Biocontrol Efficiency of Bacillus subtilis SL-13 and Characterization of an Antifungal Chitinase*
LIU Yan (劉燕), TAO Jing (陶晶), YAN Yujun (閻豫君), LI Bin (李彬), LI Hui (李暉) and LI Chun (李春),,**
?
Biocontrol Efficiency ofSL-13 and Characterization of an Antifungal Chitinase*
LIU Yan (劉燕)1, TAO Jing (陶晶)2, YAN Yujun (閻豫君)2, LI Bin (李彬)1, LI Hui (李暉)2and LI Chun (李春)1,2,**
1School of Life Science & Technology, Beijing Institute of Technology, Beijing 100081, China2School of Agriculture/Key Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan, Shihezi University, Xinjiang 832003, China
The seed germination and tomato seedling tests showed thatSL-13 could promote the sprouting and seedling growth of tomato. The fresh and dry weight of tomato seedlings increased 42.86% and 18.75%, respectively. The control efficacies of the SL-13 to tomatorot were 20.65% and 35.23% in the greenhouse and field, respectively. The growth of the plant-pathogenic funguswas considerably inhibited in the presence of the strain SL-13 culture supernatant. The main antifungal protein was detected to be chitinase through vitro assay. The chitinase was purified with DEAE-Sepharose fast flow ion exchange column chromatography and Sephadex G-75 gel filtration for further characterization. The optimal pH and temperature for the chitinase activity were 7.0 and 50°C, respectively. It was demonstrated that the enzyme was stable at pH 5-9 and 40-60°C. 70% of the enzyme activity was retained when incubated at 121°C and 0.11 MPa for 20 min, and the enzyme was not sensitive to protease K and ultraviolet radiation. Thus it is suitable for effective biological control in relatively unstable environment.
, growth-promoting, diseases control, antifungal chitinase, purification,
1 Introduction
Biological control, using microorganisms to repress plant diseases, offers an environmentally friendly strategy for controlling agricultural phytopathogens. It has been studied for more than 70 years and is becoming a realistic alternative to chemical treatments that are still widely used to control diseases caused by plant pathogens. The emergence of fungicide-resistant strains and deregistration of fungicides may provide non-chemical methods especially biocontrol methods [1].species are widely used in the biocontrol of plant diseases for more than 50 years, because they have a well developed secretary system producing structurally diverse secondary metabolites such as glucanase, protease inhibitors, ribosome inactivating proteins, chitinase and chitinase-like proteins with a wide spectrum of antibiotic activity [2, 3]. However, little is known about the antifungal activity of these secreted proteins.
Chitin, an insoluble linear-1,4--acetylglucosamine polymer, is the second most abundant polysaccharide in nature [4]. It is a major component of most fungal cell walls, insect exoskeletons, and the shells of crustaceans [5]. Chitin is degraded by chitinase, which are produced by higher plants, vertebrates, and bacteria [6-8]. The enzymes found in various bacteria, fungi, insects, plants, and animals are involved in natural protection mechanism [9-11]. It is considered that bacteria produce chitinase to degrade chitin primarily to provide carbon and nitrogen as nutrients [6, 12].
We have succeeded in isolating a.strain SL-13 from Xinjiang region of China to control., a notorious plant pathogen. In this study, the influence of strain SL-13 on tomato seedling growth and the antifungal activity of SL-13 against pathogen.are assessed in greenhouse and field bioassays. The isolation, characterization, and antifungal activity of a novel antifungal chitinase secreted in.SL-13 and its beneficial role in ecosystems are investigated.
2 Materials and methods
2.1 Microorganism and medium
.SL-13 strain (GenBank accession number EF508705) was isolated from processed tomato fields of Xinjiang and the pathogenic fungus.used in this study was kindly provided by Shihezi University. For fermentation, the seed medium contains 0.5% beef extract, 0.5% yeast extract, 1% peptone, 0.5% NaCl, and 1% C6H6O6, with pH 7.0; the fermentation medium contains 0.5% beef extract, 0.5% yeast extract, 1% peptone, 0.5% NaCl, and 1% colloidal chitin, with pH 7.0 [13, 14]. The.was cultivated in potato dextrose agar (PDA).
2.2 Enzyme production and purification
.SL-13 was cultivated in 50 ml shaking flasks for 36 h. 10% of the seed culture was transferred into the fermentation medium in 250 ml flasks and grown at 30°C for 36-48 h in the shaker (170 r·min-1) [15-17]. The culture supernatant was centrifuged for 10 min at 10000×(4°C) and used for enzyme purification. Ammonium sulfate was added to the supernatant at 80% saturation. The solution was kept overnight at 4°C. After centrifugation for 10 min at 11000×(4°C), the precipitate was collected and dialyzed against 0.02 mol·L-1sodium phosphate buffer with pH 6.8 (molecular cut off 20000) to obtain crude chitinase. The enzyme mixture (2 ml) was loaded to DEAE-Sepharose Fast Flow (bed: 1.6 mm′300 mm) equilibrated with the 0.02 mol·L-1sodium phosphate buffer [18]. The enzyme was eluted with a linear gradient of 0-0.5 mol·L-1NaCl in the 0.02 mol·L-1sodium phosphate buffer of pH 6.8 at a flow rate of 2 ml×min-1. The active fractions were pooled and concentrated by vacuum freeze-drying. The concentrated active fractions (2 ml) were separated by Sephadex G-75 column (1.6 mm′700 mm) chromatography with the 0.02 mol·L-1sodium phosphate buffer at a flow rate of 1.5 ml×min-1[19, 20]. The SDS-PAGE was used to confirm the molecular mass of enzyme.
2.3 Evaluation of growth promotion and disease control efficacy of SL-13
2.3.1
The tomato seeds were treated with sterile medium and.SL-13 (108-109cfu×ml-1). The treated seeds were sown in Styrofoam trays with 128 cavities per tray. In each treatment, one tray was filled with sterile soil from the field and 60 cavities were maintained. Treatments were arranged in a greenhouse maintained at (25±2)°C with natural light and (20±2)°C at night. During the growing period, plants were watered regularly. Two weeks later, the germination rate, lengths of shoot and root, and mass of fresh plant were recorded by randomly selecting 20 cavities per treatment. After five weeks, 20 randomly selected seedlings per treatment were harvested, and the height of plant, mass of fresh and dry plants were measured [21].
2.3.2
In the glasshouse, the sieved sterile field soil was placed in clean plastic pots (9 cm′9.5 cm). The seeds were first surface sterilized with sodium hypochlorite (1%) and then treated with sterile medium and.SL-13 (5′1010cfu×g-1) according to the treatment requirement..was added except for the control. Ten seeds were sown in each pot and watered periodically. There were three controls: CK1, seeds treated with sterile medium and pathogen.inoculated in soil; CK2, with the medium and in sterile soil; CK3, with pentachloronitrobenzene (PCNB) and in soil. Three replications were used for each treatment. The design adopted for the experiment was a completely randomized design (CRD). Germination rate was recorded after sowing. The shoot length, the percentage of diseased plants, and the incidence of disease were recorded on the 25th day [21, 22].
Field experiments were carried out at the experimental research station of agricultural college of Shihezi University, using processed tomato as test plant during the year 2006-2007. The soil at the site was a sandy loam. The field was 2.0-2.5 m plots, with rows 30 cm apart in each plot. The seeds treated with.SL-13 were sowed in each row with a distance of 15 cm between them..was added in all the treatments. There were three controls, in which the seeds were treated by: CK1, the medium; CK2, pesticide Vitavax (Uniroyal Chemical Company); CK3, pesticide PCNB. Three replicates were used for each treatment. The Statistical Program for Social Sciences (SPSS) was used to analyze the data. The one-way analysis of variance (ANOVA) was used to examine the differences between the treated group and control.
The design of experiment was a randomized complete block design (RCBD). After sowing, germination rate was recorded on the 15th day, the percentage of diseased plants and the incidence of disease were recorded on the 30th day, the crop was harvested on the 45th day, with the plant height and fresh mass recorded. Dry mass of plant samples were recorded after drying to constant mass in an oven at 80°C.
2.4 Measurement of antifungal activity
The antifungal activity of the supernatant was estimated through a growth inhibition assay..grew on PDA at 30°C for 5 days and the fungi were divided into several disks (0.9 cm diameter) [23]. Each.disk was placed in the center of a plate containing PDA medium, in which colloidal chitin was added for detection of hydrolysis activities. The supernatant (20 μl) of.SL-13 was added to one of the aperture (0.9 cm diameter) in the plate incubated at 30°C for 3 days [24]. Equal amount of medium served as the control. The diameters of the largest and smallest inhibition distance were recorded. The inhibition activity was determined by the smallest inhibition diameter.
2.5 Measurement of chitinase activity
For the detection of chitinase activity of supernatant, the plate (incubated at 30°C for 3 days) was stained by 1% Congo red for 5 minutes. Then the stained plate was washed by 50% ethanol until the hydrolyzed zone appeared.
Chitinase activities were measured with colloidal chitin as substrate. The reaction mixture, consisting of 1 ml of enzyme solution and 1ml of 0.1% colloidal chitin, was incubated at 50°C, with centrifuge at 9000 r·min-1for 5 min. The amount of reducing sugar produced was measured by the DNS methods [25], with C6H6O6as the standard sugar. One unit of chitinase activity was defined as the amount of the enzyme that produced 1 μmol of reducing sugar per minute [26]. Proteinconcentration was determined by Bradford method.

Table 1 Influence of B. subtili SL-13 on tomato sprouting and seedling growth
①Statistically highly significant in comparison with control,<0.01.②Statistically significant in comparison with control,<0.05.
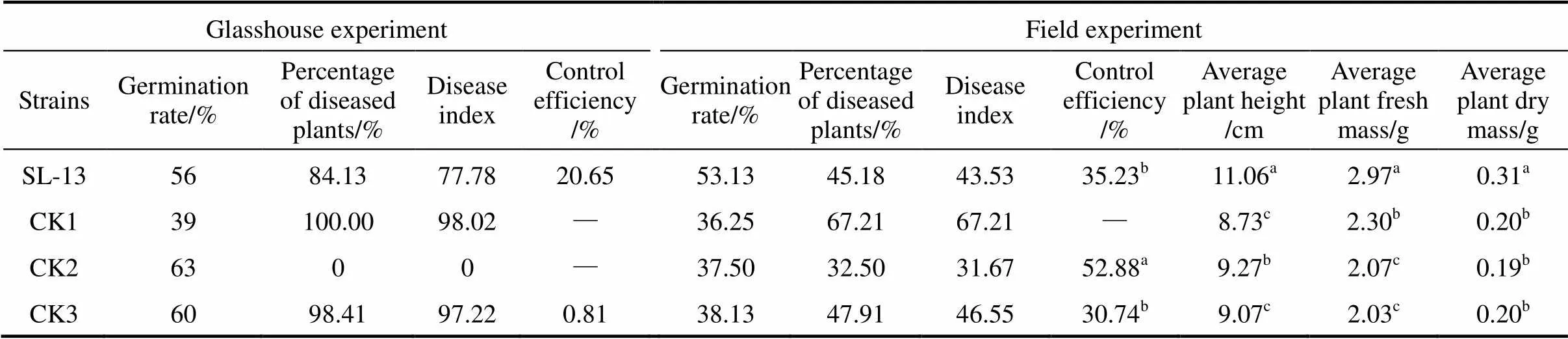
Table 2 The control efficacy of B. subtilis SL-13 to R. solani of tomato
Note: Values with the different letter superscripts in the same row means significant difference (<0. 05), with the same letter superscripts in the same row means no significant difference (>0. 05). Disease index (Di) and relative efficiency are calculated with the following formula:

Glasshouse: CK1, seeds treated by medium, pathogen.inoculated in soil; CK2, by medium, in sterile soil; CK3, by PCNB, in soil; field: CK1, seed treated by medium; CK2, by pesticide WeiFu; CK3, by pesticide PCNB.
2.6 Light microscopic examination
For understanding the antifungal mechanism,.was treated with sterile water and purified enzyme for 48 hours separately, and then the mycelium was observed under light microscopic with magnification power of 400 times [26].
2.7 Characterization of chitinase
To investigate the effect of temperature, reaction mixtures were incubated at various temperatures for 1 h in sodium phosphate buffer at pH 6.8 [24, 27]. The thermal stability was analyzed by measuring the residual activity after 20 min of pre-incubation at the optimum pH, 121°C, and 0.11 MPa. To further study the stability, the enzyme was exposed in protease K (15 mg·ml-1) and ultraviolet radiation (300lx) for 1.5 h. The untreated enzyme served as the control. The optimum pH of enzyme was determined at 50°C. The pH was adjusted by using the following buffers: citrate buffer (pH 3.0-5.0), NaH2PO4-Na2HPO4buffer (pH 6.0-8.0), Tris-Cl buffer (pH 8.0-9.0) [3].
3 RESULTS AND DISCUSSION
3.1 Growth promotion and disease control efficacy of SL-13
3.1.1
The results of growth promotion of processed tomato treated with.strain SL-13 (activity of chitinase of 2.96 U·ml-1) are listed in Table 1. SL-13 improves significantly all the biometric parameters compared with the control. The shoot length, root length and fresh mass are increased by 75%, 94.42% and 53.28%, respectively, at the germination stage. Five weeks later, the plant height, fresh and dry mass are increased by 20.11%, 42.86% and 31.25%, respectively, in the seeding stage.
3.1.2
In greenhouse experiments, data were collected after 3 weeks of pathogen inoculation. The results of biocontrol assays of SL-13 are presented in Table 2. There are obvious differences between the healthy and pathogen control. The control treated by PCNB gives 1.6% healthy plants and the diseased plants is 100% in the pathogen control. With the SL-13, the result is significantly different from the control, and the control efficiency is 20.65%. In general, SL-13 increases the percentage of healthy plants.
For further verification of growth-promotion and disease-resistance effects of.SL-13, we designed the field tests. The results are also given in Table 2. The disease control effect of SL-13 in the field is similar to that in the greenhouse. The plant is significantly higher with the treatment of SL-13 (11.06 cm) than that in all controls. Biomass in control plots (without any microbial inoculation but with normal fertilization) is 0.2 g, which is significantly lower than that in the plots inoculated with SL-13 (0.31 g). The yield parameters of processed tomato are enhanced by SL-13 inoculation. The control efficacy of SL-13 is 35.23%, higher than that treated with pesticide PCNB. Thus.SL-13 has a remarkable ability for disease control and growth promotion.
3.2 Antifungal and hydrolysis activities of the supernatant
In the petri plates,.is highly sensitive to the supernatant, so the inhibition zone is observed clearly in Fig. 1. Beside this zone, the mycelium appears yellow, atrophic and curly irregularly. After stained with Congo red, the chitinase activity is observed. The place around the aperture is not stained red, a hydrolysis zone is significant, and the size of hydrolysis zone is consistent with the inhibition zone. Therefore, the supernatant of strain SL-13 shows chitinase activity, which may hydrolyze chitin. It is the main constituent of cell wall supporting the whole structure of the cell in most fungi, is synthesized primarily on the top of mycelium, and is the main target of antibacterial proteins [Fig. 1 (b)].
Casein may also be hydrolyzed by fermentation of supernatant of strain SL-13, since there is protease in the fermentation supernatant. Protease also plays an important role in biocontrol, which is believed to facilitate the penetration into the fungal tissue by degrading the protein linkage in the fungal external layers and/or the utilization of the fungal proteins for nutrition [28, 29].
3.3 Enzyme production and antifungal activity
.SL-13 was grown for 24 h to achieve stationary phase, and after an incubation of 5 h, the chitinase activity and antifungal activity were determined, which increased gradually (Fig. 2). With the extension of incubation, the chitinase production increased with the antifungal activity. The antifungal activity was weak in the initial period of incubation, because the concentration of chitinase was low in the broth so that the chitin in the cell wall of.could not be hydrolyzed effectively.
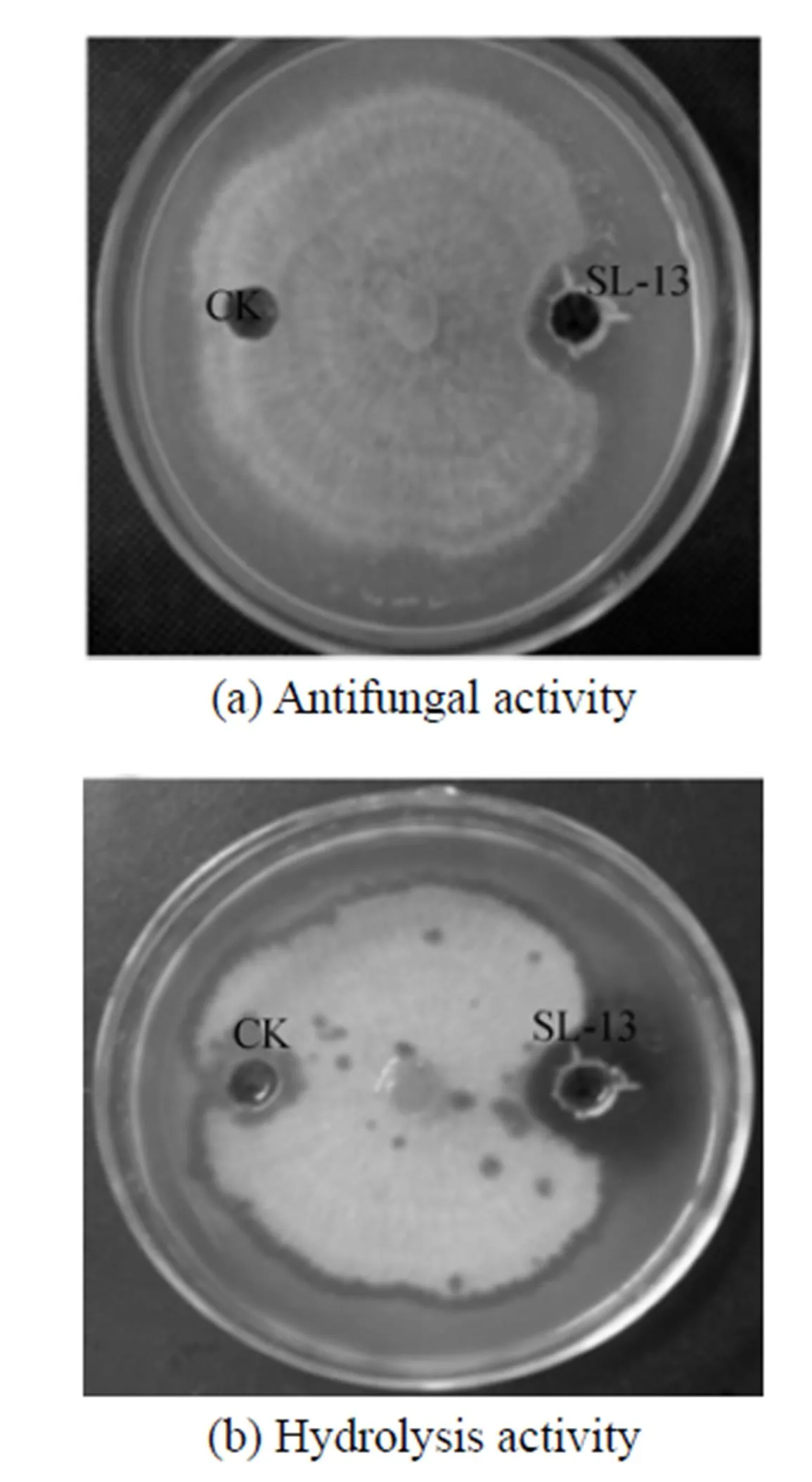
Figure 1 The antifungal activity and hydrolysis activity of supernatant toward.
Figure 2 Time course of growth, chitinase activity, and inhibitory activity in culture of.SL-13
●?time course of growth;■?inhibitory activity;▲?chitinase activity
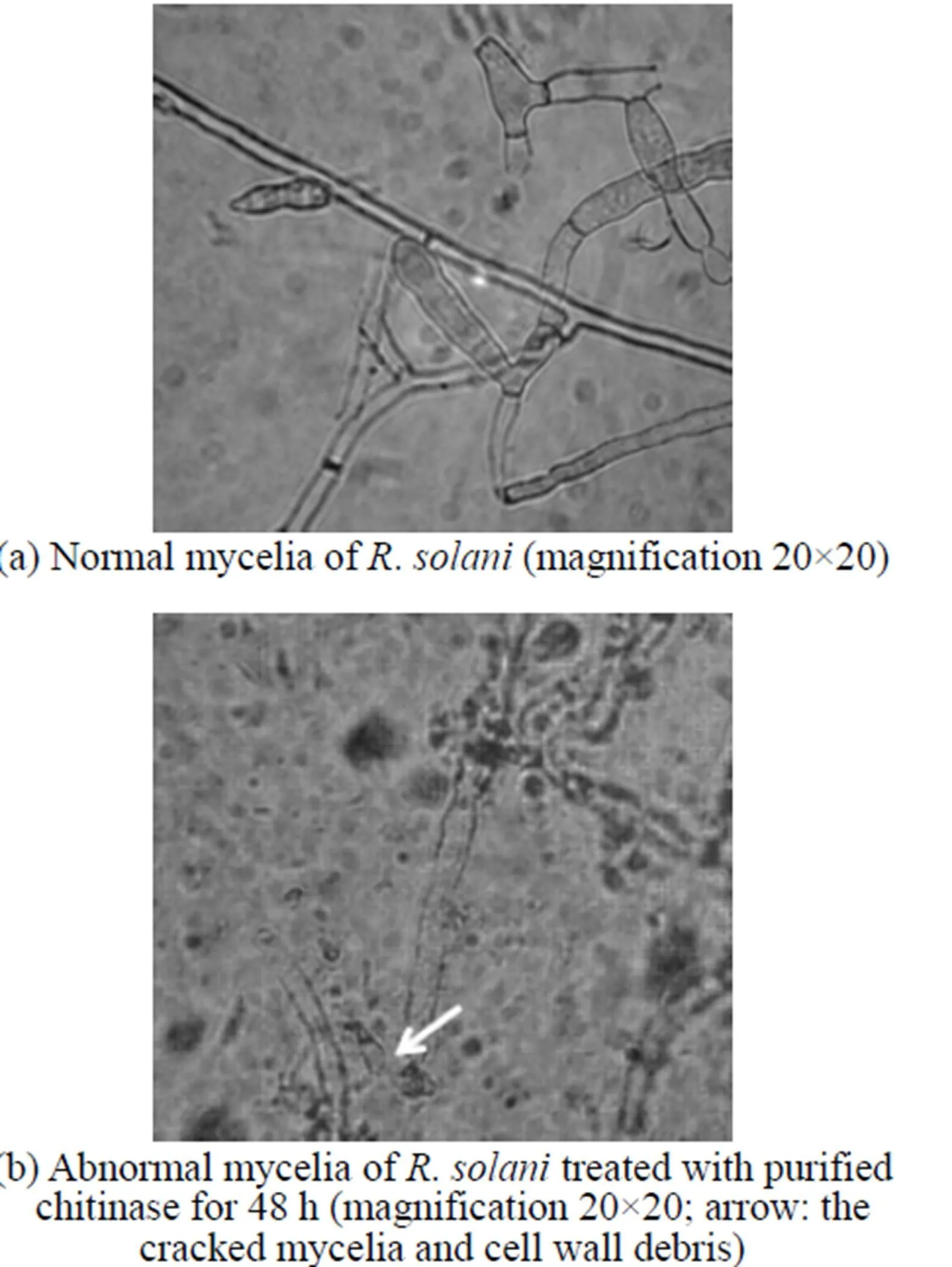
Figure 4 Inhibitory effect of chitinase on the morphology of.as seen under the light microscope
Figure 5 The effect of temperature on chitinase activity
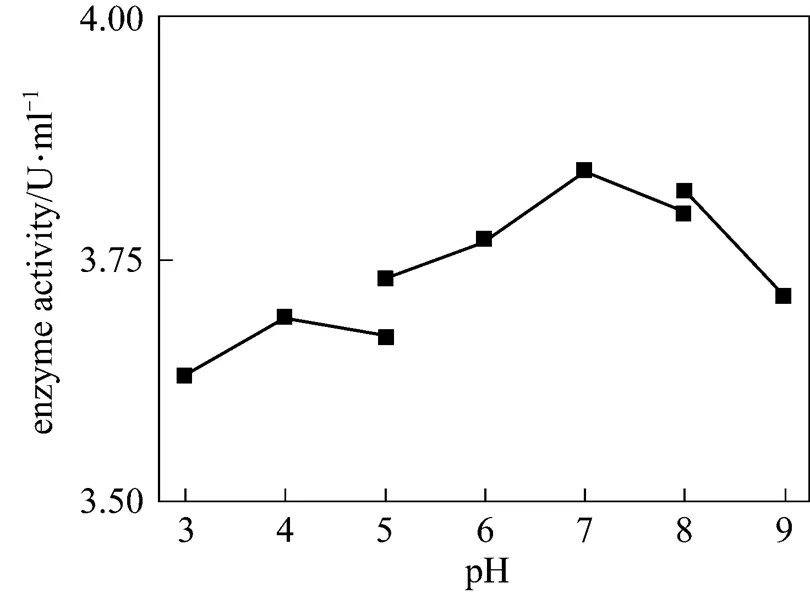
Figure 6 The effect of pH on chitinase activity
3.4 Purification of chitinase from the broth of strain SL-13
Ion exchange chromatography of.SL-13 culture extract by a DEAE-Sepharose Fast Flow column produced an unadsorbed fraction a, and two adsorbed fractions b (0.4 mol·L-1NaCl) and c (0.5 mol·L-1NaCl) [Fig. 3 (a)]. Among these fractions, fraction b showed high antifungal activity. After being concentrated and purified by Sephadex G-75 column chromatography fraction b produced two fractions d and e [Fig. 3 (b)]. Only fraction e showed high antifungal activity and was collected for further enzyme assay. When detecting antifungal activity, fraction c also inhibited the growth of., but not obviously. There may be other antifungal proteins in the supernatant, which need to be identified and purified from the supernatant for analysis.
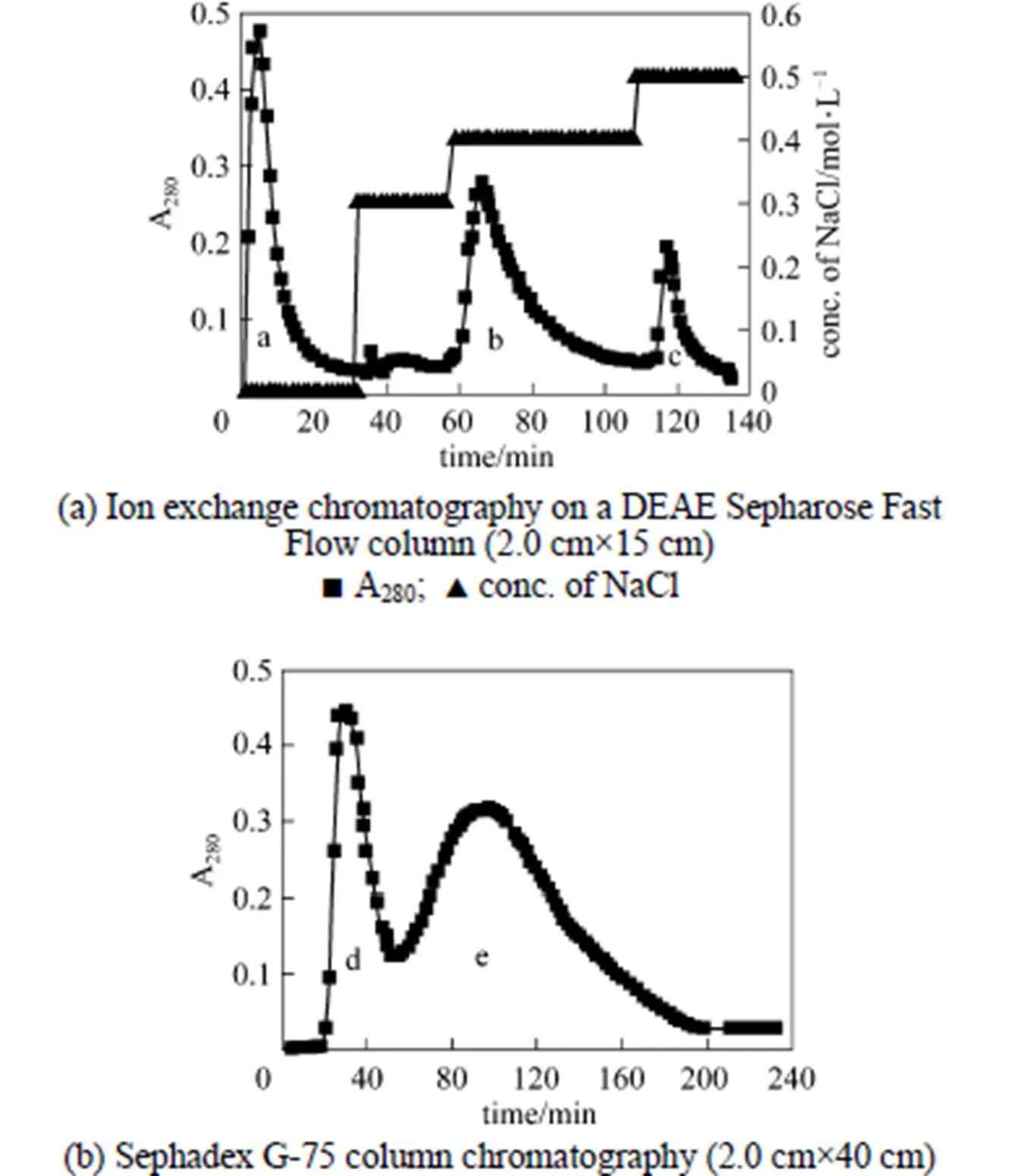
Figure 3 Chromatography for isolation of chitinase
3.5 Light microscopic examination for antifungal activity of purified chitinase
Compared to the plate treated by sterilized water [Fig. 4 (a)], the.treated by purified enzyme could not last long, since its cell wall disintegrated, protoplasm leaked out, and the mycelia cracked [Fig. 4 (b)]. The microscopic analysis shows that the main effect of the purified enzyme is to degrade the cell wall. This is also the main effect from chitinase, which is one of the most important antifungal proteins produced by.. The chitinase plays an important role in biocontrol, since it will degrade chitin present in the cell wall of phytopathogen and restrain the new material of the cell deposition. With the induction of cell wall debris degraded by chitinase, the host plant may be stimulated to have resistance response [30].
3.6 Characteristics of chitinase produced by B. subtilis SL-13
The effect of temperature on enzyme activity was investigated. The purified enzyme was incubated with the colloidal chitin substrate at specific temperature. The optimum temperature was 50°C (Fig. 5). The purified enzyme was also incubated with the colloidal chitin at specific pH using citrate buffer (pH 3-5), phosphate buffer (pH 5-8), and Tris-Cl buffer (pH 8-9). The enzyme was stable within the pH of 5-9 for 1 h at 50°C (Fig. 6) and the activity decreased when pH value was lowered than 5. The optimal pH was 7.0. SDS-PAGE analysis revealed that an apparent band with molecular mass of 30 kDa of purified chitinase samples appeared as the expected size (Fig. 7). These results were similar with the previous reports [31]. Thermal stability of the enzyme was determined with the incubation at 121°C and 0.11 MPa for 20 min and it was found that the enzyme retained 70% activity. Moreover, the enzyme did not lose the activity when treated with protease K and ultraviolet radiation for 1.5 h (Fig. 8). These characters are unique and have not been reported in the literature.
4 CONCLUSIONS
The.SL-13 strain, which belongs to tomato rhizosphere bacterial community, was isolated from the fields with tomato roots. The inhibitory effect of.SL-13 strain on.was considerably strong. The experimental results show that the SL-13 can promote plant growth and disease control in pot experiments and in the field. Thus chitinase may play an essential role in inhibiting pathogen.
The results on hydrolysis activities indicate that the fermentation supernatant can hydrolyze chitin and casein. After the separation of fermentation supernatant, the main antifungal protein is chitinase, the size of which is of 36 kDa. The optimum conditions for enzyme activity of chitinase are temperature 50°C and pH 7.0. Although some characters are similar to those of the chitinase reported, the chitinase in this study is different from other chitinase in stability and anti-adversity. This chitinase is stable with high antifungal activity resisting sterilization, UV, and protease K. It is suitable for the biological control in relatively unstable soils, presenting broad application prospects.

Figure 7 SDS–PAGE of purified chitinase
Lane 1: purified enzymes; Lane 2: supernatant; Lane 3: molecular mass marker
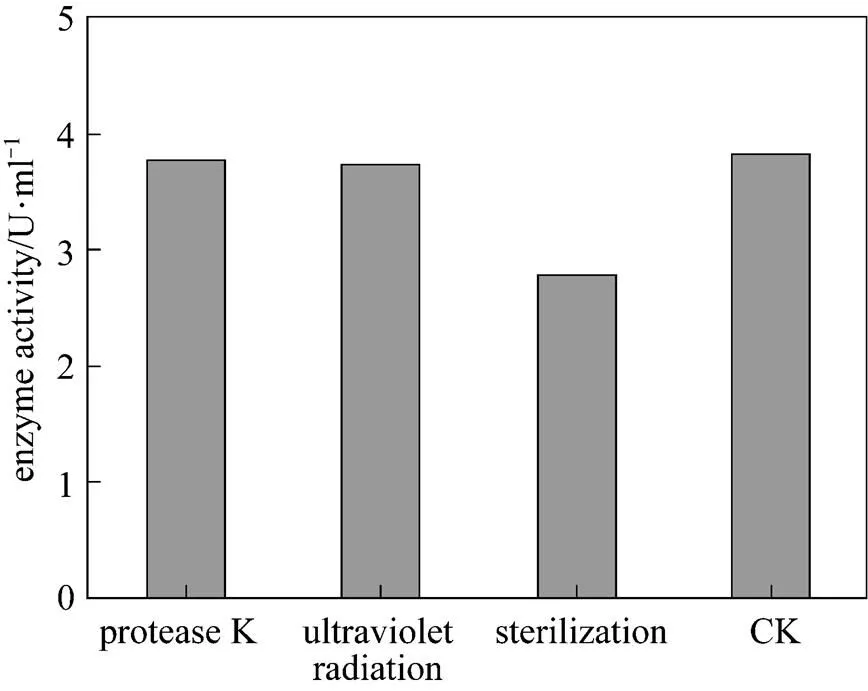
Figure 8 The effect of sterilization, UV and protease on chitinase activity
(CK: untreated enzyme)
1 Sébastien, M., Haissam, M.J., “Use of molecular techniques to elucidate the mechanisms of action of fungal biocontrol agents: A review”,..., 69, 229-241 (2007).
2 Zhang, B., Xie, C., Yang, X., “A novel small antifungal peptide fromstrain B-TL2 isolated from tobacco stems”,, 29, 350-355 (2008).
3 Liu, Y.F., Chen, Z.Y., Ng, T.B., Zhang, J., Zhou, M.G., Song, F.P., Lu, F., Liu, Y., “Bacisubin, an antifungal protein with ribonuclease and hemagglutinating activities fromstrain B-916”,, 28, 553-559 (2007).
4 Dora, M.R., Daniel, B., Christoph, M., Hollenstein, G.O., “Cell wall-associated enzymes in fungi”,, 64, 366-379 (2003).
5 Varma, R.S., George, K.J., Balaji, S., Parthasarathy, V.A., “Differential induction of chitinase inin response to inoculation with, the cause of foot rot in black pepper”,..., 16, 11-16 (2009).
6 Stephane, C., Brion, D., Jerzy, N., Christophe, C., Essad, A.B., “Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects”,..., 71, 4951-4959 (2005).
7 Liu, X.J., Jesse, L.L., Lin, H., Li, Q.W., Ma, F., “Identification and characterization of a chitinase-coding gene from Lamprey () with a role in gonadal development and innate immunity”,..., 33, 257-263 (2009).
8 Zees, A.C., Pyrpassopoulos, S., Vorgias, C.E., “Insights into the role of the (+) insertion in the TIM-barrel catalytic domain, regarding the stability and the enzymatic activity of chitinase A from”,.., 1794, 23-31 (2009).
9 Lenka, B., Katerina, S., Marcela, F., “Immunohistological localization of chitinase and-1,3-glucanase in rhizomania-diseased and benzothiadiazole treated sugar beet roots”,..., 63, 47-54 (2003).
10 Yoon, G.L., Chung, K.C., Seung, G.W., Jae, C.L., Hyeun, J.B., “Purification and properties of a chitinase from. LYG 0704”,.., 65, 244-250 (2009).
11 Raquel, C., Lourdes, G.G., “Isolation of a new fungi and wound-induced chitinase class in corms of”,.., 47, 426-434 (2009).
12 Saravanakumar, D., Vijayakumar, C., Kumar, N., Samiyappan, R., “PGPR-induced defense responses in the tea plant against blister blight disease”,, 26, 556-565 (2007).
13 Wang, S.L., Huang, J.R., “Microbial reclamation of shellfish wastes for the production of chitinases”,.., 28, 376-382 (2001).
14 Wang, S.L., Lin, T.Y., Yen, Y.H., Liao, H.F., Chen, Y.J., “Bioconversion of shellfish chitin wastes for the production ofW-118 chitinase”,.., 341, 2507-2515 (2006).
15 Zhu, Y.P., Fan, J.F., Cheng, Y.Q., Li, L.T., “Improvement of the antioxidant activity of Chinese traditional(Meitauza) usingB2”,, 19, 654-661 (2008).
16 Geng, A.L., Li, C.J., “Proteome analysis of the adaptation of a phenol-degrading bacteriumsp. EDP3 to the variation of phenol loadings”,...., 15 (6), 781-787 (2007).
17 Huang, J.M., Le, h., Sheng, Q.Y., Shan, J., Lin, D.Q., “Purification and characterization of glutamate decarboxylase ofCGMCC 1306 isolated from fresh milk”,...., 15 (2), 157-161 (2007).
18 Alamgir, K., Keith, W., Mark, P.M., Helena, N., “Purification and characterization of a serine protease and chitinases fromand detection of chitinase activity on 2D gels”,.., 32, 210-220 (2003).
19 Pirkko, H., Gleb, A., Nailia, G., Alexander, M.,Timo, K., “Lytic enzyme complex of an antagonisticsp. X-b: isolation and purification of components”,.., 758, 197-205 (2001).
20 Sun, Y.Y., Liu, W.S., Han, B.Q., Zhang, J.Q., Liu, B., “Purification and characterization of two types of chitosanase from asp”,.., 28, 1393-1399 (2006).
21 Dilfuza, E., Gisela, H., “Effect of plant growth-promoting bacteria on growth and nutrient uptake of cotton and pea in a semi-arid region of Uzbekist”,.., 56, 293-301 (2004).
22 Tao, J., Zhao, S.F., Wu, Y., Li, C., Li, H., “Study on effect of growth promotion and control diseases to processing tomato ofsp. SL-23and SL-44”,, 43, 362-365 (2006)
23 Sanket, J., Chirag, B., Anjana, J.D., “Production of biosurfactant and antifungal compound by fermented food isolate20B”,., 99, 4603-4608 (2008)
24 Brinda, M., Crawford, D.L., “Properties of the chitinase of the antifungal biocontrol agentWYEClOS”,.., 20, 489-493 (1997).
25 Yamamoto, M., Shimura, S., Itch, Y., “Anti-obesity effects of lipase inhibitor CT-II, an extract from edible herbs,, on rats fed a high-fat diet”,.., 24, 758-764 (2002).
26 Gao, X.A., Ju, W.T., Jung, W.J., Park, R.D., “Purification and characterization of chitosanase fromD-11”,.., 72, 513-520 (2008).
27 Arindam, R., Bijoy, M., Prabir, K.S., “Characteristics ofisolates from legume-based Indian fermented foods”,, 18, 1555-1564 (2007).
28 Flanagan, J., FitzGerald, R.J., “Functional properties ofproteinase hydrolysates of sodium caseinate incubated with transglutaminase pre- and post-hydrolysis”,.., 13, 135-143 (2003).
29 Chen, L., Coolbear, T., Daniel, R.M., “Characteristics of proteinases and lipases produced by sevensp. from milk powder production lines”,.., 14, 495-540 (2004).
30 Yu, T., Wang, L.P., Yin, Y., Wang, Y.X., Zheng, X.D., “Effect of chitin on the antagonistic activity ofagainstexpansum in pear fruit”,..., 122, 44-48 (2008).
31 Purwani, E.Y., Maggy, T.S., Yaya, R., Jae, K.H., Yu, R.P., “Characteristics of thermostable chitinase enzymes from the indonesiansp.13.26”,.., 35, 147-153 (2004).
** To whom correspondence should be addressed. E-mail: lichun@bit.edu.cn
2010-04-22,
2010-09-26.
the National Natural Science Foundation of China (20776017), the Xinjiang Uygur Autonomous Region High-tech Research and Development Project (20081108), the Fok Ying Tung Education Foundation (101071), and the Xinjiang Bingtuan Key Science and Technology Industry Project (2008GG24).
 Chinese Journal of Chemical Engineering2011年1期
Chinese Journal of Chemical Engineering2011年1期
- Chinese Journal of Chemical Engineering的其它文章
- Dynamic Simulation and Analysis of Industrial Purified TerephthalicAcid Solvent Dehydration Process*
- Preparation of p-Hydroxybenzaldehyde by Hydrolysis of DiazoniumSalts Using Rotating Packed Bed*
- Liquid-solid Equilibria in Quinary System Na+, K+, Mg2+//Cl-,?at 25 °C*
- Pervaporation Separation of Butanol-Water Mixtures UsingPolydimethylsiloxane/Ceramic Composite Membrane*
- Reaction Kinetics of Biodiesel Synthesis from Waste Oil Using a Carbon-based Solid Acid Catalyst
- Enzyme-catalyzed Synthesis of Vitamin E Succinate Using aChemically Modified Novozym-435*
