Progress in Research of Gas Hydrate*
SUN Changyu (孫長宇), LI Wenzhi (李文志), YANG Xin (楊新), LI Fengguang (李風(fēng)光), YUAN Qing (袁青), MU Liang (穆亮), CHEN Jun (陳俊), LIU Bei (劉蓓) and CHEN Guangjin(陳光進)
?
Progress in Research of Gas Hydrate*
SUN Changyu (孫長宇), LI Wenzhi (李文志), YANG Xin (楊新), LI Fengguang (李風(fēng)光), YUAN Qing (袁青), MU Liang (穆亮), CHEN Jun (陳俊), LIU Bei (劉蓓) and CHEN Guangjin(陳光進)**
State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing 102249, China
It is of great significance to study gas hydrate because of following reasons. (1) Most organic carbon in the earth reserves in the form of natural gas hydrate, which is considered as a potential energy resource for the survival of human being in the future. (2) A series of novel technologies are based on gas hydrate. (3) Gas hydrate may lead to many hazards including plugging of oil/gas pipelines, accelerating global warming up,. In this paper, the latest progresses in exploration and exploitation of natural gas hydrate, the development of hydrate-based technologies including gas separation, gas storage, CO2sequestrationforming hydrate, as well as the prevention of hydrate hazards are reviewed. Additionally, the progresses in the fundamental study of gas hydrate, including the thermodynamicsand kinetics are also reviewed. A prospect to the future of gas hydrate research and application is given.
hydrate, exploration/exploitation, novel technologies, inhibition, thermodynamics/kinetics
1 INTRODUCTION
Clathrate hydrates are ice-like crystalline compounds comprised of small guest molecules, such as methane or other light hydrocarbons, which are trapped in cages of a hydrogen-bonded water framework. Three types of hydrate structures have been determined: sI, sII, and sH. It has attracted intense attention in the gas and oil industry since 1934 because it was found that the formation of gas hydrates was responsible for blocking pipelines. Many researches have been conducted in this field for overcoming the hazards caused by hydrate,.., inhibiting and preventing the formation of hydrate in pipelines and facilities. However, with gradual discovery of huge reserve of natural gas hydrate in the earth and deeper understanding of the peculiar properties of gas hydrate, more and more studies focus on how to benefit from gas hydrate in recent decades. The exploration of natural gas hydrate and the corresponding exploitation researches are very active in recent years. The development of novel technologies based on hydrate also draws much attention. Since 1 m3of hydrate may contain as much as 180 m3of gas at standard temperature and pressure, hydrates may act to concentrate gas and serve as a storage and transportation medium for methane or natural gas. In addition, hydrates become a potential medium for storage of H2under relatively mild conditions. Greenhouse gases, such as CO2, can also be deposited in the form of hydrate in reservoir or seafloor. Except for storing gases by hydrates, it is also possible to separate gas mixtures by forming hydrates according to the difference in easiness of gas component to occupy the cavities of hydrates. For example, we can use this method to recover or separate hydrogen, greenhouse gases, and organic contaminants produced in oil and gas industries. Compared with other conventional separation methods, such as cryogenic fractionation, selective adsorption, gas absorption, and membrane process, the hydrate-based gas method is more effective and has many advantages.
In this work, the latest progresses in the exploration and exploitation of natural gas hydrate, the development of hydrate-based technologies, and the prevention of hydrate hazards are reviewed. In addition, the progresses in the fundamental study of gas hydrate including the thermodynamics and kinetics are also reviewed.
2 EXPLORATION AND EXPLOITATION OF NATURAL GAS HYDRATE
Natural gas hydrates are widely distributed on permafrost regions and offshore. With the growing knowledge of the distribution and saturation of gas hydrates in sediments and ongoing efforts to better constrain the volume of hydrate-bearing sediments and their gas yield, the global estimates of hydrate-bound gas decrease by at least one order of magnitude. According to Milkov [1], it is estimated that the global hydrate-bound gas is about 2.5′1015m3methane. To date over 230 natural gas hydrate deposits (NGHD) have been found in the world. A map of the discovered NGHD is shown in Fig. 1 [2]. The deposits of natural gas hydrates are found all over the world in deepwater and in the Arctic [3]. In the past 50 years natural gas hydrates have been discovered in 79 countries. More than a hundred wells are drilled for finding natural gas hydrate. In many countries, national programs have been laid for the research and production of natural gas from natural gas hydrate deposits [3].
The exploration of natural gas hydrate is very active in North America and Asia in recent years. In the shallow sediments of the Mississippi Canyon floor, strong frequency-dependent reflectivity is observed [4]. Hydrates are also abundantly detected along the northern Gulf of Mexico continental margin. Seismic reflection and drilling data are adequate to locate and map hydrates and free gases. Exploration of natural gas hydrate is also carried out in the Nankai-Trough offshore, Japan [5]. For hydrate in permafrost, it is shown that gas hydrate accumulated in Arctic regions is associated with permafrost in Canada, northern Alaska, and Russia. Onshore gas hydrates are present in the West Siberian Basin and are believed to be in other permafrost areas of northern Russia [6, 7]. Well log responses attributed to the presence of gas hydrates are obtained in about one-fifth of the wells drilled in the Mackenzie Delta. More than half of the wells in the Arctic Islands of Canada are inferred to contain gas hydrates [8]. The information from studies on Arctic gas hydrate shows that, in permafrost regions, gas hydrates may exist at subsurface depths ranging from about 130 m to 2000 m. A recent work summarized by Walsh. [9] concludes that two gas fields on the North Slope, located near the village of Barrow, may extend up into the overlying methane hydrate stability zone, which means that gas hydrate may exist in the crest of these two conventional gas fields.
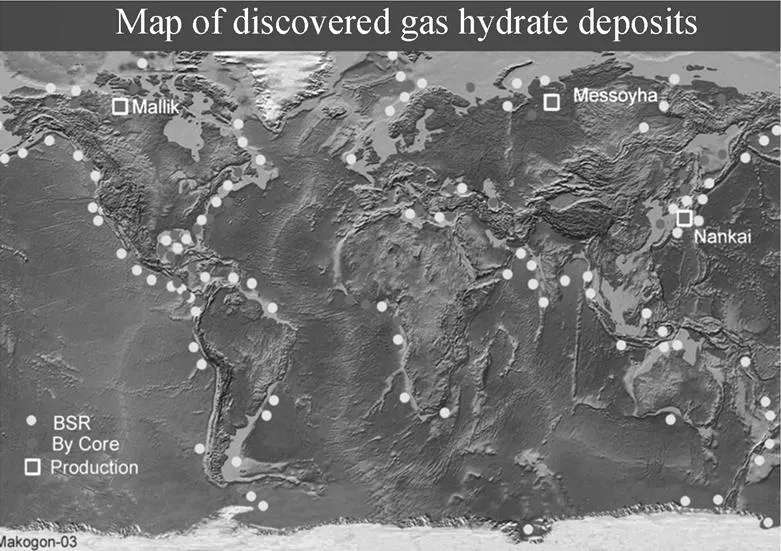
Figure 1 Map of discovered gas hydrate deposits [2]
The experimental exploitation research were carried out in four areas, including Messoyha gas field in the Siberian, the north slope area in Alaska of America, Nankai-Trough offshore in Japan, and Mackenzie Delta in the northwest area of Canada [10]. A project of getting methane from the hydrate zones under the ground was completed in Canada, which was operated by Japan, Canada, America, German, and India in 2002. Another experimental production project was completed by Japan and Canada in 2008 [11]. In this project, systematic analyses were carried out on two gas hydrate-bearing sediment core samples, HYPV4 and HYLN7, which were recovered from the test well of Mount Elbert Stratigraphic. The gas hydrates were determined to be structure I with hydration numbers of approximately 6.1. The hydrate gas composition was predominantly methane and isotopic analysis showed that methane was of thermogenic origin [12]. In addition, Japan plans to exploit hydrates under the Pacific Ocean and verify if natural gas hydrate is a feasible fuel. The essential technology needed by the commercial exploit will be completed before 2016.
In China, hydrates also exist in seafloor and permafrost abundantly. Gas hydrate resource in the north of South China Sea is estimated to be 6.435′1013-7.722′1013m3gases according to the data obtained from this area and global parameters on gas hydrate [13]. According to the digital terrain map in the southern South China Sea [14], the maximal GHSZ thickness ranges from 230 to 355 m, and the resources of gas hydrates is from 1.729′1013to 2.169′1013m3. The distributed area of hydrates in Sisha Okinawa trough is about 5242 km2and methane resource is about 4.1′1012m3[10, 15]. In 2007, several wells were drilled and gas hydrate samples were obtained in Shenhu area in the north of the South China Sea. The scientific drilling project of gas hydrate in Qilian Mountain permafrost was implemented during 2008-2009. Samples of gas hydrate were collected, which is the first discovery of gas hydrate in permafrost of China [16]. The total resources determined are (2.71-2.99)′1011m3methane gases, which illuminate a tremendous energy resource potential [17]. Corresponding exploitation work for hydrate in seafloor and permafrost is being conducted by several universities and institutes in China.
3 NOVEL TECHNOLOGIES BASED ON HYDRATE
3.1 Separation of gas mixtures
When a gas mixture forms hydrate with water partially under certain conditions, the water-free concentration of each component in the hydrate phase and that in the residual vapor phase will be different. The component that forms hydrate more easily will be enriched in the hydrate phase. Based on this principle, it is considered possible to separate gas mixtures through forming hydrate. Hydrate-based separation concepts have been proposed for many fields: (1) recovering global warming gases, such as hydrofluorocarbon from air, sulfur hexafluoride (SF6) from nitrogen, and CO2and H2S from flue gas; (2) recovering organic contaminants from gaseous or aqueous mixtures; (3) recovering hydrogen from hydrogen-containing light hydrocarbon gas mixtures; (4) recovering methane from low-concentration coal mine methane; and (5) separation of methane and ethane, which is required in natural gas, oil processing, and ethylene production. The hydrate-based gas separation is more effective and has many advantages over conventional separation methods, such as cryogenic fractionation, selective adsorption, gas absorption, and membrane process.
The high-pressure requirement for hydrate formation is one of the decisive obstacles to commercialize the hydrate-based separation process. To solve this inherent problem and to compete with other conventional methods, some hydrate promoters, such as THF, which remarkably reduce the required hydrate formation pressure, have been used as additives, without compromising the separation efficiency or gas recovery of the process. Besides THF, propane [18] and cyclo-pentane (CP) [19] are also used as promoters. According to Zhang. [20], CP has a potential to be a better thermodynamic promoter than THF for a hydrate-based precombustion CO2capture. To remove H2S from biogas, Kamata. [21] have conducted separation experiments using tetra--butyl ammonium bromide (TBAB) semi-clathrate hydrate. In the case of separating HFC-134a from gas mixtures containing N2and HFC-134a, however, no additive is needed to lower the hydrate formation pressure, since hydrate formation process can be carried out at mild temperature and low pressure ranges [22]. Among all of the additives mentioned above, THF is widely used as a promoter. However, THF sometimes acts as a selective inhibitor instead of a promoter. In the experimental study on the vapor-hydrate equilibrium of (methane + ethane + THF + water) system [23], it is found that the presence of THF may drastically change the partition of methane and ethane between hydrate phase and vapor phase,.., ethane is remarkably enriched in vapor phase in the absence of THF. This phenomenon may be attributed to the formation of structure II (sII) hydrate when THF is present in aqueous solution. As ethane molecules essentially can not occupy the small cages of hydrate at moderate pressure and the occupancy of large cages is dominated by THF, the overall occupancy of ethane molecules in cages of sII hydrate will be low and most of them therefore remain in vapor phase. Under this circumstance, THF acts as a selective inhibitor for the separation of methane and ethane [24].
Kinetic or transport processes also significantly affect and determine the performance of hydrate-based separation processes. The gas-liquid interfacial area, the driving force, and kinetic constant affects hydrate formation. A way to increase these factors is necessary for improving hydrate formation. Several efficient processes to increase the interfacial area for gas hydrate formation have been demonstrated, including spray [25], or jet reactor [26, 27], and bubble column [28, 29]. A static mixer has also been used for continuous gas hydrate formation and recovery of a gas separation system [30]. The separation rate can be enhanced by dispersing water in a porous media [31]. In addition, additives such as sodium-alkylsulfate (SDS) [32] and TBAB [33] also have the ability to accelerate hydrate formation.
Separation efficiency is an important parameter for evaluating hydrate-based gas separation technique. Since it takes a much longer time to reach vapor-hydrate- water equilibrium than to reach vapor-liquid equilibrium, multi-stage separation such as distillation is more difficult to realize for hydration separation technology. Thus, it is of more significance to increase the separation efficiency of a single equilibrium stage for hydration separation technology. For this, many efforts have been made. For example, with THF added in water, the partition coefficient of ethane between vapor and hydrate phase increases dramatically for CH4+ C2H6mixture. This can be attributed to the reason that ethane is remarkably enriched in the vapor phase in the presence of THF [34]. This also occurs for methane + ethylene mixture system [35]. If SDS is used instead of THF, the hydrate formation process will also be accelerated, and the partition coefficient of ethylene between hydrate and vapor phase increases drastically [36]. The separation efficiency for hydrogen + methane gas mixtures in pure water and THF aqueous solution are also examined at different temperatures, pressures, and feed gas compositions. Hydrogen is remarkably enriched in vapor phase in a single equilibrium stage with 6% (by mole) THF added [37]. Besides, hydrate-based separation is also combined with other separation methods to improve the separation efficiency, such as the medium-pressure clathrate hydrate/membrane hybrid process for postcombustion capture of CO2[38, 39]. In order to examine the feasibility of gas separation based on hydrate, it is necessary to compare the separation efficiency with other gas separation methods. Liquefaction and gas hydrate formation are compared in terms of the equilibrium recovery ratio for the separation of gaseous mixtures of HFC-134a and nitrogen [40]. It is found that more HFC-134a can be recovered by the hydrate-based separation than the liquefaction separation method for a given feed. In addition, the cost for CO2capture from a gas mixture by hydrate crystallization is lower than that by other classical technologies, such as membranes and amines,[41].
3.2 Storage of natural gas
Natural gas storage in hydrates is investigated because of high store capacity of natural gas [42]. The storage of NGH is appealing for industrial utilization because of not only its high storage capacity, but also its high safety. Gas hydrate can be stored at-15 °C under atmospheric pressure for 15 days, retaining almost all the gas [43, 44]. Methane hydrate remains metastable at 0.1 MPa and temperatures slightly below 0 °C for a certain time [45]. The preservation is improved if the pressure is kept at 0.2 or 0.3 MPa and depends on the concentration of CH4gaseous molecules in the hydrate [46]. Anomalous preservation has potential applications for successful retrieval of natural gas hydrate or hydrate-bearing sediments from remote settings, as well as for temporary low-pressure transport and storage of natural gas [45]. It is suggested that a relation exists between anomalous preservation and stacking-fault of ice due to transformations from cubic ice to hexagonal ice [47]. A confocal scanning microscope is used to explain the anomalous preservation behavior of CH4hydrate [48], that is, the increase in storage stability of CH4hydrate above 240 K is likely related to the formation of the ice. With high energy density, gas hydrate can be used in storage and transportation of natural gas, which has certain advantages over highly compressed or liquefied gas at higher temperatures and/or lower pressures. Particularly, for a medium- or small-scale natural gas field where it is not appropriate to use liquefied natural gas or pipeline transportation, natural gas hydrate is a more economical solution.
However, industrial applications of hydrate storage processes are hindered by some problems, such as slow formation rates, unreacted interstitial water as a large percentage of the hydrate mass, reliability of hydrate storage capacity, and economy of process scale-up [49]. Because the solubility of natural gas in water is very low, only a thin hydrate film is formed at the interface between the water and gas without stirring or other enhancing measures. To solve these problems, two approaches (mechanical and chemical means) are generally adopted, which are similar to those methods used in hydrate-based gas separation processes. The mechanical method includes stirring [50, 51], spraying of liquid in continuous gas phase [25, 52], bubbling of gas in continuous liquid phase [28], microbubbling [53], and icing [54]. Gas hydrate can also be shaped into pellets using mechanical method, and treated as a slurry medium with some cooling medium or type of oil. The pelletized NGH is expected to be able to improve the transportation and storage efficiency, self-preservation effect, and handling efficiency [55].
The chemical method consists of changing the properties of reactant system by adding low dose of surfactants to decrease gas/liquid interfacial tension and to increase the solubility of gas in liquid water. For example, surfactant such as SDS is used to reduce the formation time of hydrates and increase the gas storage efficiency [49, 56, 57]. The surfactant molecules help minimize mass-transfer and kinetic difficulties during hydrate formation. It promotes the hydrate formation rate by a factor greater than 700 above the critical micellar concentration [32]. In the presence of SDS, hydrates formed from ethane or methane grow as very fine particles [49, 58], increasing the storage capacities under the unstirring condition. The addition of a heat-transfer plate for the surfactant-aided hydrate formation or the presence of a large heat transfer surface area in the formation vessel is a prospective way to realize high-performance hydrate-forming reactors with no power consumption for gas and liquid mixing [58, 59]. A multi-deck cell-type vessel is also devised as the internals of the reactor to eliminate the scale-up effect, where water is loaded in each cell of the vessel instead of being loaded in the reactor directly and the hydrate forms in all cells of the vessel simultaneously [60]. The gas storage capacity of gas hydrate is also influenced by the concentration of additives as well as the kind of additives. A critical SDS concentration of 650 mg·L-1is assumed corresponding to a maximum storage capacity of 170V/V [61]. Besides SDS, the following additives on hydrate formation rate and storage capacity were also examined: calcium hypochlorite [62], linear alkyl benzene sulfonic acid [63], para-toluene sulfonic acid [64], dodecyl polysaccharide glycoside [65], alkylpolyglycuside, sodium dodecyl benzene sulfonate, potassium oxalate monohydrate [23], linear alkyl benzene sulfonate (LABS), cetyl trimethyl ammonium bromide, and ethoxylated nonylphenol (ENP) [66]. The optimum concentration of above surfactants for hydrate formation was also determined correspondingly. Recently, propane hydrate was studied as a possible storage medium for methane gas to form the double hydrate efficiently under near ambient conditions for practical purposes [67].
The stability of the hydrate formed is vital for long distance and period transportations. Lin. [61] studied the stability of methane hydrate with and without SDS as hydrate promoter and found that SDS decreases the hydrate stability below ice point. Ganji. [66], and Mandal and Laik [49] also considered that surfactants decrease the stability of methane hydrate and increase the dissociation rate compared to pure water-methane hydrates. Therefore, there should exist a balance between promoting hydrate formation and decreasing its stability when using surfactant, such as SDS, as an additive in gas storage. In addition, with addition of a minor amount of xanthan or starch, the dissociation rate of methane hydrate formed in the presence of SDS is decreased effectively [68]. Besides, if gas hydrate is shaped into pellets and soaked in ethylene glycol or silicone oil, it is also advantageous to have functions of preventing the dissociation reaction of gas hydrate and controlling dissociation rate [69].
Compared with other natural gas storage and transportation methods, such as liquefied transportation, a substantial cost saving (18%-24%) is expected for the transport of natural gas in the form of hydrates [70, 71]. For distant markets, the most popular method of transporting the natural gas is LNG (liquefied natural gas). This technology, however, requires expensive capital investment and so is suitable for huge gas reserves. The NGH method avoids the capital cost investment for the infrastructure constructions of the LNG method, which is an important point for the transportation of stranded gas. Therefore, especially for the stranded gas, the NGH method can be considered as an alternative for transportation of natural gas [72].
3.3 Storage of hydrogen
Efficient storage and transportation of H2under moderate conditions are essential for developing new economy sustained by H2energies. The conventional technologies for hydrogen storage include compressing hydrogen gas to high pressures to be stored in carbon-fiber-jacketed aluminum-alloy cylinders, cooling hydrogen to about 20 K to keep it in a liquid state in cryogenic dewars, compressing and cooling hydrogen to be absorbed by metal hydrides,[73]. With better understanding of the hydrate, it is also quite possible to store H2by hydrate. Mao. [74] reported that a hydrogen hydrate with an ordinary clathrate structure II was formed in the pressure range from 0.18 to 0.22 GPa at 249 K. The real breakthrough for hydrogen storage applications by hydrate was reported for binary clathrate hydrate H2O + H2+ THF. Formation of this hydrogen clathrate hydrate was observed at remarkably low pressures of 15 MPa and at temperatures very close to the room temperature (270 K) [75].
An additive component that constructs the clathrate hydrate under moderate conditions plays an important role for effective storage of gases by hydrate. To store H2, the clathrate hydrates of additive need some empty cages that can entrap H2molecules [76]. A suitable additive needs to satisfy the following requests simultaneously: high stability, high H2storage density, and reversible storage and release of H2for an additive hydrate [77]. Some kinds of second guest substance have been checked for hydrogen storage, such as THF [78-80], TBAB [81, 82], tetra--butylammonium fluoride (TBAF) [82, 83], Trimethylamine (TMA) [77], tetrahydrothiophene and furan [76]. The above guest substance forms by itself a sII clathrate hydrate or a semiclathrate hydrate at much lower pressures.
The stability is one of important parameters for hydrogen storage. Lee. [75] used Raman measurements to show that the stoichiometric THF/H2hydrate contained two hydrogen molecules per small cavity. However, some studies suggested that the maximum hydrogen occupancy in the small cavity of the THF + H2hydrate is only one hydrogen molecule [79, 84, 85]. The hydrogen occupancy is important to the stability of hydrate lattice. For hydrogen contained within the small cavities of a semi-clathrate with TBAB, the stability is even greater than that of THF + H2hydrate, and if TBAF is used instead of TBAB, the stability is increased further [86]. As pointed out by Ogata. [77] very recently, the H2+ TBAF semi- clathrate hydrate is stable even at room temperature (around 301 K).
However, the use of a second guest substance is accompanied by a substantial decrease in hydrogen occupancy in the hydrate cages [79, 80]. Lee. [75] claimed that hydrogen storage capacity of binary clathrate depends on THF content in the mixture and can be increased to 4% (by mass) for 0.15% (by mole) of THF. The parameters of hydrogen uptake and dependence of hydrogen storage capacity on THF content found in their work were questioned by other researchers. For example, Strobel. [79] did not observe the dependence of hydrogen uptake on THF concentration, while the maximal hydrogen content was found on the significantly lower level of ~1% (by mass). Raman spectroscopic measurement revealed that it reached the maximum value of 2.0 mol (H2)/mol (THF) at about 85 MPa [80]. Talyzin [87] used a fine dispersion of ice grains to improve kinetics of hydrogen uptake, but the maximum amount of stored hydrogen was found on the level of 0.2% (by mass).
As for the volumetric or mass-based hydrogen storage capacity, the hydrates have no advantage over conventional hydrogen-storage technologies for simply compressing or liquefying hydrogen gas. However, it is acceptable from a district-safety and energy saving viewpoint. According to a comparative study on various hydrogen-storage technologies, the ratio of the energy required for producing a hydrogen-storing material to the energy that the material can store is by far the highest for liquefied hydrogen, while those for compressed hydrogen gas (20-70MPa) and for a binary hydrogen + THF hydrate are similar to each other [88]. Therefore, the hydrate based technology is considered to be a practical choice for the purpose of stationary, large-capacity hydrogen storage [73]. Although it is far from being optimized, it may be competitive with other approaches.
3.4 CO2 sequestration
Carbon sequestration is defined as the removal of greenhouse gases from industrial or utility plant streams and their long term storage in such a way that they cannot interact with the climate system [89]. One volume of CO2hydrate can contain as much as 160 volumes of gaseous CO2. Carbon dioxide and water form a stable system when pressure and temperature fall within the hydrate formation region. For example, in the ocean, a long-lived system is formed. Additionally, CO2hydrate formation will impede the flow of liquid CO2[89, 90]. Since CO2-containing hydrates are considerably more stable thermodynamically than methane hydrates, it is a possible way to replace the original hydrate bound hydrocarbons by CO2. Two goals can be accomplished at the same time: safe storage of carbon dioxide in hydrate reservoirs, and in situ release of hydrocarbon gas. The hydrate and the matrix mineral surfaces are separated by liquid-containing channels, which will serve as escape routes for released natural gas, as well as distribution channels for injected CO2[91]. For CO2disposal in the form of hydrate, the preservation of CO2hydrate containing different quantities of CO2at pressures between 0.1 and 0.3 MPa and temperatures between-3 and 0 °C show that CO2hydrate does not present any anomalous self-preservation effect, and its dissociation is not affected by subcooling before storage. More than pressure, which is very important for methane hydrate, temperature affects the preservation of CO2hydrate. The temperature of-3 °C assures a good stability at atmospheric pressure, provided CO2saturation in the hydrate is not too high [89].
4 PREVENTION OF HYDRATE HAZARDS
It is well known that gas hydrates are crystalline compounds that can block transportation pipelines and production facilities in oil and gas industry. Traditional researches focused on how to prevent hydrate plug in these multiphase pipelines. Recently, some new technologies based on the chemical or physical characteristic properties of hydrate were developed as described above. Two basic processes, continuous formation and continuous transference of hydrate slurry, are needed for development of these techniques. Thus, the hydrate slurry must be kept under flow conditions without blockages.
Some methods have been used for hydrate prevention, among which the traditional thermodynamic method and addition of low dosage of hydrate inhibitors (LDHIs) are two typical examples. With respect to the traditional thermodynamic method, we can change either operating conditions by increasing temperatures and/or reducing pressures or change the hydrate formation conditions by injecting thermodynamic inhibitors such as methanol and glycol. To achieve remarkable effect, the concentration of methanol and glycol sometimes need reach 50% (by mass) on the free water basis. High cost and environmental concern of these thermodynamic inhibitors make them a critical burden on oil and gas companies [92]. Therefore, it is highly necessary to have new types of hydrate inhibitors. Recently, low dosage hydrate inhibitors are being widely utilized in the oil and gas industry, with a concentration below 1.0% (by mass) typically. Unlike thermodynamic inhibitors, the LDHIs do not change the hydrate formation conditions. There are two kinds of LDHIs: kinetic inhibitors (KIs) and anti-agglomerates (AAs) [93]. The KIs interfere with the nucleation and the growth of hydrate crystals, most of which are polymers or copolymers. In contrast, the AAs do not retard the formation of hydrate particles, but prevent them from agglomerating and accumulating into large masses.
The history of LDHIs was introduced in detail by Kelland [93]. In recent years, the research on LDHIs mainly focused on further development of LDHI based on previous hydrate inhibitors by changing structures or other parameters. For KHIs, Colle. [94] and Leinweber. [95, 96] performed molecular design based on the previous polyesteramide/amide classification KHI molecular structures. Leinweber and Feustel [97-99] applied patents on polyalkoxylated amine structure. Villano. [100] synthesized a class of kinetic hydrate inhibitors from polysuccinimide, which appears to have good biodegradability. The performance of new kinetic hydrate inhibitors were tested in stirred autoclaves using a natural gas blend and saline water. It is found that in the presence of solvents, the new inhibitors perform fairly well.
With respect to AAs, Dahlmann and Feustel [101, 102], and Rivers. [103] patented applications on new quaternary surfactant AAs. York and Firoozabadi [104] carried out the experiments with a multiple screening-tube rocking apparatus and they found that rhamnolipids is an effective AA comparable to other chemical surfactants. The operation subcooling, residence time, shut-in, and emulsion stability were chosen as the indicators of inhibition performance. Their results indicate that the use of bio-surfactants have huge potential for green practices in industry.
5 FUNDAMENTAL STUDY
5.1 Thermodynamics of hydrate
Natural gas hydrates in sediments are in general dispersed in pores of fine-grained silts, muds and clays or fractures in geological formations. It is known that the sediments inhibit hydrate formation and change the stability condition. It is important to determine the stable existence condition of hydrate in porous media for the exploration and exploitation of natural gas hydrate. Capillary inhibition of hydrate stability in narrow pores has been suggested as a possible explanation for differences between the predicted and actual boundary of hydrate stability zones. Handa and Stupin [105] were probably the first who measured the hydrate inhibition in mesoporous media where the inhibition was attributed to the depression of water activity. Then experimental studies on the effect of narrow pores [106, 107] and thermodynamic models for prediction of hydrate equilibria in porous media [108, 109] were reported. In the phase equilibrium models with porous media, van der Waals-Platteeuw model [110] based on statistic thermodynamics was used with Gibbs-Thomson relationship to determine the interfacial tension between hydrate and water from experimental data. In addition, in stead of the traditional van der Waals-Platteeuw type models, a thermodynamics model based on the concepts of reaction, adsorption two-step formation mechanism [111], was also improved to predict the hydrate-liquid-vapor equilibrium in pores [112].
In the development of techniques based on hydrate, a series of additives was involved to decrease the hydrate formation pressure, to increase hydrate formation rate, to inhibit the formation of hydrate, or change the morphology of hydrate. Therefore, it is important to obtain the knowledge of phase equilibria after the addition of additives. THF was widely used in hydrate-based gas separation and hydrogen storage. The three-phase equilibria of hydrate, liquid, and vapor were measured for the systems comprising THF. The hydrate formation conditions for different gas species, such as CO2, N2, CO2+ H2, were reported [39, 113, 114]. Relevant thermodynamic data at different content of THF were obtained. Thermodynamic study suggested that the use of THF as a hydrate stabilizer could reduce the hydrate formation pressure. In addition, thermodynamic models were successfully applied to describe hydrate formation systems containing THF, based on the CSMGem framework [115] or two-step hydrate formation mechanism [24].
Besides THF, other cyclic ethers, such as propylene oxide and 1,4-dioxane, were also examined for CO2system. The stabilization effect of the mixed hydrate was found to be the highest for THF followed by propylene oxide, while no stabilization effect was observed for 1,4-dioxane in the studied temperature and pressure range [116]. With CP as a promoter, the equilibrium pressure of H2+ CP hydrates is lower than that of H2+ THF hydrates [19]. Therefore, CP has a potential to be a better thermodynamic promoter than THF for a hydrate-based precombustion CO2capture. The phase equilibria of quaternary CO2+ H2+ CP + water systems containing gas hydrates were also determined and the thermodynamic analysis provides a conceptual design for developing a process of pre-combustion CO2capture in IGCC plants [20]. Semiclathrate hydrates were also used for gas separation and gas storage. Thermodynamic equilibrium of CO2-N2hydrates was investigated by using TBAB as an additive. The presence of TBAB decreased considerably the formation pressure of simple hydrates (CO2or N2) and mixed hydrates (CO2-N2) [41]. Similar to TBAB, the thermodynamic stabilities of semiclathrate hydrates were investigated in the tetra--butyl ammonium chloride (TBAC) aqueous solution + H2, + N2, + CH4, + CO2, and +C2H6systems [117].
5.2 Kinetics of hydrate formation
The porous medium is related to natural gas hydrate in the sediments and the development of gas hydrate storage. In the porous medium systems, with the existing experimental equipment, the kinetics and mass and heat transfer in pores are difficult to control, and especially, the reaction surface area is difficult to estimate because it is difficult to visually monitor the hydrates inside pores [118]. The study on the formation of methane hydrate in wet activated carbon [119] demonstrates that the formation of methane hydrate is enhanced by immersing activated carbon in water. Similar to pure water system, the hydrate formation rate increases with the increase of pressure or the decrease of temperature, but the formation rate decreases with time more rapidly at lower temperature. Porous media are also used to control the hydrogen clathrate particle size in order to accelerate its formation kinetics. The formation for the THF-H2binary clathrate hydrates in a porous medium with a median pore diameter of 4.9 nm is 6-22 times faster than that in the bulk ice. The clathrate formation time increases with pore size of the porous medium [120]. Silica gels with controlled size are used for formation kinetics to evaluate the formation behavior of CO2hydrates in porous media. The results show that CO2hydrates form faster with larger driving force [121]. Compared to mechanical stirring and ice powder methods, the hydrate formation in silica gel pores shows a more rapid rate of hydrate formation and shorter induction time [122]. In addition, SDS is also used as a kinetic promoter in order to identify the promotional effect on the formation kinetics in porous media. Experimental results suggest that there can be an optimum value for added promoter concentration and that an excess amount of promoter plays an inhibition role, which is accordance with the results obtained in systems without porous media [123].
The success of the potential applications based on hydrate is mainly hindered by some technological problems associated with hydrate formation, including slow formation rates, low conversions, and the economics of process scale-up [124]. Some kinds of additive have been used to overcome such difficulties and a better understanding of hydrate formation kinetics is required. The addition of THF reduces the induction time and the hydrate formation pressure. However, the rate of hydrate growth is reduced [39]. By means of a molecular dynamic simulation for the growth kinetics of a THF clathrate hydrate at the interface between the clathrate and an aqueous THF solution, Nada [125] found the same anisotropic growth as that observed in real systems, which suggests that the rate-determining process for clathrate growth is the rearrangement of THF molecules at the interface from a disordered state to a state in which THF molecules are ideally arranged at large cage sites only. Some surfactants such as SDS are found to be excellent kinetic promoters for hydrocarbon hydrates. Surfactant shows a favorable effect on the reduction of induction time and acceleration of hydrate formation with or without mechanical stirring. At a SDS concentration of 284 mg·L-1, ethane hydrates grow at a rate that is 700 times higher than that in a pure water system because of SDS micelle formation [32]. Gayet. [126] suggest that dissolved SDS prevents hydrate particles from agglomerating. They then grow along the reactor wall as a porous structure, which absorbs the liquid from the bulk to the crystallization front where the gas-liquid interface is renewed and hydrates grow at a high rate without any mechanical agitation. The experimental results on hydrate formation [61] show that the formation rate of methane hydrate can be speeded up by adding SDS to water. SDS not only increases hydrate nucleation rate by reducing the interfacial tension between hydrate and liquid but also accelerates hydrate growth rate by increasing the total surface area of hydrate particles, the solubility of gas molecules in the interface, and the gas-liquid interfacial area [123, 127]. This promotion effect does not increase any more when the concentration of SDS exceeds a certain value. The SDS molecules may be adsorbed on the hydrate particles and inhibit the further growth of the particles at higher SDS concentration [123]. In addition, by adding a small amount of cyclopentane, the growth rate of CO2hydrates and water conversion to the hydrates are accelerated [19]. The measurement shows that growth rates of TBAB semi-clathrate hydrate crystals are extremely dependent on interfacial kinetics [128].
The thermodynamics and kinetics of emulsion are important for the safe flow of hydrate containing systems. Up to now, there are no measurable differences between bulk solutions and emulsions from thermodynamic point of view [129, 130]. For the kinetics of emulsion, Jakobsen. [131] adopted permittivity technique to measure gas hydrate formation in water-in-oil emulsions. They confirmed that formation of hydrate changes the permittivity in bulk solution and formation of hydrate in emulsion can be accurately detected by using this property. Aichele. analyzed methane hydrate formation in water-in-oil emulsions with nuclear magnetic resonance (NMR) [132]. Combining the drop size distribution with transverse relaxation data, they yielded quantitative information about the relationship between drop size distribution and methane hydrate formation. Boxall. [133] used particle video microscope (PVM) and focused beam reflectance measurement (FBRM) to study the gas hydrate formation and dissociation in water-in-oil emulsions. They confirmed that droplet size has a major effect on whether or not agglomeration will occur and agglomeration is extremely dramatic due to the creation of surface water on the particles. Palermo. [134] investigated the agglomeration between hydrate particles in oil using differential scanning calorimetry (DSC). They showed that the kinetics of hydrate formation may differ strongly depending on the anti-agglomerant properties of the crude. According to the observation, they proposed a new mechanism of agglomeration between hydrate particles: hydrate agglomerates grow, not due to the adhesion between hydrate particles, but due to the contact between a water droplet and a hydrate particle, followed by an immediate crystallization of the water droplet. Using DSC method, Dalmazzone. [135] measured methane hydrate formation in water-in-oil emulsions. Combining with their previous work [136], they used four parameters to set up a model. The model based on crystal growth theory, coupled with a normal distribution of induction time to take into account the germination in a population of micro-sized droplets, is to represent the hydrate formation rate versus time in the particular case of water-in-oil emulsions and it may be useful to assess the risk of hydrate formation in the drilling fluid under industrial conditions.
5.3 Kinetics of hydrate dissociation
The kinetics of dissociation is important for the development of hydrate-based technique. For example, the stability is an important performance in the applications such as gas storage, which is described above. Furthermore, the kinetics of hydrate dissociation is essentially important on developing gas production technology from natural gas hydrate. Usually, the methods of hydrate dissociation include depressurization, thermal stimulation, and inhibitor injection. Compared with intrinsic kinetics of pure hydrate dissociation, the dissociation kinetics of gas hydrate- bearing sediments involves fluid flow and heat transfer through a porous medium whose properties evolve as the hydrate phase disappears. To investigate dissociation kinetics of hydrate-bearing sediments, more information on the heat transfer, flow characteristics, and dissociation behavior of the hydrate-bearing sediments is necessary. Although natural gas hydrates are known to occur in numerous marine and permafrost regions, few investigations about gas production from natural gas hydrate reservoir have been conducted due to huge fees, risk, and difficulty in these investigations. At present, the recovery of gas from hydrate reservoirs is mainly performed through laboratory experimental investigations and numerical simulations.
Very recently Lee. [137] observed that hydrate reformed during gas production by depressurization in a core holder when the outlet pressure was 70% of the equilibrium pressure. However, it did not happen when the outlet pressure was 74% of the equilibrium pressure. In addition, it was observed that the dissociation was fast corresponding to a small pressure difference between the outlet pressure and the equilibrium pressure, which is contrary to previous theory of hydrate dissociation kinetics. Lee. [137] believed that the pore was plugged by hydrate regeneration, resulting in the contradictory results. It is noted that the highest gas production rate is less than 0.1 L·min-1in their investigations, which does not satisfy the commercial production. Zhou. [138] observed a cooling effect during the dissociationdepressurization scheme, which was caused by the endothermic dissociation reaction. In addition, the temperature jumped to 0°C in an extremely short period. The interpretation of this phenomenon is ice formation in the transition regime where hydrate decomposes to gas and ice instead of gas and liquid. Oyama. [139] found that the sensible heat of the sediment was immediately consumed during the depressurization process by comparing the pressure and temperature behavior before and after the depressurization. It was derived from the temperature changes that hydrate dissociation was driven by heat transfer surrounding the core . The rate of gas and water production increased with the decrease of the production pressure. For gas production from gas hydrates by thermal injection, Selim and Sloan [140] found that the dissociation rate was a strong function of the thermal properties of the system and the porosity of the porous medium. Kamata. [141] applied thermal recovery method to dissociate methane hydrate in hydrate sediment sample by hot-water injection from one side and gas production from another side. It was found that temperature and pressure in the sample fluctuated between stability region and decomposition region of methane hydrate sample when temperature of the hot water was high. Li. [142] investigated the influence of salinity and temperature on gas production of methane hydrate in porous sediment. The results indicated that the dissociation rate was fast with the increase of the salinity within a certain range. For hydrate samples synthesized in different size volume beds, Linga. [143] found that the rate of methane recovery depended on the bed size when dissociated by thermal stimulation. In the gas production experiments by injecting brine scheme, Lee [144] found that the permeability of rock is one of the important factors that influence production from gas hydrates by injecting brine. Numerical simulations were also used to investigate the hydrate dissociation. Nihous. [145] provided important information about the model of gas hydrate dissociation by comparing the equilibrium model and the kinetic model. Their results indicated that the intrinsic kinetic formalism was only suitable for hydrate dissociation by depressurization. At present, TOUGH?+?HYDRATE, the popular code for the simulation of the behavior of hydrate-bearing geologic systems, has been used widely to simulate gas production under different conditions from different classes hydrate reservoirs [146].
6 CONCLUSIONS AND PROSPECTS
In this work, the exploration and exploitation of natural gas hydrate, the novel techniques developed based on hydrate, the inhibition and agglomeration of hydrate, and the progresses about the thermodynamic and kinetic studies on hydrate are reviewed.
It is well known that natural gas hydrates are widely distributed on permafrost and offshore and the huge amounts of natural gas hydrates will be a considerable future sustainable energy source. Though they are very important for our future life, the exploitation method and the environmental risk should be assessed first.
For the hydrate-based gas separation, the high- pressure requirement for hydrate formation has been overcome by adding hydrate promoter. Several efficient methods to enhance the hydrate formation process have been invented. Compared with other gas separation methods, such as cryogenic fractionation, the hydrate-based gas separation method is very promising. To make the hydrate-based gas separation process more efficient and economical, it is important to operate the equipment for hydrate formation and dissociation continuously and steadily and keep the hydrate slurry flowing continuously.
NGH has certain advantages over CNG (compressed natural gas) or LNG (liquefied natural gas), particularly for small natural gas fields where it is not appropriate to use liquefied approach or pipeline transportation. In industrial applications, however, NGH approach has encountered many problems, such as slow hydrate formation rates, low conversion ratio of water, low hydrate storage capacity, and difficulty in process scale-up. To solve these problems, many mechanical and chemical methods have been proposed. It should be pointed out that the stability of hydrate is also important for hydrate storage processes.
For hydrogen storage application, one can obtain the hydrate at relatively low pressures by adding some additives. The primary concern for hydrogen storage by forming hydrate is the hydrogen storage capacity. The level of the U.S. DOE targets for on-board hydrogen storage capacity is 6% (by mass) by 2010, which is still beyond the capacity of hydrate-based hydrogen storage based on the current status of research.
For the inhibition and prevention of agglomeration of hydrate in pipelines and facilities, instead of the traditional thermodynamics methods, many low dosage hydrate inhibitors are being developed and utilized in recent years. In order to prevent the agglomeration of hydrate particles in pipelines more efficiently, we do need to know the mechanisms of hydrate particle nucleation, growth, and aggregation.
1 Milkov, A.V., “Global estimates of hydrate-bound gas in marine sediments: how much is really out there?”,-.., 66 (3/4), 183-197 (2004).
2 Makogon, Y.F., “Natural gas hydrates-A promising source of energy”,...., 2 (1), 49-59 (2010).
3 Makogon, Y.F., Holditch, S.A., Makogon. T.Y., “Natural gas-hydrates—A potential energy source for the 21st Century”,...., 56 (1-3), 14-31 (2007).
4 Wood, W.T., Hart, P.E., Hutchinson, D.R., Dutta, N., Snyder, F., Coffin, R.B., Gettrust, J.F., “Gas and gas hydrate distribution around seafloor seeps in Mississippi Canyon, Northern Gulf of Mexico, using multi-resolution seismic imagery”,..., 25 (9), 952-959 (2008).
5 Takahashi, H., Tetsuo, Y., Yoshikazu, T., “Exploration for natural hydrate in Nankai-Trough wells offshore Japan”,....., 66 (6), 652-665 (2001).
6 Collett, T.S., “Energy resource potential of natural gas hydrates”,...., 86 (11), 1971-1992 (2002).
7 Collett, T., Johnson, A., Knapp, C., Boswell, R., Natural Gas Hydrates-—Energy Resource Potential and Associated Geologic Hazards, 89 (AAPG Memoir), American Association of Petroleum Geologists, 505-524 (2009).
8 Osadetz, K.G., Chen, Z., “A re-examination of Beaufort Sea-Mackenzie Delta Basin gas hydrate resource potential using a petroleum system approach”, In: Proceedings of the Fifth International Conference on Gas Hydrates, Trondheim, Norway (2005).
9 Walsh, T.P., Stokes, P.J., Singh, P.K., “Characterization and quantification of the methane hydrate resource potential associated with the Barrow Gas Fields”, In: Proceedings of the 6th International Conference on Gas Hydrotes (ICGH), Vancouver, British Columbia, Canada (2008).
10 Xu, W.S., Yu, X.H., Liu, N.N., Liu, W.L., “The development perspective and environmental problems of natural gas hydrates”,, 16 (5), 680-683 (2005). (in Chinese)
11 Hao, L., Cui, Y.X., “Resource potential and exploiting prospects of gas hydrate”,.., 7, 5-6 (2009). (in Chinese)
12 Lu, H., Lorenson, T.D., Moudrakovski, I.L., Ripmeester, J.A., Collett, T.S., Hunter, R.B., Ratcliffe, C.I., “The characteristics of gas hydrates recovered from Mount Elbert gas hydrate stratigraphic test well, Alaska North Slope”,..., 1-8 (2010).
13 Yao, B.C., “The gas hydrate in the south China sea”,., 20 (2), 20-28 (2001). (in Chinese)
14 Zeng, W.P., Zhou, D., “GIS-aided estimation of gas hydrate resources in southern south China sea”,., 22 (6), 35-45 (2003). (in Chinese)
15 Jiang, H.Y., Qiao, W.J., Zhong, T.X., Song, X.M., Qi, R.L., An, X.X., “Status and forecast of world’s natural gas hydrate exploration and development”,-, 13 (6), 19-25 (2008). (in Chinese)
16 Zhu, Y.H., Zhang, Y.Q., Wen, H.J., Lu, Z.Q., Wang, P.K., “Gas hydrates in the Qilian Mountain permafrost and their basic characteristics”,, 31 (1), 7-16 (2010). (in Chinese)
17 Xu, S.S., Wang, T., Liu, T.J., Wang, D., Cao, D.Y., “Resource quantity estimation of gas hydrate in Muli coalfield, Qinhai Province”,, 21 (9), 1-3 (2009). (in Chinese)
18 Kumar, R., Wu, H.J., Englezos, P., “Incipient hydrate phase equilibrium for gas mixtures containing hydrogen, carbon dioxide and propane”,, 244 (2), 167-171 (2006).
19 Zhang, J.S., Lee, J.W., “Equilibrium of hydrogen?+?cyclopentane and carbon dioxide?+?cyclopentane binary hydrates”,..., 54 (2), 659-661 (2009).
20 Zhang, J.S., Yedlapalli, P., Lee, J.W., “Thermodynamic analysis of hydrate-based pre-combustion capture of CO2”,..., 64 (22), 4732-4736 (2009).
21 Kamata, Y., Yamakoshi, Y., Ebinuma, T., Oyama, H., Shimada, W., Narita, H., “Hydrogen sulfide separation using tetra--butyl ammonium bromide semi-clathrate (TBAB) hydrate”,, 19 (4), 1717-1722 (2005).
22 Seo, Y., Tajima, H., Yamasaki, A., Takeya, S., Ebinuma,T., Kiyono, F., “A new method for separating HFC-134a from gas mixtures using clathrate hydrate formation”,..., 38 (17), 4635-4639 (2004).
23 Zhang, C.S., Fan, S.S., Liang, D.Q., Guo, K.H., “Effect of additives on formation of natural gas hydrates”,, 83 (16), 2115-2121 (2004).
24 Sun, C.Y., Chen, G.J., Zhang, L.W., “Hydrate phase equilibrium and structure for (methane?+?ethane?+?tetrahydrofuran?+?water) system”,.., 42 (9), 1173-1179 (2010).
25 Fukumoto, K., Tobe, J., Ohmura, R., Mori, Y.H., “Hydrate formation using water spraying in a hydrophobic gas: A preliminary study”,., 47 (8), 1899-1904 (2001).
26 Warzinski, R.P., Riestenberg, D.E., Gabitto, J., Haljasmaa, I.V., Lynn, R.J., Tsouris, C., “Formation and behavior of composite CO2hydrate particles in a high-pressure water tunnel facility”,..., 63 (12), 3235-3248 (2008).
27 Szymcek, P., McCallum, S.D., Taboada-Serrano, P., Tsouris, C., “A pilot-scale continuous-jet hydrate reactor”,..., 135 (1/2), 71-77 (2008).
28 Luo, Y.T., Zhu, J.H., Fan, S.S., Chen, G.J., “Study on the kinetics of hydrate formation in a bubble column”,..., 62 (4), 1000-1009 (2007).
29 Hashemi, S., Macchi, A., Servio, P., “Gas-liquid mass transfer in a slurry bubble column operated at gas hydrate forming conditions”,..., 64 (16), 3709-3716 (2009).
30 Tajima, H., Nagata, T., Abe, Y., Yamasaki, A., Kiyono, F., Yamagiwa, K., “HFC-134a hydrate formation kinetics during continuous gas hydrate formation with a kenics static mixer for gas separation”,...., 49 (5), 2525-2532 (2010).
31 Seo, Y.T., Moudrakovski, I.L., Ripmeester, J.A., Lee, J.W., Lee, H., “Efficient recovery of CO2from flue gas by clathrate hydrate formation in porous silica gels”,..., 39 (7), 2315-2319 (2005).
32 Zhong, Y., Rogers, R.E., “Surfactant effects on gas hydrate formation”,..., 55 (19), 4175-4187 (2000).
33 Li, S.F., Fan, S.S., Wang, J.Q., Lang, X.M., Liang, D.Q., “CO2capture from binary mixtureforming hydrate with the help of tetra--butyl ammonium bromide”,.., 18 (1), 15-20 (2009).
34 Zhang, L.W., Chen, G.J., Guo, X.Q., Sun, C.Y., Yang, L.Y., “The partition coefficients of ethane between vapor and hydrate phase for methane plus ethane plus water and methane plus ethane plus THF plus water systems”,, 225 (1/2), 141-144 (2004).
35 Zhang, L.W., Chen, G.J., Sun, C.Y., Ding, Y.M., Yang, L.Y., “The partition coefficients of ethylene between vapor and hydrate phase for methane plus ethylene plus THF plus water systems”,, 245 (2), 134-139 (2006).
36 Zhang, L.W., Chen, G.J., Sun, C.Y., Fan, S.S., Ding, Y.M., Wang, X.L., Yang, L.Y., “The partition coefficients of ethylene between hydrate and vapor for methane?+?ethylene?+?water and methane?+?ethylene?+?SDS?+?water systems”,..., 60 (19), 5356-5362 (2005).
37 Sun, C.Y., Ma, C.F., Chen, G.J., Zhang, S.X., “Experimental and simulation of single equilibrium stage separation of (methane + hydrogen) mixturesforming hydrate”,, 261 (1/2), 85-91 (2007).
38 Linga, P., Kumar, P., Englezos, P., “The clathrate hydrate process for post and pre-combustion capture of carbon dioxide”,..., 149 (3), 625-629 (2007).
39 Linga, P., Adeyemo, A., Englezos, P., “Medium-pressure clathrate hydrate/ membrane hybrid process for postcombustion capture of carbon dioxide”,..., 42 (1), 315-320 (2008).
40 Nagata, T., Tajima, H., Yamasaki, A., Kiyono, F., Abe, Y., “An analysis of gas separation processes of HFC-134a from gaseous mixtures with nitrogen—Comparison of two types of gas separation methods, liquefaction and hydrate-based methods, in terms of the equilibrium recovery ratio”,..., 64 (3), 351-356 (2009).
41 Duc, N.H., Chauvy, F., Herri, J.M., “CO2capture by hydrate crystallization—A potential solution for gas emission of steelmaking industry”,, 48 (4), 1313-1322 (2007).
42 Makogon, Y.F., Hydrates of Hdrocarbon, Pennwell Publishing Co.,Tulsa, Oklahoma (1997).
43 Gudmundsson, J.S., Khokhar, A.A., Parlaktuna, M., “Storage of natural gas as frozen hydrate”, In: Proceedings of the 67th Annual Technical Conference and Exhibition of Society of Petroleum Engineers (SPE), SPE 24924, Washington, D.C., 699-707 (1990).
44 Gudmundsson, J.S., Parlaktuna, M., Khokhar, A.A., “Storing natural gas as frozen hydrates”,.., 9 (1), 69-73 (1994).
45 Stern, L.A., Circone, S., Kirby, S.H., Durham, W.B., “Anomalous preservation of pure methane hydrate at 1 atm”,..., 105 (9), 1756-1762 (2001).
46 Giavarini, C., Maccioni, F., “Self-preservation at low pressures of methane hydrates with various gas contents”,...., 43 (20), 6616-6621 (2004).
47 Kuhs, W.F., Genov, G., Satykova, D.K., Hansen, T., “Ice perfection and onset of anomalous preservation of gas hydrates”,...., 6, 4917-4920 (2004).
48 Shimada, W., Takeya, S., Kamata, Y., Uchida, T., Nagao, J., Ebinuma, T., Narita, H., “Texture change of ice on anomalously preserved methane clathrate hydrate”,..., 109 (12), 5802-5807 (2005).
49 Mandal, A., Laik, S., “Effect of the promoter on gas hydrate formation and dissociation”,, 22 (4), 2527-2532 (2008).
50 Iwasaki, T., Katoh, Y., Nagamori, S., Takahashi, S., “Continuous natural gas hydrate pellet production (NGHP) by process development unit (PDU)”, In: Proceedings of the Fifth International Conference on Gas Hydrates, Trondheim, Norway, 1107-1115 (2005).
51 Takaoki, T., Hirai, K., Kamei, M., Kanda, H., “Study of natural gas hydrate (NGH) carriers”, In: Proceedings of the Fifth International Conference on Gas Hydrates, Trondheim, Norway, 1258-1265 (2005).
52 Ohmura, R., Kashiwazaki, S., Shiota, S., Tsuji, H., Mori, Y.H., “Structure-I and structure-H hydrate formation using water spraying”,, 16 (5), 1141-1147 (2002).
53 Takahashi, M., Oonari, H., Yamamoto, Y., “A novel manufacturing method of gas hydrate using the micro-bubble technology”, In: Proceedings of the 4th International Conference on Natural Gas Hydrates, Yokohama, Japan, 825-828 (2002).
54 Stern, L.A., Kirby, S.H., Durham, W.B., “Peculiarities of methane clathrate hydrate formation and solid-state deformation, including possible superheating of water ice”,, 273 (5283), 1843-1848 (1996).
55 Takaoki, T., Iwasaki, T., Katoh, Y., Arai, T., Horiguchi, K., “Use of hydrate pellets for transportation of natural gas, Advantage of pellet form of natural gas hydrate in sea transportation”, In: Proceedings of the 4th International Conference on Natural Gas Hydrates, Yokohama, Japan, 982-986 (2002).
56 Karaaslan, U., Parlaktuna, M., “Surfactants as hydrate promoters?”,, 14 (5), 1103-1107 (2000).
57 Sun, Z.G., Wang, R.Z., Ma, R.S., Guo, K.H, Fan, S.S., “Natural gas storage in hydrate with the presence of promoters”,, 44 (17), 2733-2742 (2003).
58 Lee, J., Shin, C., Lee, Y., “Experimental investigation to improve the storage potentials of gas hydrate under the unstirring condition”,, 24, 1129-1134 (2010).
59 Rogers, R.E., Zhong, Y., Etheridge, J.A., Arunkumar, R., Pearson, L.E., Hogancamp, T.K., “Micellar gas hydrates storage process”, In: Proceedings of the Fifth International Conference on Gas Hydrates, Trondheim, Norway, 1361-1365 (2005).
60 Pang, W.X., Chen, G.J., Dandekar, A., Sun, C.Y., Zhang, C.L., “Experimental study on the scale-up effect of gas storage in the form of hydrate in a quiescent reactor”,..., 62 (8), 2198-2208 (2007).
61 Lin, W., Chen, G.J., Sun, C.Y., Guo, X.Q., Wu, Z.K., Liang, M.Y., Chen, L.T., Yang, L.Y., “Effect of surfactant on the formation and dissociation kinetic behavior of methane hydrate”,..., 59 (21), 4449-4455 (2004).
62 Guo, Y.K., Fan, S.S., Guo, K.H., Chen, Y., “Storage capacity of methane in hydrate using calcium hypochlorite as additive”, In: Proceedings of the Fourth International Conference on Natural Gas Hydrates, Yokohama, Japan, 1040-1043 (2002).
63 Karaaslan, U., Uluneye, E., Parlaktuna, M., “Effect of an anionic surfactant on different type of hydrate structures”,...., 35 (1/2), 49-57 (2002).
64 Ganandran, N., Amin, R., “The effect of hydrotropes on gas hydrates formation”,...., 40 (1/2), 37-46 (2003).
65 Sun, Z.G., Ma, R.S., Wang, R.Z., Guo, K.H., Fan, S.S., “Experimental studying of additives effects on gas storage in hydrates”,, 17 (5), 1180-1185 (2003).
66 Ganji, H., Manteghian, M., Zadeh, K.S., Omidkhah, M.R., Mofrad, H.R., “Effect of different surfactants on methane hydrate formation rate, stability and storage capacity”,, 86 (3), 434-441 (2007).
67 Prado, M.R., Cazares, Y., Janda, K.C., “Toward the efficient production of methane/propane double hydrate”,...., 48 (11), 5160-5164 (2009).
68 Ganji, H., Manteghian, M., Mofrad, H.R., “Effect of mixed compounds on methane hydrate formation and dissociation rates and storage capacity”,.., 88 (9), 891-895 (2007).
69 Kawamura, T., Sakamoto, Y., Ohtake, M., Yamamoto, Y., Komai, T., Haneda, H., Yoon, J.H., “Dissociation behavior of pellet-shaped methane hydrate in ethylene glycol and silicone oil. Part 1: Dissociation above ice point”,...., 45 (1), 360-364 (2006).
70 Gudmundsson, J.S., Mork, M., Graff, O.F., “Hydrate non-pipeline technology”, In: Proceedings of the Fourth International Conference on Gas Hydrate, Yokohama, Japan, 997-1002 (2002).
71 Kanda, H., “Economics study on natural gas transportation with natural gas hydrate pellets”, In: 23rd World Gas Conference, Amsterdam (2006).
72 Javanmardi, J., Nasrifar, K., Najibi, S.H., Moshfeghian, M., “Economic evaluation of natural gas hydrate as an alternative for natural gas transportation”,..., 25 (11/12), 1708-1723 (2005).
73 Nakayama, T., Tomura, S., Ozaki, M., Ohmura, R., Mori, Y.H., “Engineering investigation of hydrogen storage in the form of clathrate hydrates: Conceptual design of hydrate production plants”,, 24, 2576-2588 (2010).
74 Mao, W.L., Mao, H.K., Goncharov, A.F., Struzhkin, V.V., Guo, Q.Z., Hu, J.Z., Shu, J.F., Hemley, R.J., Somayazulu, M., Zhao, Y.S., “Hydrogen clusters in clathrate hydrate”,, 297 (5990), 2247-2249 (2002).
75 Lee, H., Lee, J.W., Kim, D.Y., Park, J., Seo, Y.T., Zeng, H., Moudrakovski, I.L., Ratcliffe, C.I., Ripmeester, J.A., “Tuning clathrate hydrates for hydrogen storage”,, 434 (7034), 743-746 (2005).
76 Tsuda, T., Ogata, K., Hashimoto, S., Sugahara, T., Moritoki, M., Ohgaki, K., “Storage capacity of hydrogen in tetrahydrothiophene and furan clathrate hydrates”,..., 64 (19), 4150-4154 (2009).
77 Ogata, K., Tsuda, T., Amano, S., Hashimoto, S., Sugahara, T., Ohgaki, K., “Hydrogen storage in trimethylamine hydrate: Thermodynamic stability and hydrogen storage capacity of hydrogen + trimethylamine mixed semi-clathrate hydrate”,..., 65 (5), 1616-1620 (2010).
78 Florusse, L.J., Peters, C.J., Schoonman, J., Hester, K.C., Koh, C.A., Dec, S.F., Marsh, K.N., Sloan, E.D., “Stable low-pressure hydrogen clusters stored in a binary clathrate hydrate”,, 306 (5695), 469-471 (2004).
79 Strobel, T.A., Taylor, C.J., Hester, K.C., Dec, S.F., Koh, C.A., Miller, K.T., Sloan, E.D., “Molecular hydrogen storage in binary THF-H2clathrate hydrates”,..., 110 (34), 17121-17125 (2006).
80 Ogata, K., Hashimoto, S., Sugahara, T., Moritoki, M., Sato, H., Ohgaki, K., “Storage capacity of hydrogen in tetrahydrofuran hydrate”,..., 63 (23), 5714-5718 (2008).
81 Hashimoto, S., Murayama, S., Sugahara, T., Sato, H., Ohgaki, K., “Thermodynamic and Raman spectroscopic studies on H2+?tetrahydrofuran?+?water and H2+?tetra--butyl ammonium bromide?+?water mixtures containing gas hydrates”,..., 61 (24), 7884-7888 (2006).
82 Chapoy, A., Anderson, R., Tohidi, B., “Low-pressure molecular hydrogen storage in semi-clathrate hydrates of quaternary ammonium compounds”,...., 129 (4), 746-747 (2007).
83 Sakamoto, J., Hashimoto, S., Tsuda, T., Sugahara, T., Inoue, Y., Ohgaki, K., “Thermodynamic and Raman spectroscopic studies on hydrogen?+?tetra--butyl ammonium fluoride semi-clathrate hydrates”,..., 63 (24), 5789-5794 (2008).
84 Hester, K.C., Strobel, T.A., Sloan, E.D., Koh, C.A., Huq, A., Schultz, A.J., “Molecular hydrogen occupancy in binary THF-H2clathrate hydrates by high resolution neutron diffraction”,..., 110 (29), 14024-14027 (2006).
85 Anderson, R., Chapoy, A., Tohidi, B., “Phase relations and binary clathrate hydrate formation in the system H2-THF-H2O”,, 23 (6), 3440-3444 (2007).
86 Strobel, T.A., Koh, C.A., Sloan, E.D., “Hydrogen storage properties of clathrate hydrate materials”,, 261 (1/2), 382-389 (2007).
87 Talyzin, A., “Feasibility of H2-THF-H2O clathrate hydrates for hydrogen storage applications”,.., 33 (1), 111-115 (2008).
88 Profio, D.P., Arca, S., Rossi, F., Filipponi, M., “Comparison of hydrogen hydrates with existing hydrogen storage technologies: Energetic and economic evaluations”,.., 34 (22), 9173-9180 (2009).
89 Giavarini, C., Maccioni, F., Politi, M., Santarelli, M.L., “CO2hydrate: Formation and dissociation compared to methane hydrate”,, 21 (6), 3284-3291 (2007).
90 House, K.Z., Schrag, D.P., Harvey, C.F., Lackner, K.S., “Permanent carbon dioxide storage in deep-sea sediments”,......., 103 (33), 12291-12295 (2006).
91 Kvamme, B., Graue, A., Buanes, T., Kuznetsova, T., Ersland, G., “Storage of CO2in natural gas hydrate reservoirs and the effect of hydrate as an extra sealing in cold aquifers”,, 1 (2), 236-246 (2007).
92 Sloan, E.D., Koh, C.A., Clathrate Hydrates of Natural Gases, 3rd edition, CRC Press, New York (2008).
93 Kelland, M.A., “History of the development of low dosage hydrate inhibitors”,, 20 (3), 825-847 (2006).
94 Colle, K.S., Talley, L.D., Longo, J.M., “Method for inhibiting hydroate formation”, U.S. Pat., 0205603 (2006).
95 Leinweber, D., Roesch, A., Feustel, M., “Use of 1-alkyl-5-oxopyrrolidine-3- carboxylic esters as gas hydrate inhibitors with improved biodegradabiltiy”, U.S. Pat., 0042747 (2009).
96 Leinweber, D., Roesch, A., Feustel, M., “Use of substituted polyethyleneimines as gas hydrate inhibitors with improved biodegradability”, U.S. Pat., 0054268 (2009).
97 Leinweber, D., Feustel, M., “Polymers and their production and use as gas hydrate inhibitors”, U.S. Pat., 0113878 (2008).
98 Leinweber, D., Feustel, M., “Biologically degradable gas hydrate inhibitors”, U.S. Pat., 0177103 (2008).
99 Leinweber, D., Feustel, M., “Corrosion and gas hydrate inhibitors with an increased biological degradability and a reduced toxicity”, U.S. Pat., 0173663 (2009).
100 Villano, L.D., Kommedal, R., Kelland, M.A., “Class of kinetic hydrate inhibitors with good biodegradability”,, 22 (5), 3143-3149 (2008).
101 Dahlmann, U., Feustel, M., “Additives for inhibiting the formation of gas hydrates”, U.S. Pat., 0129256 (2007).
102 Dahlmann, U., Feustel, M., “Additives for inhibiting gas hydrate formation”, U.S. Pat., 0173672 (2007).
103 Rivers, G.T., Tian, J., Hackerott, J.A., “Oxazolidinium compounds and hydrate inhibitors”, U.S. Pat., 0130747 (2010).
104 York, J.D., Firoozabadi, A., “Comparing effectiveness of rhamnolipid biosurfactant with a quaternary ammonium salt surfactant for hydrate anti-agglomeration”,..., 112 (3), 845-851 (2008).
105 Handa, Y.P., Stupin, D., “Thermodynamic properties and dissociation characteristics of methane and propane hydrates in 70-?-radius silica gel pores”,..., 96 (21), 8599-8603 (1992).
106 Anderson, R., Llamedo, M., Tohidi, B., Burgass, R.W., “Experimental measurements of methane and carbon dioxide clathrate hydrate equilibria in mesoporous silica”,..., 107 (15), 3507-3514 (2003).
107 Seo, Y., Lee, S., Cha, I., Lee, J.D., Lee, H., “Phase equilibria and thermodynamic modeling of ethane and propane hydrates in porous silica gels”,..., 113 (16), 5487-5492 (2009).
108 Klauda, J.B., Sandler, S.I., “Modeling gas hydrate phase equilibria in laboratory and natural porous media”,...., 40 (20), 4197-4208 (2001).
109 Sun, R., Duan, Z.H., “An accurate model to predict the thermodynamic stability of methane hydrate and methane solubility in marine environments”,, 244 (1/2), 248-262 (2007).
110 van der Waals, J.H., Platteeuw, J.C., “Clathrate solutions”,, 2 (1), 1-57 (1959).
111 Chen, G.J., Guo, T.M., “A new approach to gas hydrate modeling”,..., 71 (8), 145-151 (1998).
112 Chen, L.T., Sun, C.Y., Chen, G.J., Nie, Y.Q., “Thermodynamics model of predicting gas hydrate in porous media based on reaction-adsorption two-step formation mechanism”,...., 49 (8), 3936-3943 (2010).
113 Kang, S.P., Lee, H., “Recovery of CO2from flue gas using gas hydrate: Thermodynamic verification through phase equilibrium measurements”,..., 34 (20), 4397-4400 (2000).
114 Hashimoto, S., Murayama, S., Sugahara, T., Ohgaki, K., “Phase equilibria for H2plus CO2plus tetrahydrofuran plus water mixtures containing gas hydrates”,..., 51 (5), 1884-1886 (2006).
115 Strobel, T.A., Koh, C.A., Sloan, E.D., “Thermodynamic predictions of various tetrahydrofuran and hydrogen clathrate hydrates”,, 280 (1/2), 61-67 (2009).
116 Seo, Y., Kang, S.P., Lee, S., Lee, H., “Experimental measurements of hydrate phase equilibria for carbon dioxide in the presence of THF, propylene oxide, and 1,4-dioxane”,..., 53 (12), 2833-2837 (2008).
117 Makino, T., Yamamoto, T., Nagata, K., Sakamoto, H., Hashimoto, S., Sugahara, T., Ohgaki, K., “Thermodynamic stabilities of tetra--butyl ammonium chloride?+?H2, N2, CH4, CO2, or C2H6semiclathrate hydrate systems”,..., 55 (2), 839-841 (2010).
118 Sun, X., Mohanty, K.K., “Kinetic simulation of methane hydrate formation and dissociation in porous media”,..., 61 (11), 3476-3495 (2006).
119 Yan, L.J., Chen, G.J., Pang, W.X., Liu, J., “Experimental and modeling study on hydrate formation in wet activated carbon”,..., 109 (12), 6025-6030 (2005).
120 Saha, D., Deng, S.G., “Accelerated formation of THF-H2clathrate hydrate in porous media”,, 26 (11), 8414-8418 (2010).
121 Kang, S.P., Lee, J.W., “Kinetic behaviors of CO2hydrates in porous media and effect of kinetic promoter on the formation kinetics”,..., 65 (5), 1840-1845 (2010).
122 Kang, S.P., Seo, Y., Jang, W., “Kinetics of methane and carbon dioxide hydrate formation in silica gel pores”,, 23 (7), 3711-3715 (2009).
123 Sun, C.Y., Chen, G.J., Ma, C.F., Huang, Q., Luo, H., Li, Q.P., “The growth kinetics of hydrate film on the surface of gas bubble suspended in water or aqueous surfactant solution”,, 306 (2), 491-499 (2007).
124 Ribeiro, C.P., Lage, P.L.C., “Modelling of hydrate formation kinetics: State-of-the-art and future directions”,..., 63 (8), 2007-2034 (2008).
125 Nada, H., “Anisotropy in growth kinetics of tetrahydrofuran clathrate hydrate: A molecular dynamics study”,..., 113 (14), 4790-4798 (2009).
126 Gayet, P., Dicharry, C., Marion, G., Graciaa, A., Lachaise, J., Nesteroy, A., “Experimental determination of methane hydrate dissociation curve up to 55 MPa by using a small amount of surfactants as hydrate promoter”,..., 60 (21), 5751-5758 (2005).
127 Zhang, J.S., Lee, S.Y., Lee, J.W., “Kinetics of methane hydrate formation from SDS solution”,...., 46 (19), 6353-6359 (2007).
128 Shimada, W., Ebinuma, T., Oyama, H., Kamata, Y., Narita. H., “Free-growth forms and growth kinetics of tetra--butyl ammonium bromide semi-clathrate hydrate crystals”,., 274 (1/2), 246-250 (2005).
129 Dalmazzone, D., Kharrat, M., Lachet, V., Fouconnier, B., Clausse, D., “DSC and PVT measurement methane and trichlorofluoromethane hydrate dissociation equilibria”,...., 70 (2), 493-505 (2002).
130 Dalmazzone, D., Hamed, N., Dalmazzone, C., Rousseau, L., “Application of high pressure DSC to the kinetics of formation of methane hydrate in water-in-oil emulsion”,...., 85 (2), 361-368 (2006).
131 Jakobsen, T., Folger?, K., “Dielectric measurements of gas hydrate formation in water-in-oil emulsions using open-ended coaxial probes”,..., 8 (9), 1006-1015 (1997).
132 Aichele, C.P., Chapman, W.G., Rhyne, L.D., Subramani, H.J., Montesi, A., Creek, J.L., House, W., “Nuclear magnetic resonance analysis of methane hydrate formation in water-in-oil emulsions”,, 23 (1), 835-841 (2009).
133 Boxall, J.A., Greaves, D.P., Mulligan, J., Koh, C.A., Sloan, E.D., “Gas hydrate formation and dissociation from water-in-oil emulsions studied using PVM and FBRM particle size analysis”, In: Proceedings of the 6th International Conference on Gas Hydrate, Vancouver, British Columbia, Canada (2008).
134 Palermo, T., Arla, D., Borregales, M., Dalmazzone, C., Rousseau, L., “Study of the agglomeration between hydrate particles in oil using differential scanning calorimetry (DSC)”, In: Proceedings of Fifth International Conference on Gas Hydrates, Trondheim, Norway, 1, 332-339 (2005).
135 Dalmazzone, D., Hamed, N., Dalmazzone, C., “DSC measurements and modelling of the kinetics of methane hydrate formation in water-in-oil emulsion”,..., 64 (9), 2020-2026 (2009).
136 Dalmazzone, D., Hamed, N., Clausse, D., Pezron, I., Luong, A.T., Dalmazzone, C., “Study of the kinetics of formation of trichlorofluoromethane hydrates and methane hydrates in water-in-oil emulsion by microcalorimetry”, In: Proceedings of the 6th International Conference on Gas Hydrate, Vancouver, British Columbia, Canada (2008).
137 Lee, J., Park, S., Sung, W., “An experimental study on the productivity of dissociated gas from gas hydrate by depressurization scheme”,, 51 (12), 2510-2515 (2010).
138 Zhou, Y., Castaldi, M.J., Yegulalp, T.M., “Experimental investigation of methane gas production from methane hydrate”,...., 48 (6), 3142-3149 (2009).
139 Oyama, H., Konno, Y., Masuda, Y., Narita, H., “Dependence of depressurization-induced dissociation of methane hydrate bearing laboratory cores on heat transfer”,, 23, 4995-5002 (2009).
140 Selim, M.S., Sloan, E.D., “Hydrate dissociation in sediments”,.., 5 (2), 245-251 (1990).
141 Kamata, Y., Ebinuma, T., Omura, R., Minagawa, H., Narita, H., Masuda, Y., Konno, Y., “Decomposition experiment of methane hydrate sediment by thermal recovery method”, In: Proceedings of the Fifth International Conference on Gas Hydrates, Trondheim, Norway, 81-85 (2005).
142 Li, X.S., Wan, L.H., Li, G., Li, Q.P., Chen, Z.Y., Yan, K.F., “Experimental investigation into the production behavior of methane hydrate in porous sediment with hot brine stimulation”,...., 47 (23), 9696-9702 (2008).
143 Linga, P., Haligva, C., Nam, S.C., Ripmeester, J.A., Englezos, P., “Recovery of methane from hydrate formed in a variable volume bed of silica sand particles”,, 23 (11), 5508-5516 (2009).
144 Lee, J., “Experimental study on the dissociation behavior and productivity of gas hydrate by brine injection scheme in porous rock”,, 24, 456-463 (2010).
145 Nihous, G.C., Kuroda, K., Lobos-Gonzalez, J.R., Kurasaki, R.J., Masutani, S.M., “An analysis of gas hydrate dissociation in the presence of thermodynamic inhibitors”,..., 65 (5), 1748-1761 (2010).
146 Moridis, G.J., Collett, T.S., Boswell, R., Kurihara, M., Reagan, M.T., Koh, C., Sloan, E.D., “Toward production from gas hydrates: current status, assessment of resources, and simulation-based evaluation of technology and potential”,., 12 (5), 745-771 (2009).
** To whom correspondence should be addressed. E-mail: gjchen@cup.edu.cn
2010-09-24,
2010-11-07.
the National Natural Science Foundation of China (20925623, 21076225), the National High Technology Research and Development Program of China (2007AA09Z311), the National Science & Technology Major Project (2008ZX05026-004-03), and the National Basic Research Program of China (2009CB219504).
 Chinese Journal of Chemical Engineering2011年1期
Chinese Journal of Chemical Engineering2011年1期
- Chinese Journal of Chemical Engineering的其它文章
- Dynamic Simulation and Analysis of Industrial Purified TerephthalicAcid Solvent Dehydration Process*
- Preparation of p-Hydroxybenzaldehyde by Hydrolysis of DiazoniumSalts Using Rotating Packed Bed*
- Liquid-solid Equilibria in Quinary System Na+, K+, Mg2+//Cl-,?at 25 °C*
- Pervaporation Separation of Butanol-Water Mixtures UsingPolydimethylsiloxane/Ceramic Composite Membrane*
- Reaction Kinetics of Biodiesel Synthesis from Waste Oil Using a Carbon-based Solid Acid Catalyst
- Enzyme-catalyzed Synthesis of Vitamin E Succinate Using aChemically Modified Novozym-435*
