Reaction Kinetics of Biodiesel Synthesis from Waste Oil Using a Carbon-based Solid Acid Catalyst
SHU Qing (舒慶), GAO Jixian (高繼賢), LIAO Yuhui (廖玉會) and WANG Jinfu (王金福),*
?
Reaction Kinetics of Biodiesel Synthesis from Waste Oil Using a Carbon-based Solid Acid Catalyst
SHU Qing (舒慶)1,2, GAO Jixian (高繼賢)1, LIAO Yuhui (廖玉會)1and WANG Jinfu (王金福)1,*
1Beijing Key Laboratory of Green Chemical Reaction Engineering and Technology, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China2School of Material and Chemical Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, China
The kinetics of simultaneous transesterification and esterification with a carbon-based solid acid catalyst was studied. Two solid acid catalysts were prepared by the sulfonation of carbonized vegetable oil asphalt and petroleum asphalt. These catalysts were characterized on the basis of elemental analysis, acidity site concentration, the Brunauer-Emmett-Teller (BET) surface area and pore size. The kinetic parameters with the two catalysts were determined, and the reaction system can be described as a pseudo homogeneous catalyzed reaction. All the forward and reverse reactions follow second order kinetics. The calculated concentration values from the kinetic equations are in good agreement with experimental values.
biodiesel, carbon-based solid acid catalyst, heterogeneous catalysis, simultaneous transesterification and esterification reaction, kinetics
1 INTRODUCTION
Due to the concerns for the pending shortage of fossil fuels and environment, liquid fuels of agricultural origin are being increasingly considered as alternatives to gasoline. In this respect, biodiesel (fatty acid methyl ester, FAME) is becoming popular around the world because it is an excellent substitute for diesel. Biodiesel can be prepared from the transesterification of triglycerides (the main component of vegetable oils or animal fats) or the esterification of free fatty acid (FFA) with methanol [1-4].
A wide variety of vegetable oils,.., soybean oil and rapeseed oil, can be used as the raw material for production of biodiesel. However, in China, there is a consumption need of approximately 22 million tonnes of edible oils annually (50% has to be imported), so vegetable oils are not favored as a feedstock. Only waste oils, such as used frying oil, trap grease and soap stock (byproduct of vegetable oil refineries), which are available cheaply, should be considered as feedstock for biodiesel [5-8]. However, waste oils are rich in FFAs. The base-catalyzed method is not suitable for these waste oils because soap is produced from the reaction of an FFA with a base catalyst. The formation of soap not only consumes the catalyst, but also causes the emulsification of FAME and glycerol (byproduct of biodiesel), which would make the separation of FAME-glycerol mixtures difficult. A homogeneous acid (H2SO4) shows a better performance with FFAs than the base catalysts, and it can simultaneously catalyze esterification and transesterification [9]. However, it suffers from several drawbacks, such as equipment corrosion and the waste from the neutralization of H2SO4. The use of a heterogeneous acid catalyst may solve many problems associated with homogeneous acid catalysts.
In recent years, the sulfonation of incompletely carbonized polymers (such as naphthalene) and carbon material (such as carbon nanotube) to synthesize carbon- based solid acid catalysts has received more attention. The carbon-based solid acid catalyst is reported as promising catalyst for the production of biodiesel [10-14]. However, the studies were focused on the influence of the variables in the synthesis of biodiesel from triglyceride transesterification or FFA esterification. There are a few attempts to develop kinetic models for the transesterification or esterification reaction [15-19], but the kinetic model for simultaneous transesterification and esterification catalyzed by a heterogeneous acid catalyst has not been reported. In order to design a suitable reactor, it is necessary to know the kinetics of ester production. Our group has reported the kinetics of the transesterification of cottonseed oil with methanol with a homogeneous base catalyst (KOH), in which the forward and reverse reactions follow second order kinetics. The calculated concentrations of feeds at different compositions are in good agreement with experimental data [20]. Based on the above study, we assume here that the forward and reverse reactions of the simultaneous transesterification and esterification also follow pseudo-second order kinetics with a heterogeneous acid catalyst, and develop a kinetic model.
In this work, we use two carbon-based solid acid catalysts, namely, catalysts from the sulfonation of an incompletely carbonized vegetable oil asphalt V-C-600-S-210 and petroleum asphalt P-C-750-S-210 [in the code V(P)-C-M-S-N, V is the vegetable oil asphalt, P is the petroleum asphalt, C is the carbonization, M is the carbonization temperature, S is the sulfonation, and N is the sulfonation temperature]. The kinetics with these two catalysts is studied when model waste oil is used to prepare biodiesel. The rate constants of the forward and reverse reactions and the activation energies and pre-exponential factors are obtained. It is expected that the study provides the theoretical basis and basic data for the research and design of an industrial reactor.
2 KINETIC MODEL
2.1 Reaction mechanism
The reaction scheme for the methanolysis of model waste oil is presented in Fig. 1. For the transesterification reaction, the reaction stoichiometry requires 3 mol of methanol (M) and 1 mol of triglyceride (TG) to give 3 mol of FAME (ME) and 1 mol of glycerol (GL). This reaction actually comprises three consecutive reversible reactions, where 1 mol of ME is produced in each step and monoglycerides (MG) and diglycerides (DG) are intermediate products. For the esterification reaction, the reaction stoichiometry requires 1 mol of methanol (M) and 1 mol of RCOOH (FFA) to give 1 mol of FAME (ME) and 1 mol of H2O.1,3,5and7are the forward rate constants and2,4,6and8are the reverse rate constants. The model waste oil feed and methanol are immiscible, so the reaction system consists of two layers in the initial stage. Once the reaction is started, since the reaction is carried out at high pressure with stirring, the miscibility of the oil feed and methanol is greatly improved. Thus, although the mass transfer controls the kinetics at the beginning, the effect can be neglected after the reaction starts, due to a large amount of FAME produced. FAME acts as a co-solvent because it is soluble in the oil and methanol. Therefore, the system can be treated as pseudo-homogeneous, where the chemical reaction controls the kinetics.
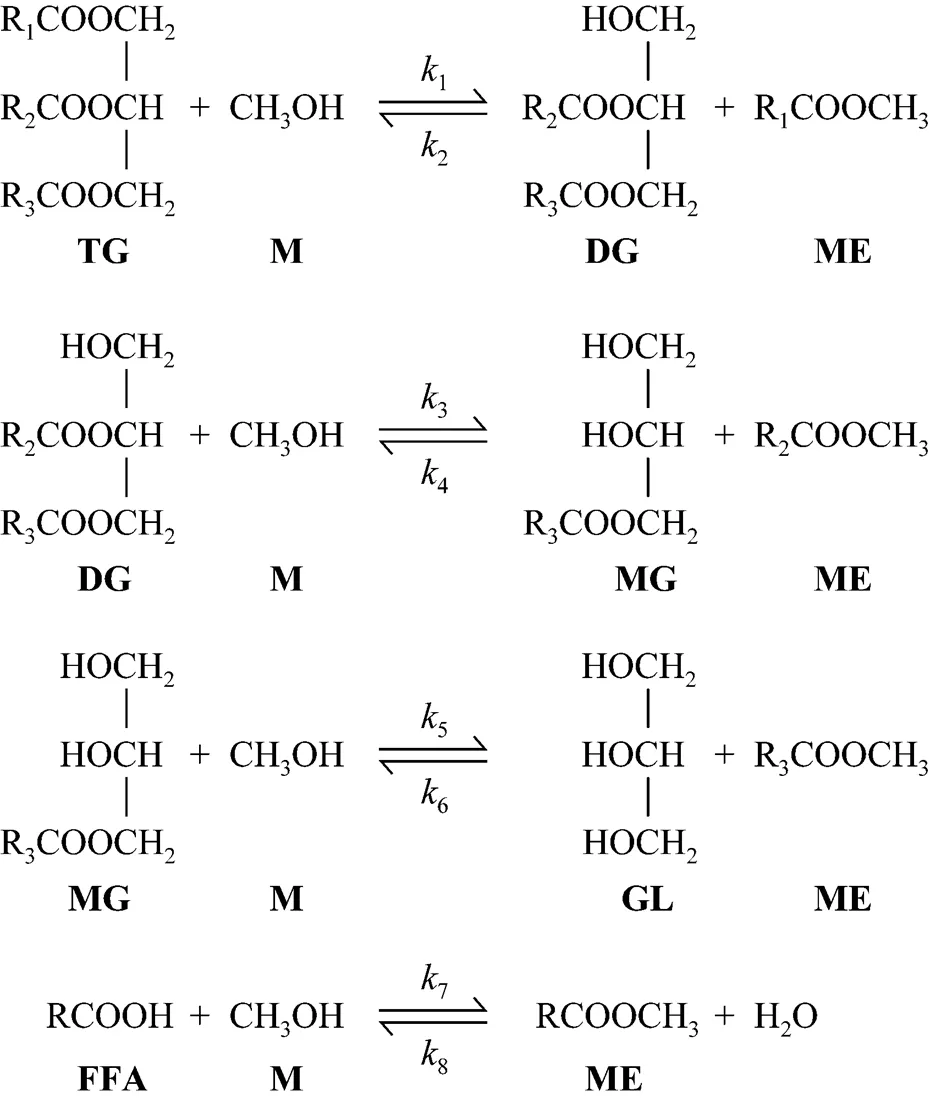
Figure 1 The reaction scheme of simultaneous transesterification and esterification
2.2 Mathematical analysis
In the proposed kinetic model, we make following assumptions. (1) The chemical reactions control the reaction rate and the catalyst particle size remains constant in the reaction. (2) Cottonseed oil is a mixture of triglycerides, mainly triglycerides of palmitic acid, oleic acid and linoleic acid, and all of the different isomers have the same reaction rate and reaction mechanism. (3) The catalyst concentration is constant, and the forward and reverse reaction rates follow the law of mass action. (4) The reaction rates of the non-catalyzed reactions are negligible compared to the catalyzed ones. (5) Both the forward and reverse reactions follow pseudo-second order kinetics in the liquid phase. From the five assumptions and the reaction scheme, the differential equations governing each component are as follows.
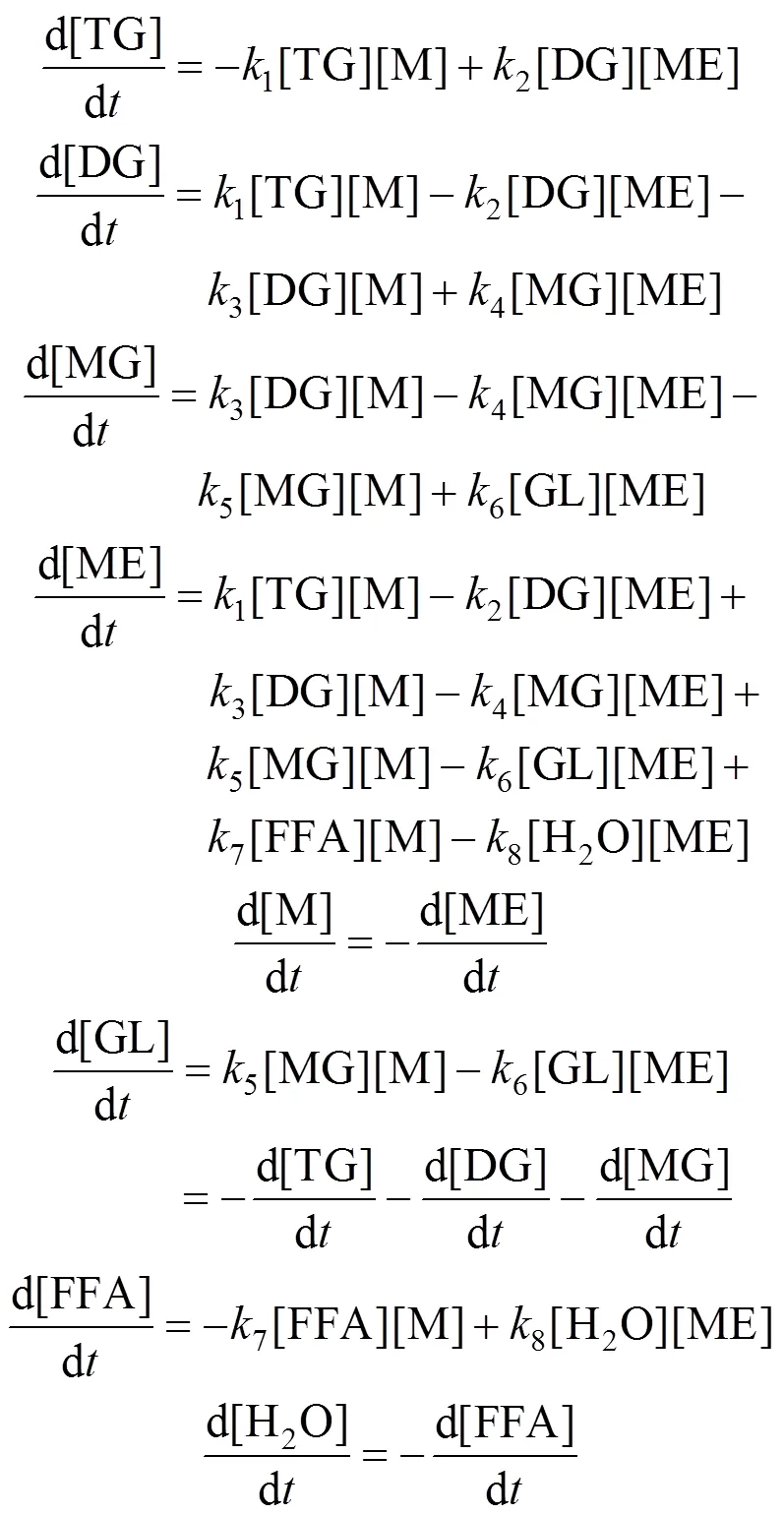
The initial concentrations of DG, MG, GL, ME and H2O are zero. Hence, the mole balance conditions are
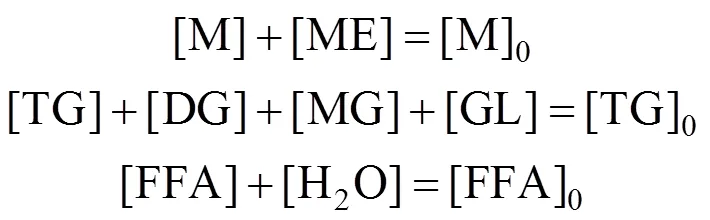
The kinetic parameters are determined by a nonlinear regression program, which iteratively adjusted these parameters until a predefined criterion is satisfied. The criterion is the minimization of the objective function
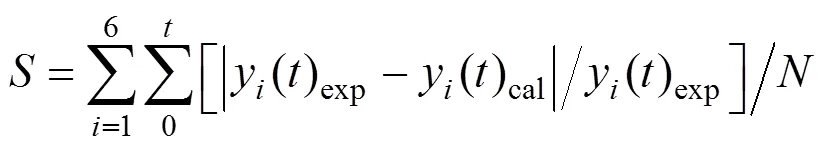
wherey() is the concentration of componentat time,is the total number of the experimental concentrations of component, andis the average relative error.
An iteration of the optimization search is performed as follows. First, the reaction system Eq. (1) is integrated with time with a fourth-order Runge-Kutta algorithm. Second, the rate constants at each temperature are obtained. Third, the pre-exponential factors and activation energies are obtained by plotting the logarithm of the rate constants versus the reciprocal of absolute temperature using the Arrhenius equation
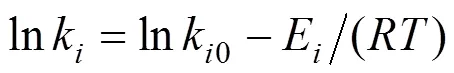
wherekis the reaction constant,k0is the frequency or pre-exponential factor,Eis the activation energy of the reaction,is the gas constant, andis the absolute temperature.
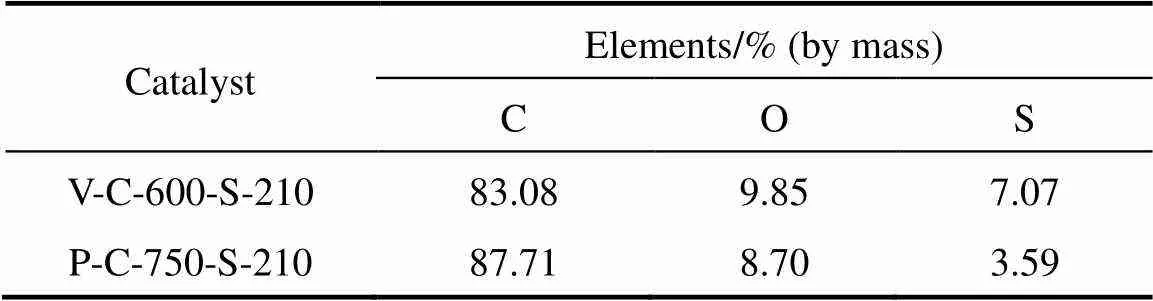
Table 1 Elements present in the carbon-based solid acid catalysts
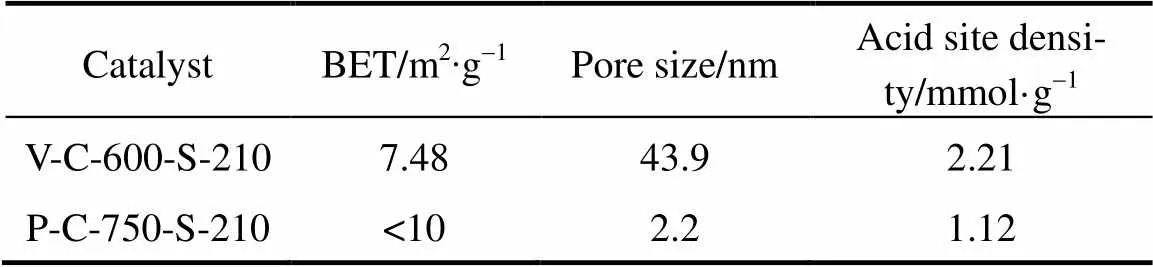
Table 2 BET, pore size and acid site density of thecarbon-based solid acid catalysts
3 EXPERIMENTAL
3.1 Preparation of catalyst
The carbon-based solid acid catalyst precursors were obtained by carbonizing vegetable oil asphalt and petroleum asphalt. The vegetable oil asphalt from a biodiesel plant (Linyi Qingda New Energy Co., Ltd, China) was pretreated to remove water and residual esters. The pretreatment process was carried out as follows. Residual esters were converted to methyl ester, and then the methyl ester and water were removed by reduced pressure distillation. Batches of 10 g of extracted vegetable oil asphalt or petroleum asphalt were oxidized for 1 h at 280°C in a stream of air (300 ml·min-1). They were heated to 500-700°C at a rate of 2°C·min-1under an argon atmosphere (100 ml·min-1).
The sulfonation of the carbon precursors was performed as follows. 5 g carbonized vegetable oil asphalt (or petroleum asphalt) and 100 ml concentrated H2SO4(96%) solution were put into a 250 ml flask controlled at 210°C in an oil bath. It was kept under reflux and agitation for 10 h. After the treatment, the suspension was washed with hot deionized water (>353 K) to remove any physically adsorbed species until sulfate ions were no longer detected in the filtration water [sulfate ions were detected with 6 mol?L-1Ba(NO3)2solution]. After the filtration, the samples were dried at 120 °C under vacuum for 4 h to obtain the sulfonated vegetable oil asphalt or petroleum asphalt catalyst.
3.2 Characterization of catalyst
The elements present in the samples (O, C and S) from energy dispersive spectroscopy (EDS) characterization are given in Table 1. The Brunauer-Emmett- Teller (BET) surface area and pore size were measured by the multipoint N2adsorption-desorption method at liquid nitrogen temperature (-196°C). The pore size distribution of the catalyst was calculated by the method of Barrett-Joyner-Hallenda (BJH) pore size analysis. In order to estimate the acid site density, the sulfur content in the vegetable oil asphalt catalyst and petroleum asphalt catalyst was used. The BET, pore size and acid site density of different carbon-based solid acid catalysts are given in Table 2.
3.3 Catalytic reaction procedure
In industrial production, waste vegetable oils used as feedstock include rapeseed, cottonseed and soybean acidified oils (derived from soap stocks by acidification). In this work, the mixed oil was used as the model feed. It was made by adding 50% (by mass) oleic acid (a common type of FFA in oils) to refined cottonseed oil. The required molar ratio of methanol to mixed oil was calculated by treating 3 mol of FFA as 1 mol of triglyceride. The reaction was carried out in a 250 ml autoclave equipped with a magnetic stirrer. The mixed oil and a known amount of catalyst were charged into the reactor. When the required temperature was reached, methanol was added into the reactor by a pump. The reaction was started with stirring (at 240 r·min-1). The simultaneous transesterification and esterification were stopped after 6 h.
3.4 Analysis of product
Samples for analysis were taken at different time. A high performance liquid chromatograph (HPLC, Shimadzu LC-10A) equipped with an ultraviolet photometric detector was used for analyzing the samples. A spherisorb ODS 2 column (250 mm′4.6 mm, 8 nm pore size and 5 μm particle size) was used for the separation. The mobile phase was a mixture of acetone and acetonitrile in the volumetric ratio of 50︰50. The flow rate of the mobile phase was 1.0 ml·min-1. The column temperature was 40 °C. The components measured by HPLC included oleic acid, methyl esters, triglyceride, diglyceride and monoglyceride. Standard samples were used to establish the calibration charts, which were used to calculate the weight percentage of the individual components by the integration of the peak areas using the external standard method.
3.5 Mathematical treatment
The calculation of the rate constants needs the solution of differential equation system Eq. (1). We treat this equation system as the one where the solution changes abruptly in a small part of the whole integration interval, while it changes very little in the rest of the interval. The optimization is based on the simulation of the differential equation system starting with different values for the rate constants. The methodology consists of the following steps.
(1) Selection of the rate constant ranges. This is based on the rate constant values found in the bibliography for similar reactions and also based on our previous experiments. (2) Simulation. Differential equation system Eq. (1) is solved for all the possible combinations of the rate constants. In each range, the values for the rate constants are varied to four decimal points. (3) Comparison of the calculated concentrations and experimental values and calculation of the average relative error. (4) Selection of the solution with the best combination of reaction constants. It is the one with the smallest average relative error. (5) Graphical verification of the kinetic model and experimental results.
4 Results and discussion
4.1 Effect of particle size and stirring speed
The particle size distribution of carbon-based solid acid catalyst was within the range of 10-163 μm. The particles with different sizes were separated using standard sieves (160 to 60 μm), and the effect of particle size on the conversion of oleic acid was studied. For a specified catalyst loading, no effect was observed for the variation in the particle size from 60-160 μm, so that the intra-particle diffusion resistance of the reactant in the carbon-based solid acid catalyst was insignificant. Hence, all further experiments were conducted directly with the carbon-based solid acid catalyst without any size screening.
Preliminary experiments were also conducted with different stirring speeds to evaluate the external resistances to heat and mass transfer. These experiments showed that the effect of agitation speed on the overall rate of reaction was little in the range of 180-300 r·min-1. Hence, all further experiments were conducted at a stirring speed of 240 r·min-1to eliminate the external mass transfer resistance.
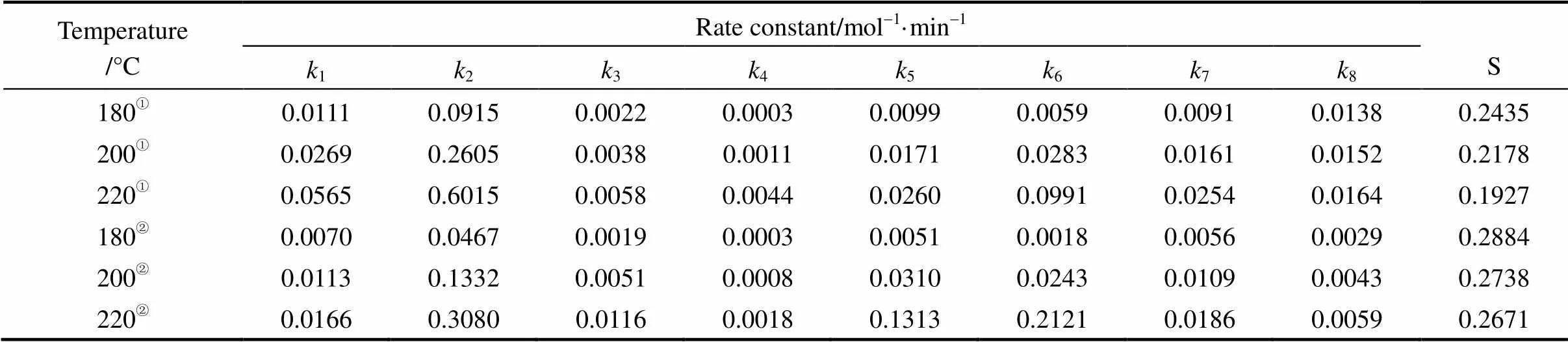
Table 3 Rate constant (ki) and average relative error (S) of V-C-600-S-210 and P-C-750-S-210
① V-C-600-S-210; ② P-C-750-S-210.
4.2 Calculation of the kinetics parameters
Both FFA and triglyceride initially require the activation of their respective carboxylic/carbonyl functions by protonation (under acid-catalyzed conditions) to start the reaction. The large alkyl chains in a bulky triglyceride molecule can interfere with the activation of its carbonyl group. Hence, the triglyceride is more difficult to activate than the FFA. In order to promote methanol nucleophilic attack on the triglyceride, a comparatively high reaction temperature was needed to activate its carbonyl group. Thus, the experiments using the asphalt based carbon catalyst (V-C-600-S-210 and P-C-750-S-210) were conducted at 180, 200 and 220°C. Other experimental conditions were: mixed oil feed (50% cottonseed oil and 50% oleic acid), catalyst (V-C-600-S-210)/mixed oil mass ratio 0.5% and molar ratio of methanol/mixed oil 21, and the stirring speed of 240 r·min-1.
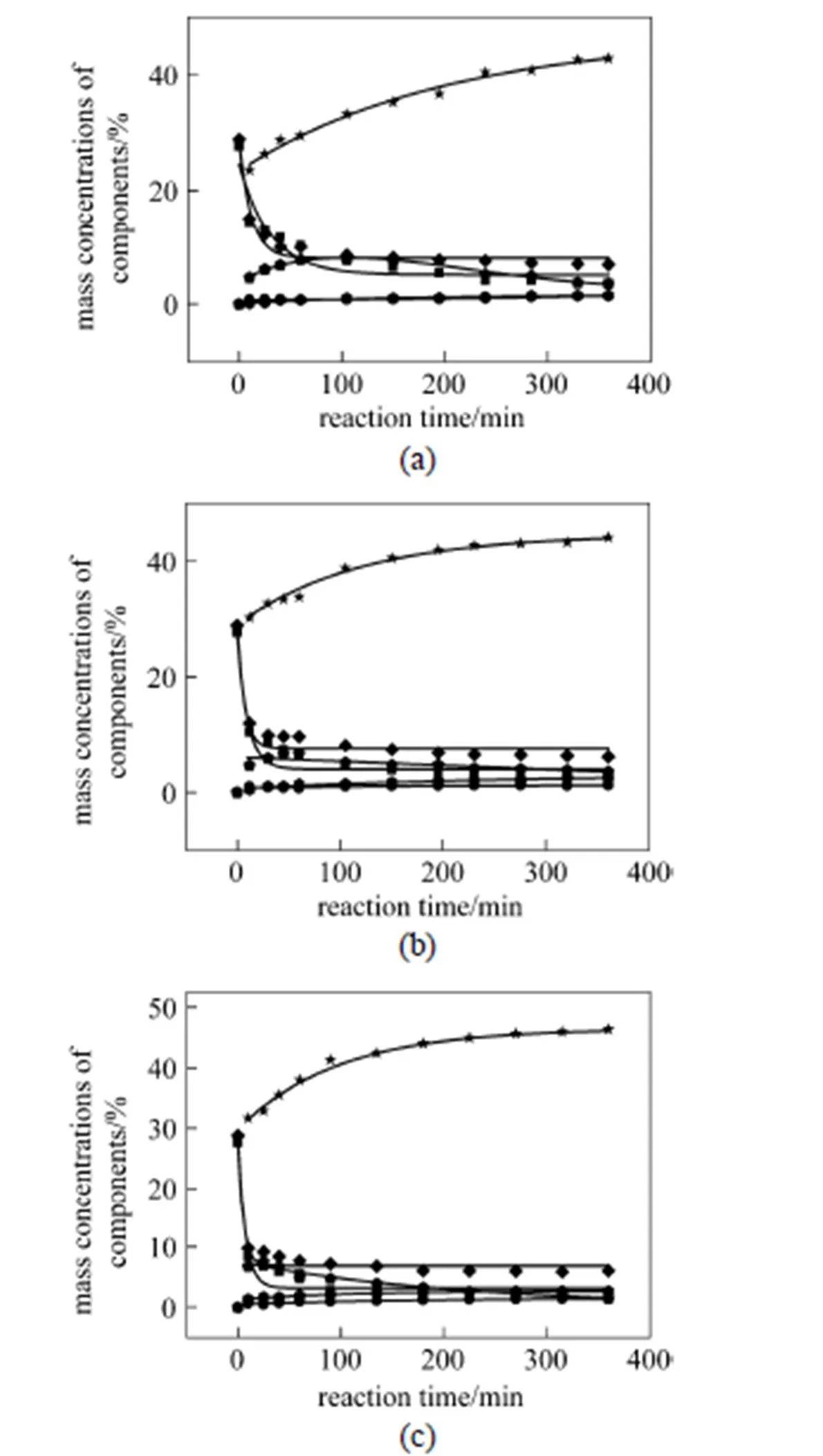
Figure 2 Kinetic modeling curves and experimental points for concentrations of components
[Reaction conditions: mixed oil feed (50% cottonseed oil and 50% oleic acid), 21 mole ratio of methanol to mixed oil, 0.5% mass ratio of the catalyst (V-C-600-S-210) to mixed oil, reaction temperature (a: 180 °C; b: 200 °C; c: 220 °C) and 240 r·min-1]

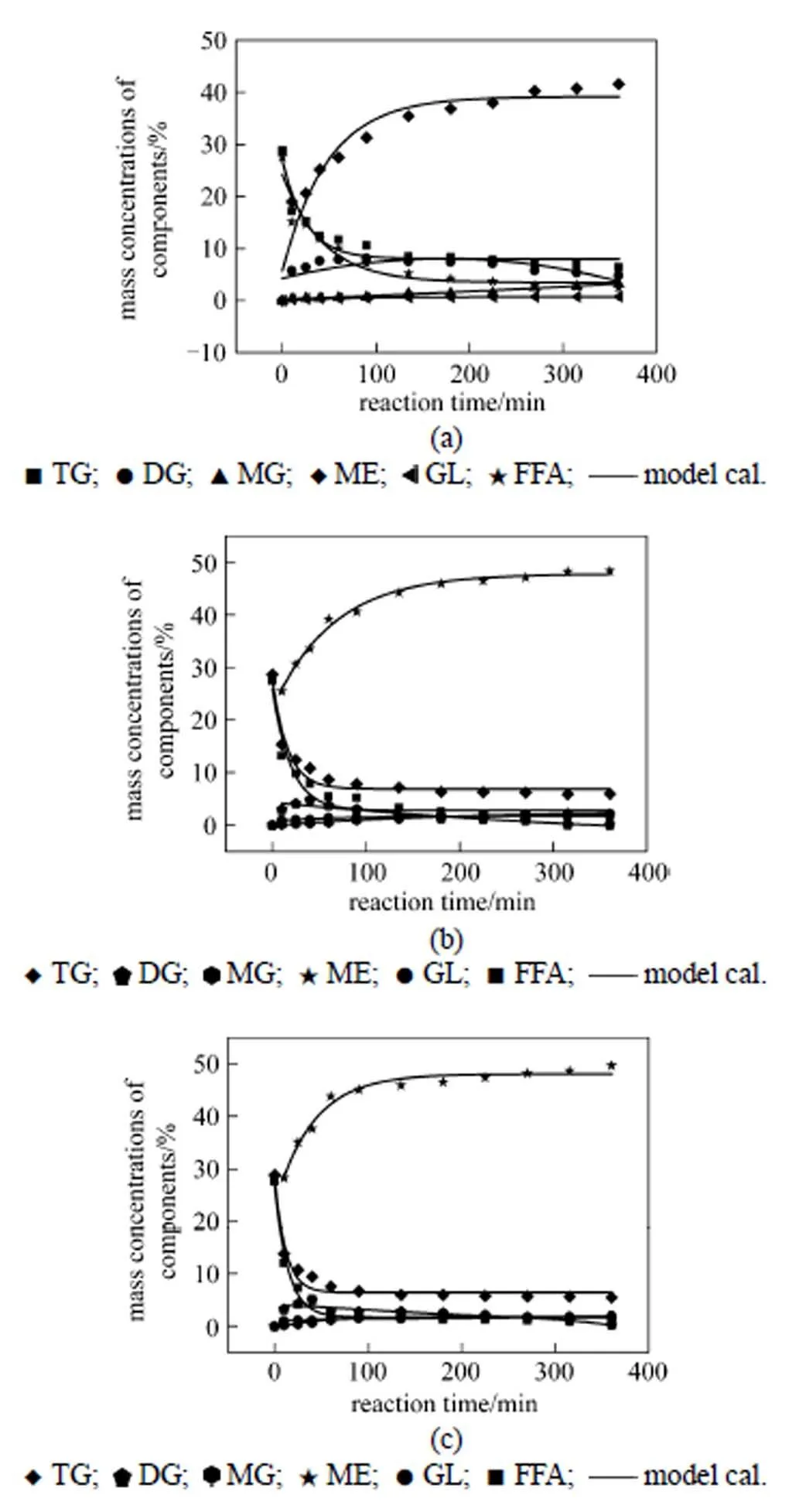
Figure 3 Kinetic modeling curves and experimental points for concentrations of components
[Reaction conditions: mixed oil feed (50% cottonseed oil and 50% oleic acid), 21 mole ratio of methanol to mixed oil, 0.5% mass ratio of the catalyst (P-C-750-S-210) to mixed oil, reaction temperature (a: 180 °C; b: 200 °C; c: 220 °C) and 240 r·min-1]
A computerized kinetics program, described previously, was used to determine whether the proposed kinetic equations were reasonable. The program required a specific kinetic scheme for each reaction studied. The reactions studied are shown in Fig. 1. Additional inputs were the concentration of each component versus time. The program used the data to produce plots of concentration versus time, which are shown in Figs. 2 and 3. A separate plot is produced for each temperature. Based on the kinetic scheme being tested, the program will draw a line through the points. A close fit of the line to the points indicates an adequate kinetic scheme, from which a reaction order can be obtained. On the other hand, a poor fit of the line to the points indicates an incorrect scheme.
The calculated results for the reaction rate constants are summarized in Table 3.1is much smaller than2, and3is much larger than4(at all the reaction temperatures studied), which will result in low concentration of DG in the entire reaction process. It should be noted that5is much smaller than6at higher reaction temperature, which will lead high concentration of MG but it is not beneficial to the formation of ME.7is larger than8, so it is beneficial to the formation of ME from the esterification.
The activation energy and pre-exponential factor of each consecutive reaction was obtained by plotting the logarithm of the calculated rate constant (ln) versus-1. The calculated results of the activation energy and pre-exponential factor are summarized in Table 4. The activation energy (E) of the esterification is much lower than that of the transesterification, so the esterification is easier to occur when both reactions are carried out at a low reaction temperature. Furthermore, the formation of FAME from the tranesterification will be reduced since so much FAME is formed from the esterification. The reaction rate constants and differential equation system Eq. (1) are combined to calculate the concentration of each component at different reaction time, and the conversions of reactants are calculated. The proposed model is assessed by comparing the calculated values for each composition with experimental values. The comparison results are shown in Figs. 2 and 3, demonstrating that the proposed kinetic model describes the experimental results well.
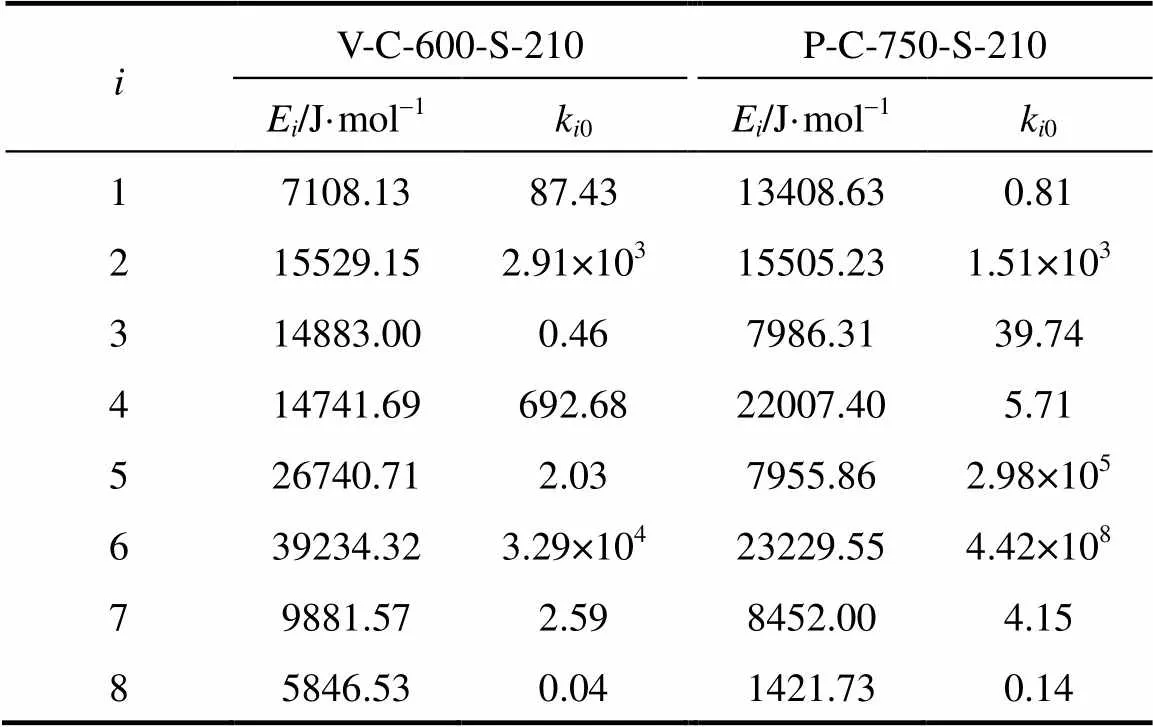
Table 4 Activation energy (Ei) and pre-exponential factor (ki0) of V-C-600-S-210 and P-C-750-S-210
In this work, we report the kinetic parameters for the carbon-based solid acid-catalyzed simultaneous transesterification and esterification reaction of waste oil with methanol. Two carbon-based solid acid catalysts prepared by the sulfonation of carbonized vegetable oil asphalt and petroleum asphalt were used in this study. A four-consecutive reversible-reaction model was developed to reflect the reaction mechanism and simplify the reaction model. Based on the proposed kinetic model, the reaction was described as a pseudo homogeneous reaction and the kinetic parameters with these two catalysts were determined. The experiments demonstrate that all the forward and reverse reactions follow second order kinetics. The proposed kinetic model describes the experimental results well.
The rate constants follow the Arrhenius equation. The concentration of MG is high in the entire reaction process, which is not beneficial to the formation of ME. The activation energy of the esterification is much lower than that of the tranesterification. Taking into account the above reaction characteristics, the coupling of reaction and separation can be applied to improve the conversion of waste oil. This study may provide some useful information for the research and design of an industrial reactor to produce biodiesel from waste oils with higher energy efficiency.
NOMENCLATURE
Eactivation energy, J·mol-1
kreaction constant, mol-1·min-1
k0pre-exponential factor
total number of experimental concentrations of component
gas constant
average relative error
absolute temperature, K
y() concentration of componentat, mol·L-1
1 Sharma, Y.C., Singh, B., Upadhyay, S.N., “Advancements in development and characterization of biodiesel: A review”,, 87, 2355-2373 (2008).
2 Gerpen, J.V., “Biodiesel processing and production”,..., 86, 1097-1107 (2005).
3 Shu, Q., Zhang, Q., Xu, G.H., Nawaz, Z., Wang, D.Z., Wang, J.F., “Synthesis of biodiesel from cottonseed oil and methanol using a carbon-based solid acid catalyst”,..., 90, 1002-1008 (2009).
4 Vicente, G., Martinez, M., Aracil, J.A., “Comparative study of vegetable oils for biodiesel production in spain”,.., 20, 394-398 (2006).
5 Shu, Q., Nawaz, Z., Gao, J.X., Liao, Y.H., Zhang, Q., Wang, D.Z., Wang, J. F., “Synthesis of biodiesel from waste oil feedstocks using a carbon-based solid acid catalyst: Reaction and separation”,.., 101, 5374-5384 (2010).
6 Han, M.H., Yi, W.L., Wu, Q., Liu, Y., Hong, Y.C., Wang D.Z., “Preparation of biodiesel from waste oils catalyzed by a Br?nsted acidic ionic liquid”,.., 100, 2308-2310 (2009).
7 Zhang, J.J., Jiang, L.F., “Acid-catalyzed esterification of zanthoxylum bungeanum seed oil with high free fatty acids for biodiesel production”,.., 99, 8995-8998 (2008).
8 Felizardo, P., Correia, M.J.N., Raposo, I., “Production of biodiesel from waste frying oils”,.., 26, 487-494 (2006).
9 Lotero, E., Liu, Y.J., Lopez, D.E., Suwannakarn, K., Bruce, D.A., Goodwin, J.G., “Synthesis of biodieselacid catalysis”,...., 44, 5353-5363 (2005).
10 Toda, M., Takagaki, A., Okamura, M., “Biodiesel made with sugar catalyst”,, 438, 178 (2005).
11 Takagaki, A., Toda, M., Okamura, M., “Esterification of higher fatty acids by a novel strong solid acid”,., 116, 157-167 (2006).
12 Mo, X.H., Lotero, E., Lu, C.Q., “A novel sulfonated carbon composite solid acid catalyst for biodiesel synthesis”,.., 123, 1-6 (2008).
13 Shu, Q., zhang, Q., Xu, G.H., Wang, D.Z., Wang, J.F., “Preparation of biodiesel using s-MWCNT catalysts and the coupling of reaction and separation”,..., 87, 164-170 (2009).
14 Zong, M.H., Duan, Z.Q., Lou, W.Y., Smith, T.J., Wu, H., “Preparation of a sugar catalyst and its use for highly efficient production of biodiesel”,.., 9, 434-437 (2007).
15 Minamia, E., Saka, S., “Kinetics of hydrolysis and methyl esterification for biodiesel production in two-step supercritical methanol process”,, 85, 2479-2483 (2006).
16 Tesser, R., Serio, M.D., Guida, M., “Kinetics of oleic acid esterification with methanol in the presence of triglycerides”,...., 44, 7978-7982 (2005).
17 Freedman, B., Butterfield, R.O., Pryde, E.H., “Transesterification kinetics of soybean oil”,....., 63, 1375-1380 (1986).
18 Noureddini, H., Zhu, D., “Kinetics of soybean oil”,....., 74, 1457-1463 (1997).
19 Gan, M.Y., Pan, D., Ma, L., Yue, E., Hong, J.B., “The kinetics of the esterification of free fatty acids in waste cooking oil using Fe2(SO4)3/C catalyst”,...., 17, 83-87 (2009).
20 Chen, H., Wang, J.F., “Kinetics of KOH catalyzed tranesterification of cottonseed oil for biodiesel production”,.... (), 56, 1971-1974 (2005). (in Chinese)
* To whom correspondence should be addressed. E-mail: wangjf@flotu.org
2010-06-21,
2010-12-01.
 Chinese Journal of Chemical Engineering2011年1期
Chinese Journal of Chemical Engineering2011年1期
- Chinese Journal of Chemical Engineering的其它文章
- Dynamic Simulation and Analysis of Industrial Purified TerephthalicAcid Solvent Dehydration Process*
- Preparation of p-Hydroxybenzaldehyde by Hydrolysis of DiazoniumSalts Using Rotating Packed Bed*
- Liquid-solid Equilibria in Quinary System Na+, K+, Mg2+//Cl-,?at 25 °C*
- Pervaporation Separation of Butanol-Water Mixtures UsingPolydimethylsiloxane/Ceramic Composite Membrane*
- Enzyme-catalyzed Synthesis of Vitamin E Succinate Using aChemically Modified Novozym-435*
- Alkylation of p-Cresol with tert-Butanol Catalyzed by NovelMultiple-SO3H Functioned Ionic Liquid*
