficient of phenol decrease with the increase of initial phenol concentration.
Combining Eqs. (3) and (4), we have

Figure 5 indicates the relationship between distribution coefficient D and the molar concentration of phenol in water, in which ln D and ln[C6H5OH] has a linear relationship, ln D=?0.6660ln[C6H5OH]+0.4964, with the correlation coefficient R2of 0.9945. Therefore, (n?1)=?0.6660 and n=0.3340.

Figure 5Dependence of distribution ratio of phenol on phenol concentration
3.3The influence of concentration of castor oil acid
With initial phenol concentration at 0.1277 mol·L?1,temperature at 25°C, volume ratio of oil and water phases of 1︰5, mixing time of 30 min, pH at 7.18, concentration of sodium sulfate at 200 g·L?1, the influences of concentration of castor oil acid on the extraction ratio and distribution coefficient are shown in Fig. 6, both of which increase with castor oil acid concentration. When the initial concentration of castor oil acid increases from 0.63 to 3.15 mol·L?1, the extraction ratio and distribution coefficient increase from 30% to 78% and from 2.2 to 18, respectively. The extractant is good for phenol recovery.

Figure 6The influence of castor oil acid concentration on E and D ([C6H5OH]a=0.1277 mol·L?1; Na2SO4=200 g·L?1; T=25 °C; oil/water=1/5; pH=7.18; t=30 min)
To obtain the value of m and extraction equilibrium constant Kexat 25 °C, Eq. (5) is rearranged as
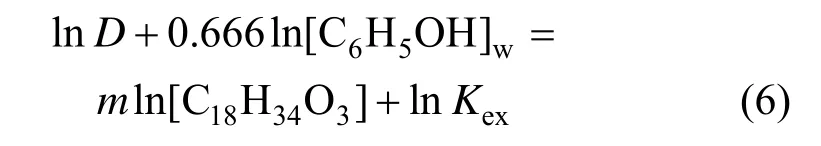
Figure 7 shows the linear relationship between ln D+0.666ln[C6H5OH]wand ln[C18H34O3], from which m=1.46 and Kex=8.41 at 25 °C are obtained. The Gibbs free energy ΔG is calculated by
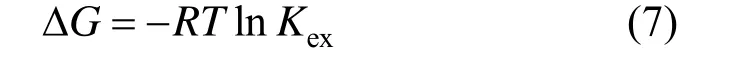
For castor oil acid to extract phenol at 25 °C, ΔG=?5.277 kJ·mol?1.
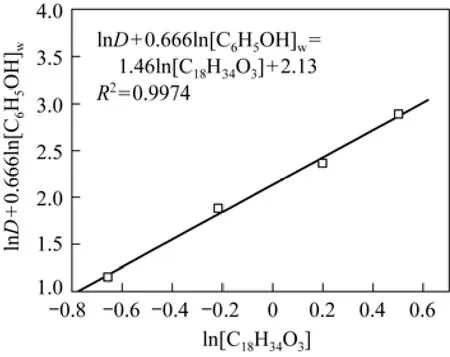
Figure 7Dependence of distribution ratio of phenol on castor oil acid concentration
3.4The influence of temperature
At the initial concentration of phenol 0.1277 mol·L?1, castor oil acid concentration 3.15 mol·L?1, volume ratio of oil and water phases of 1︰5, mixing time 30 min, and pH value of 7.18, the extraction ratio and the distribution coefficient at different temperatures are shown in Fig. 8. As the temperature increases from 25 °C to 70 °C, the extraction ratio increases from 78.3% to 80.4%, and the distribution coefficient increases from 18.02 to 20.54. It indicates that the extraction process is an endothermic process, but the absorption heat is not much, since the influence of temperature on the extraction is small. The reason is that as the temperature increases, the solubility of phenol in water and in castor oil acid increases, but because higher temperature will reduce the viscosity of castor oil acid, the solubility of phenol in castor oil acid is better than that in water, so that the extraction ratio and the distribution coefficient increase. Taking into account the viscosity of castor oil acid, the extraction process must be maintained at certain temperature (30 °C or so).
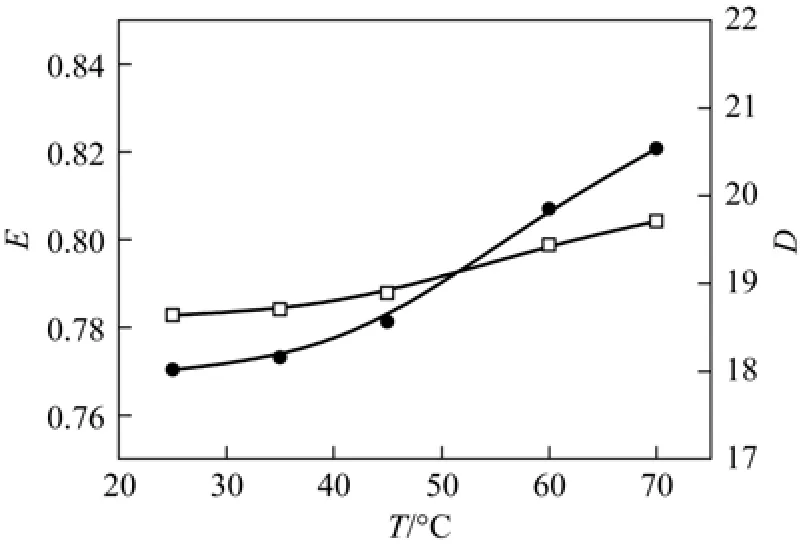
Figure 8The influence of temperature on E and D ([C6H5OH]a=0.1277 mol·L?1; [C18H34O3]=3.15 mol·L?1; N2SO4=200 g·L?1; t=30 min; oil/solution=1/5; pH=7.18)
According to literature [18, 21],
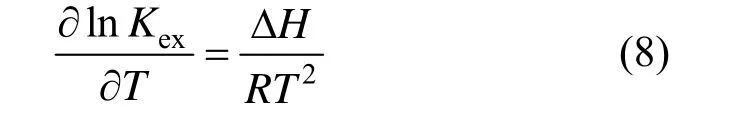
Adding Eq. (8) to Eq. (5), we have
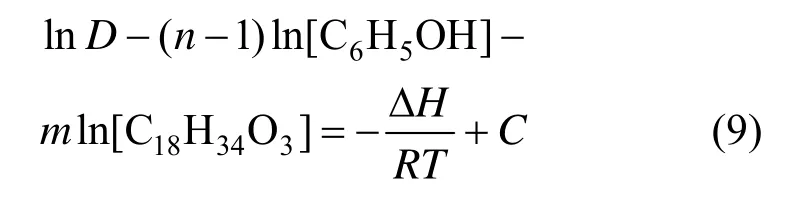
The linear correlation is shown in Fig. 9, which is ln D?(n?1)ln[C6H5OH]=165(?1/T)?0.578, with the correlation coefficient R2=0.9918. The endothermic enthalpy ΔH is calculated as 1.372 kJ·mol?1. The small endothermic enthalpy value also shows the strong extraction ability of castor oil acid for phenol. According to the formula ΔG=ΔH?TΔS, the entropy for the process at 25 °C is 22.3 J·mol?1·K?1.

Figure 9Relationship between ln D and ?1/T
3.5The influence of sodium sulfate concentration
At the initial concentration of phenol 0.1277 mol·L?1, castor oil acid concentration 3.15 mol·L?1, temperature 25°C, volume ratio of oil and water phases of 1︰5, and mixing time 30 min, the effects of sodium sulfate concentration in the wastewater on extraction ratio and distribution coefficient are shown in Fig. 10. With the increase of sodium sulfate concentration, the oil and water stratifies faster. As the sodium sulfate concentration increases from 100 g·L?1to 300 g·L?1, the extraction ratio of phenol gradually increases from 0.74 to 0.86. The reason is that phenol is an organic material whose solubility in castor oil acid is far greater than that in water. If sodium sulfate is present in water, it will increase the ionic strength, increasing salting-out effect and reducing the solubility of phenol in water, thereby enhancing the extraction ratio and the distribution coefficient.

Figure 10The influence of concentration of sodium sulfate on E and D ([C6H5OH]a=0.1277 mol·L?1; [C18H34O3]=3.15 mol·L?1; pH=7.18; T=25 °C; t=30 min; oil/solution=1/5)
3.6The influence of pH
The pH is an important factor to affect the extraction efficiency. With sodium sulfate concentration at 200 g·L?1, phenol concentration at 0.1277 mol·L?1, castor oil acid concentration at 3.15 mol·L?1, temperature at 25°C, mixing time of 30 min, and volume ratio of oil and water phases of 1︰5, the influence of the initial pH value on the extraction ratio and distribution coefficient is shown in Fig. 11. When the pH value is less than 8, the extraction ratio is 0.78 and the distribution coefficient is 16-19; when the pH value is greater than 8, the extraction ratio and distribution coefficient decline sharply; when the pH value is higher than 9, the extraction ratio approaches 0. The reason is as follows. When the pH value is less than 8, castor oil acid and phenol exist mainly in molecular state; when the pH value is more than 9, a large quantity of castor oil acid reacts with the hydroxide in the solution, forming castor oil acid salt that has greater solubility in water; phenol also reacts to form salt and the castor oil acid salt will dissolve in water to form an emulsion, so that it cannot extract phenol anymore. Thus, in the extraction of phenol by castor oil acid, it is necessary to use an appropriate pH value .

Figure 11The influence of pH value on E and D (Na2SO4= 200 g·L?1; [C6H5OH]a=0.1277 mol·L?1; [C18H34O3]=3.15 mol·L?1; T=25 °C; t=10 min; oil/water=1/5)
3.7The influence of extraction times
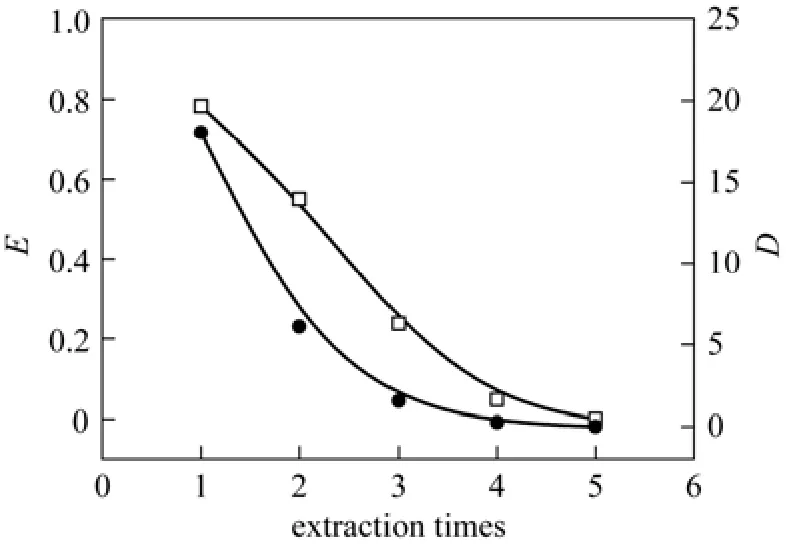
Figure 12The influence of extraction times on E and D (Na2SO4=200 g·L?1; [C6H5OH]a=0.1277 mol·L?1; [C18H34O3]= 3.15 mol·L?1; T=25 °C; t=10 min; oil/water=1/5; pH=7.18)
To determine the maximum extraction amount of castor oil acid, the wastewater was extracted by castor oil acid for 5 times under the standard conditions. The experimental result is show in Fig. 12. With the increaseof extraction times, the extraction ratio and distribution coefficient decrease significantly. When the extraction is carried out for 4 times, the extractant is saturated, with the phenol concentration in castor oil acid at 1.03 mol·L?1. Therefore, multi-stage extraction can be used in actual extraction process.
3.8The influence of oil-water volume ratio
With sodium sulfate concentration at 200 g·L?1, phenol concentration at 0.1277 mol·L?1, castor oil acid concentration at 3.15 mol·L?1, temperature at 25 °C, mixing time for 30 min, and pH value at 7.18, the effect ofoil-water phase volume ratio is shown in Fig. 12. The extraction ratio reduces as the phase ratio decreases. When the phase ratio changes from3︰1 to 1︰10, the extraction ratio declines to 0.45 from 1. The distribution coefficient firstly increases and then decreases. When the oil-water volume ratio is 1︰3, the distribution coefficient reaches a maximum of 40. The reason is that when the oil-water ratio decreases, i.e., the extractant is less, the extraction ratio declines. The distribution coefficient is determined by phenol concentration in the oil and water phases. When the oil-water ratio is large, although extracted phenol is more and castor oil acid dosage is also large, the phenol concentration in the castor oil acid will decrease since the castor oil acid volume decreases. With the oil-water ratio decreases, the amount of castor oil acid declines quickly and the volume is reduced much. Because the extraction ratio declines slowly, the concentration of phenol in the oil phase will increase substantially, so that the distribution coefficient increases. When the oil-water ratio drops to 1︰3 and lower, with the substantially reduced amount of extractant, the extraction ability is abated accordingly and the phenol concentration in water phase increases significantly, so the distribution coefficient also decreases. Therefore, the best distribution coefficient appears at oil-water ratio of 1︰3.

Figure 13The influence of oil/water ratio on E and D (Na2SO4=200 g·L?1; [C6H5OH]a=0.1277 mol·L?1; [C18H34O3]= 3.15 mol·L?1; T=25 °C; t=10 min; pH=7.18)
3.9IR spectrum of castor oil acid before and after extraction
The IR spectra of castor oil acid and the castor oil acid loaded with phenol are shown in Fig. 14. The changes of IR spectrum are some strong absorption peaks at 1610 cm?1, 1500 cm?1, 725 cm?1and 692 cm?1, which are characteristic absorption of phenol. Castor oil acid molecule has a hydroxyl and a carboxyl group, and phenol molecule has a hydroxyl group. At 3200-3400 cm?1there is no change before and after extraction, indicating no alcohol-phenol hydrogen bonding. Carboxyl combination of hydrogen bonding is in the range of 2500-3300 cm?1. Since the castor oil acid itself has hydrogen bond connections between carboxylic, the characteristic absorption peaks are the same before and after the extraction. Carboxyl and hydroxyl, carboxyl and carboxyl form hydrogen bonds. The hydroxyl in phenol and the carboxylic in castor oil acid are connected by hydrogen bonds.

Figure 14IR spectra of castor oil acid with and without phenol loaded
4CONCLUSIONS
In the extraction of phenol by castor oil acid, it is very quick to reach equilibrium. The extraction ratio increases with the increase of sodium sulfate and castor oil acid concentrations, and decreases as phenol concentration increases. When the oil-water ratio is 1︰3, there is an optimal distribution coefficient of 40. The extraction process must be maintained at a pH value of less than 8. After extraction for 4 times, the phenol saturated in castor oil acid is 1.03 mol·L?1. At 25 °C the extraction equilibrium constant Kexis 8.41, the endothermic enthalpy ΔH is 1.372 kJ·mol?1, the Gibbs free energy ΔG is ?5.277 kJ·mol?1, and the entropy is 22.3 J·mol?1· K?1. The mechanism of the extraction of phenol by castor oil acid is that the hydroxyl in phenol and the carboxylic in castor oil acid are connected by hydrogen bonding.
REFERENCES
1 Kricheldorf, H. R., Behnken, G., Schwarz, G., “Telechelic polyestersof ethane diol and adipic or sebacic acid by means of bismuth carboxylates as non-toxic catalysts”, Ploymer,46, 11219-11224 (2007).
2 Franco, L., Subirana, J.A., Puiggali, J., “Incorporation of glycine residues in even-even polyamides. Part II: Nylons 6, 10 and 12, 10”, Polymer,40(9), 2429-2438 (1999).
3 Ning, Z.Y., Zhang, Q.S., Wu, Q.P., Li, Y.Z., Ma, D.X., Chen, J.Z.,“Efficient synthesis of hydroxyl functioned polyesters from natural polyols and sebacic acid”, Chin. Chem. Lett.,22, 635-638 (2011).
4 Djordjevic, I., Choudhury, N.R., Dutta, N.K., Kumar, S., “Synthesis and characterization of novel citric acid-based polyester elastomers”, Polymer,50, 1682-1691 (2009).
5 Tang, J.C., Zhang, Z.G., Song, Z.F., “Synthesis and characterization of elastic aliphatic polyesters from sebacic acid, glycol and glycerol”, Eur. Polym. J.,42, 3360-3366 (2006).
6 Azcan, N., Demirel, E., “Obtaining 2-octanol, 2-octanone, and sebacic acid from castor oil by microwave-induced alkali fusion”, Ind. En g. Chem. Res.,47, 174-1778 (2008).
7 Vasishtha, A.K., Trivedi, R.K., Das, G., “Sebacic acid and 2-octranol from castor oil”, Journal of the American Oil Chemists Society,67, 333-337 (1990).
8 Kaneyuki, H.D., Hiratsuka, J., “Production of sebacic acid from n-decane by mutants derived from torulopsis-candida”, J. Ferment. Technol.,58, 405-410 (1980).
9 Al-Zuhair, S., EI-Naas, M., “Immobilization of pseudomonas putida in PVA gel particles for the biodegradation of phenol at high concentrations”, Biochem. Eng. J.,56, 46-50 (2011).
10 Tay, J.H., Jiang, H.L., Tay, S.T., “High-rate biodegradation of phenol by aerobically grown microbial granules”, J. Environ. Eng.,24, 243-247 (2004).
11 Zheng, H.D., Wang, B.Y., Wu, Y.X., Ren, Q.L., “Instability mechanisms of supported liquid membrane for phenol transport”, Chin. J. Chem. Eng.,17, 750-755 (2009).
12 Lu, G.C., Hao, J., Liu, L., Ma, H.W., Fang, Q.F., Wu, L.M., Wei, M.Q., Zhang, Y.H., “The adsorption of phenol by lignite activated carbon”, Chin. J. Chem. En g.,19, 380-385 (2011).
13 Fortuny, A., Bengoa, C., Font, J., “Bimetallic catalysts for continuous catalytic wet air oxidation of phenol”, J. Hazard. Mater.,64, 181-193 (1999).
14 Jiang, H., Fang, Y., Guo, Q.X., “Studies on the extraction of phenol in wastewater”, J. Hazard. Mater.,101, 179-190 (2003).
15 Zhang, J.Y., Hu, A.X., Wang, Y., Xiao, X.H., Guo, J.B., Luo, X.F.,“The separation of catechol from carbofuran phenol by extractive distillation”, Chin. J. Chem. Eng.,17, 42-46 (2009).
16 Rao, N.N., Singh, J.R., Misra, R., Nandy, T., “Liquid-liquid extraction of phenol form simulated sebacic acid”, J. Scientific and Industrial Research,68, 823-828 (2009).
17 Jacek, O., Jacek, S., Jan, S., “Extraction of phenols and phenyl acetates with diethyl carbonate”, Analytica Chimica Acta,535, 251-257 (2005).
18 Li, Z., Wu, M.H., Jiao, Z., Bao, B.R., Lu, S.L., “Extraction of phenol from wastewater by N-octanoylpyrrolidine”, J. Hazard. Mater.,114, 111-114 (2004).
19 Bernhard, B., Edwin, Z., Andre, B., “Phenol extraction with cyanex 923: Kinetics of the solvent impregnated resin application”, Reactive and Functional Polymers,69, 264-271 (2009).
20 Tanase, D., Anicuta, G.S., Octavian, F., “Reactive extraction of phenols using sulfuric acid salts of trioctylamine” Chem. Eng. S ci.,54, 1559-1563 (1999).
21 Fu, X.C., Shen, W.X., Yao, T.Y., Physical Chemistry, Higher Education Press, Beijing (1990). (in Chinese)
10.1016/S1004-9541(13)60546-7
2012-02-14, accepted 2012-11-26.
* Supported by the National Natural Science Foundation of China (21006057) and the National Science Foundation for Post-doctoral Scientists of China (20100470351).
** To whom correspondence should be addressed. E-mail: wangjfu@mail.tsinghua.edu.cn
 Chinese Journal of Chemical Engineering2013年9期
Chinese Journal of Chemical Engineering2013年9期
- Chinese Journal of Chemical Engineering的其它文章
- Soft Sensor for Inputs and Parameters Using Nonlinear Singular State Observer in Chemical Processes*
- A New Tuning Method for Two-Degree-of-Freedom Internal Model Control under Parametric Uncertainty*
- Halloysite Nanotube Composited Thermo-responsive Hydrogel System for Controlled-release*
- Recent Advances in Separation of Bioactive Natural Products*
- A Novel γ-Alumina Supported Fe-Mo Bimetallic Catalyst for Reverse Water Gas Shift Reaction*
- Experimental and Theoretical Studies of CO2Absorption Enhancement by Nano-Al2O3and Carbon Nanotube Particles
