Adsorption and Ozonation Kinetic Model for PhenolicWastewater Treatment*
Wongsarivej Pratarn**, Tongprem Pornsiri Swasdisevi Thanit, Charinpanitkul Tawatchai and Tanthapanichakoon Wiwut
?
Adsorption and Ozonation Kinetic Model for PhenolicWastewater Treatment*
Wongsarivej Pratarn1,**, Tongprem Pornsiri1, Swasdisevi Thanit2, Charinpanitkul Tawatchai3and Tanthapanichakoon Wiwut3
1National Nanotechnology Center, Thailand Science Park, Klong Luang, Pathumthani 12120, Thailand2School of Energy, Environment and Materials, King Mongkut’s University of Technology Thonburi, 126 Pracha u-tid Rd., Bangkok 10140, Thailand3Center of Excellence in Particle Technology, Faculty of Engineering, Chulalongkorn University, Payathai Rd., Wangmai, Pathumwan, Bangkok 10330, Thailand
A three phase fluidized bed reactor was used to investigate the combined effect of adsorption and oxidation for phenolic wastewater treatment. Aqueous solutions containing 10 mg·L-1of phenol and ozone were continuously fed co-currently as upward flow into the reactor at constant flow rate of 2 and 1 L·min-1, respectively. The phenolic treatment results in seven cases were compared: (a) O3only, (b) fresh granular activated carbon (GAC), (c) 1st reused GAC, (d) 2nd reused GAC, (e) fresh GAC enhanced with O3, (f) 1st reused GAC enhanced with O3, and (g) 2nd reused GAC enhanced with O3. The phenolic wastewater was re-circulated through the reactor and its concentration was measured with respect to time. The experimental results revealed that the phenolic degradation using GAC enhanced with O3provided the best result. The effect of adsorption by activated carbon was stronger than the effect of oxidation by ozone. Fresh GAC could adsorb phenol better than reused GAC. All cases of adsorption on GAC followed the Langmuir isotherm and displayed pseudo second order adsorption kinetics. Finally, a differential equation for the fluidized bed reactor model was used to describe the phenol concentration with respect to time for GAC enhanced with O3. The calculated results agree reasonably well with the experimental results.
adsorption, ozonation, kinetic model, phenol, wastewater
1 INTRODUCTION
Activated carbon, also called activated charcoal or “activated coal”, is a form of carbon that is made extremely porous and possesses a very large surface area available for adsorption and chemical reaction. Granular activated carbon (GAC) has relatively larger particle sizes than powdered activated carbon and consequently has a smaller specific external surface. Diffusion through the pores of the adsorbate is thus an important factor. The GAC is nevertheless effective for adsorption of gases and vapors as their rates of diffusion are faster than those of liquids. Granulated carbons are also used for treating water and wastewater to reduce pressure drop and elutriation loss [1].
Ozone (O3) is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope (O2). Ozone in the lower atmosphere is an air pollutant with harmful effects on the respiratory systems of animals. Exposure of 0.1×10-6to 1×10-6produces headaches, burning eyes, and irritation to the respiratory passages [2]. Even low concentrations of ozone in the air could be destructive to various organic materials such as latex, plastics, and lungs. Ozone could be generated by several methods, for example, corona discharge, ultraviolet light and cold plasma [3]. Industrially, ozone is used to chemically attack contaminants in water such as iron, arsenic, hydrogen sulfide, nitrites and complex organics, often lumped together as color.
In various industrial processes, phenol is one of the important starting or intermediate materials. Phenol is known to be carcinogenic and possesses high stability and high toxicity. It has been declared to be hazardous pollutant even at very low concentration [4]. It can damage the skin and other tissues of the human and animals. When digested, phenol-containing liquids could lead to liver damage, dark urine and irregular heart beats. Therefore, the treatment of phenolic wastewater is of considerable importance in environmental protection.
Many technologies have been attempted for treating phenolic wastewater, for example, biological treatment [5], chemical precipitation or oxidation [6], ion exchange [7], and adsorption [8]. However, there are few processes to deal with this highly toxic wastewater with reasonable costs. The degradation of aqueous phenol by simultaneous use of ozone and activated carbon or zeolite offers an environmentally friendly alternative of phenolic treatment [9-13]. In this research, the removal of phenol by adsorption on GAC without and enhanced with ozone is investigated using a laboratory scale three phase fluidized bed reactor. The adsorption isotherm and kinetics of GAC are examined and modeled. A differential equation to describe the phenol concentration with respect to time for the case of GAC enhanced with O3is derived and examined.
2 EXPERIMENTAL
2.1 Activated carbon preparation
The test GAC made from coconut shell was purchased from Carbokarn (Thailand) Co., Ltd. The GAC was sieved to select the size range of 0.4-2.0 mm particle diameter. The classified GAC was heated and held at 473 K for 4 h to eliminate its moisture and volatile impurities.
2.2 Characterization
Following the Brunauer-Emmett-Teller (BET) adsorption method, the specific surface area and porosity of the activated carbon were measuredN2adsorption-desorption isotherms. Test materials were measured at 77 K using an automatic adsorption apparatus (BELSORP 28, BEL Japan Inc.). The morphological structure of the activated carbon was characterized by scanning electron microscope (SEM, Hitachi S-3400N).
2.3 Apparatus and procedure
A laboratory scale three phase fluidized bed reactor was used as the adsorption and oxidation system to improve mixing and homogeneity. Its schematic diagram is shown in Fig. 1. The reactor with an effective volume of 272 ml was made from transparent acrylic that allowed the observation of the phenomena inside. The outside diameter and height of the reactor were 40 and 300 mm, respectively. The aqueous solution containing 10 mg·L-1of phenol was treated and 256.8 g·h-1of O3was used as oxidizing agent. A constant flow rate of 1 L·min-1of the solution and 2 L·min-1of O3were continuously fed co-currently into the reactor as upward flow. Then, the liquid effluent stream was recycled to the hold-up tank. 6 L of phenolic wastewater containing in the tank of 15 L was treated with activated carbon of 5 g. The solution temperature in the tank was monitored and controlled at 303 K using a thermocouple and a cooler. The samples were collected from the effluent stream with respect to time. The adsorption and oxidation of activated carbon and ozone were investigated in seven cases: (a) O3only, (b) fresh GAC, (c) 1st reused GAC, (d) 2nd reused GAC, (e) fresh GAC enhanced with O3, (f) 1st reused GAC enhanced with O3, and (g) 2nd reused GAC enhanced with O3. Their phenolic elimination performance was analyzed and evaluated.
2.4 Chemical analysis
The progress of the removal was followed by periodically taking the liquid samples from the reactor for immediate analysis after filtration through 0.45mm nylon filter. Phenol was identified and quantified by high performance liquid chromatography (HPLC, Shimadzu LC-20A Series) with a diode array detector at wavelengths of 210 and 254 nm. A 5mm column of C18(Inertsil ODS-3, 25 cm long, 4.6 mm diameter) was used as stationary phase and a mixture of 4 mmol·L-1aqueous sulphuric and acetonitrile (volume ratio of 4︰1) was used as mobile phase at 1 ml·min-1.
3 RESULTS AND DISCUSSION
3.1 Characterization of activated carbon
The pore size distribution of GAC measured with the MP-Plot method was found to be essentially microporous with a modal peak of 0.6 nm, and the BET surface area and total pore volume of GAC were 1154 m2·g-1and 0.49 cm3·g-1, respectively. To understand the morphology of GAC surface, its image was obtained with scanning electron microscope (see Fig. 2) to reveal the high surface area structure of GAC. The abundant micropores provide superb condition for the adsorbed material to interact with the ozone at high concentration.

Figure 1 Schematic diagram of experimental apparatus
1—hold-up tank; 2—liquid pump; 3—ball valve; 4—liquid flowmeter; 5—air flow meter; 6—ozone generator; 7—air pump; 8—three phase fluidized bed reactor
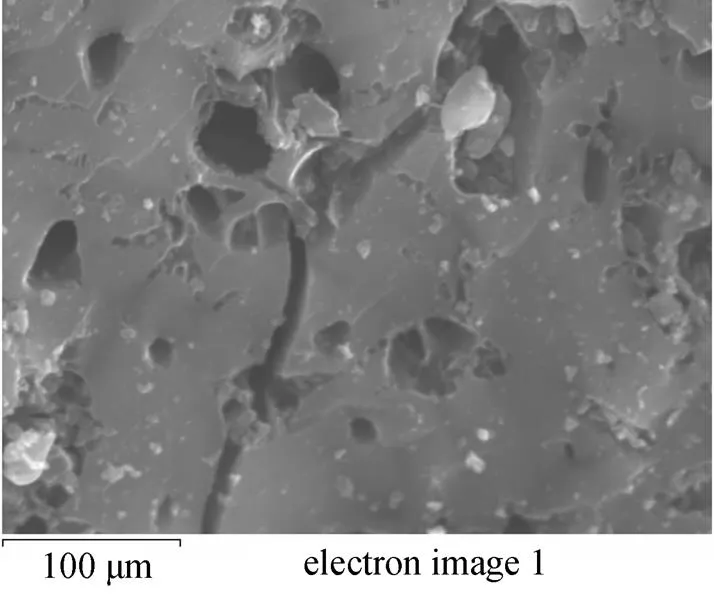
Figure 2 Scanning electron microscope image of GAC
Figure 3 Equilibrium adsorption isotherm of fresh activated carbon fitted with Langmuir and Freundlich adsorption isotherm

3.2 Adsorption isotherms
The capacity of virgin activated carbon to adsorb aqueous phenol was determined by measuring the adsorption isotherm at 298 K, as shown in Fig. 3. The analysis of the isotherm data is important to develop an equation that accurately represents the equilibrium and can be used for design purpose. In this study, two commonly used equilibrium models are selected to describe the adsorption data, namely the Langmuir and the Freundlich isotherm equations [14-20].
The Langmuir theory assumes a homogeneous monolayer adsorption within the adsorbent. The linear form of the Langmuir isotherm equation is represented as
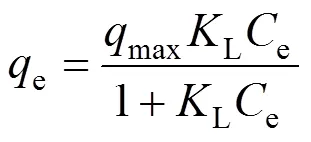
After rearrangement Eq. (1) becomes
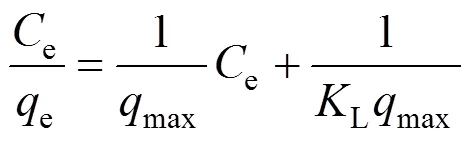
whereeis the equilibrium phenolic concentration on the adsorbent (mg·g-1),ethe equilibrium concentration of phenol in the solution (mg·L-1),maxthe maximum monolayer adsorption capacity of the adsorbent (mg·g-1), andLis the Langmuir isotherm constant (L·mg-1) related to the free energy of adsorption. A plot ofe/eversusefor the adsorption of phenol onto the activated carbon gives a straight line of slope of 1/maxand intercept of 1/maxL.
The Freundlich isotherm is an empirical equation assuming that the adsorption takes place on heterogeneous surfaces of solids and in multilayer sorption manner. The adsorption capacity is the adsorbed concentration of phenol at equilibrium. The linear form of Freundlich equation is
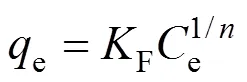
After rearrangement Eq. (3) becomes

whereF(L·g-1) andare Freundlich adsorption isotherm constants indicative of the adsorption capacity and the degree of nonlinearity between solution concentration and adsorption, respectively. The plot of lneversus lneis employed to generateFvalue from the intercept andvalue from the slope.
The Langmuir and Freundlich parameters for the adsorption of phenol onto activated carbon are listed in Table 1. This table and Fig. 3 show conclusively that Langmuir isotherm provides a significantly higher regression coefficient2than Freundlich isotherm. This means that the surface of GAC is made up of homogeneous adsorption patches, which is in good agreement with other reports [21-23].

Table 1 Adsorption isotherm constants for the phenol onto virgin activated carbon at 30 °C

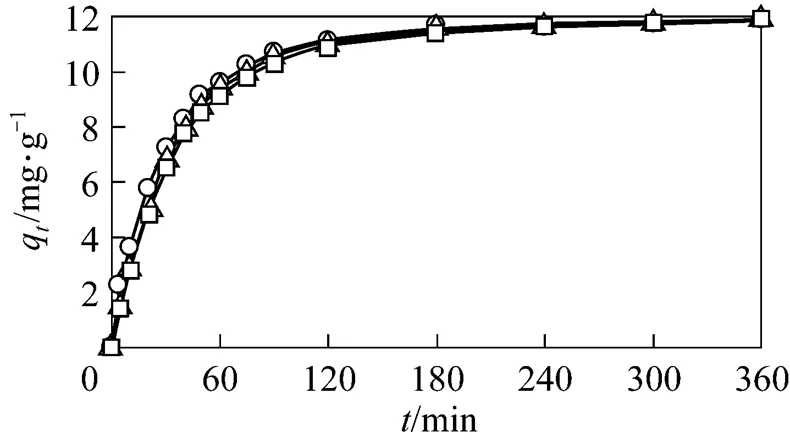
Figure 4 GAC adsorption of phenol as a function of time
○?fresh GAC;△?1st reused GAC;□?2nd reused GAC
3.3 Adsorption kinetics
Two kinetic models, the pseudo first order and pseudo second order, were tested with the experimental data to elucidate the adsorption phenomenon with the air fed co-currently instead ozone. In the case of the first order rate equation for GAC, with 1st reused GAC and 2nd reused GAC, the values ofa1andewere calculated from the slope and intercept of the plot of lg(e-q)(see Fig. 5) and summarized in Table 2. It is found that the correlation coefficients for the pseudo first order model are significantly lower than those of the pseudo second order model. Coupled with the fact that the lines in Fig. 5 are not straight, it conclusively shows that the adsorption process does not follow the first order kinetics.

Table 2 Experimental and calculated adsorption ability and rate constant of GAC with 1st reused GAC and 2nd reused GAC
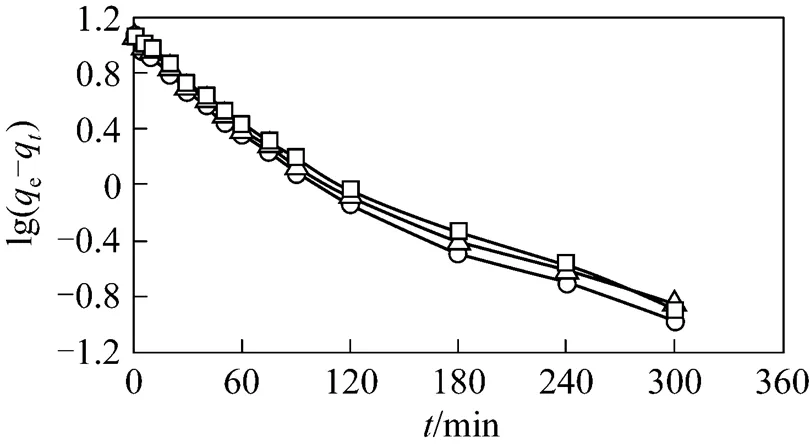
Figure 5 Pseudo first order analysis for GAC with 1st reused GAC and 2nd reused GAC
○?fresh GAC;△?1st reused GAC;□?2nd reused GAC
In the case of the second order rate equation for GAC, with 1st reused GAC and 2nd reused GAC, the values ofa2andewere calculated from the plot of/qagainst(see Fig. 6 and Table 2). The calculatedevalues agree essentially with the experimental values. The correlation coefficients for the pseudo second order kinetic plots are very high, indicating that the adsorption kinetics of both GAC and reused GAC are the pseudo second order.
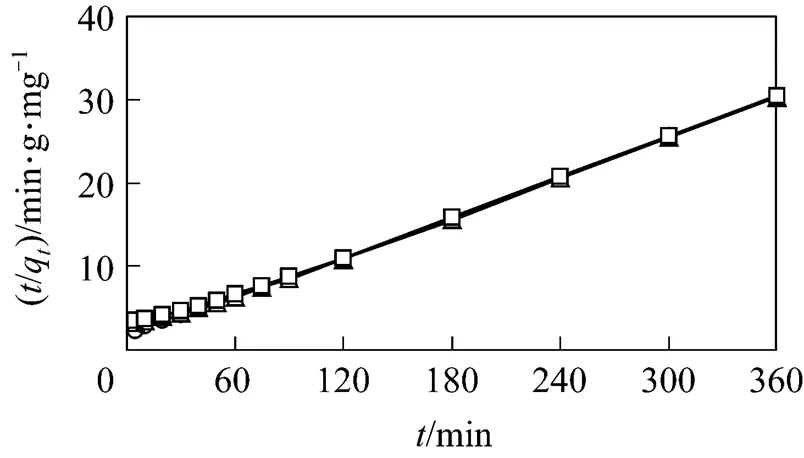
Figure 6 Pseudo second order analysis for GAC with 1st reused GAC and 2nd reused GAC
○?fresh GAC;△?1st reused GAC;□?2nd reused GAC
3.4 Ozonation kinetics


Figure 7 The plot of-ln(/0).for the reaction between phenol and ozone
3.5 The coupling between adsorption and ozonation
In three out of the seven experimental cases, the coupled adsorption and oxidation performance of GAC and ozone was investigated under the same experimental conditions. The three cases are (e) fresh GAC enhanced with O3, (f) 1st reused GAC enhanced with O3, and (g) 2nd reused GAC enhanced with O3. The results for the seven cases are presented in Fig. 8. Each of the shown data points is the average value of triplicate data. As expected, using only O3is the worst for phenol degradation. When employing only GAC and air, the degradation performance is significantly better. In the cases of GAC enhanced with O3, the performance is further improved significantly. In the best case, complete degradation of phenol is achieved within 75 min. In summary, the removal effect of adsorption by GAC is stronger than that of oxidation by ozone. GAC adsorbs phenolic compounds in aqueous solutions until it reaches equilibrium. Interestingly, when coupling the effect of adsorption together with the effect of ozonation, phenol can be eliminated faster than in the four previous cases, either O3or GAC only.
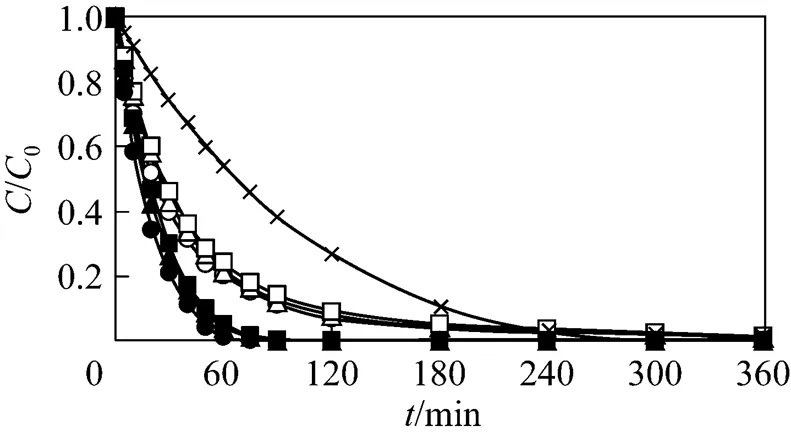
Figure 8 Phenol concentration as a function of time for fresh and reused GAC without and enhanced with O3
×?O3only;○?fresh GAC-air;△?1st reused GAC-air;□?2nd reusedGAC-air;●?fresh GAC-O3;▲?1st reused GAC-O3;■?2nd reused GAC-O3
In Fig. 8, the phenol adsorption of GAC in the three cases without ozone, fresh GAC and 1st reused GAC is the fastest and second fastest, respectively. This is consistent with the kinetic results shown in Fig. 4. Similarly, the phenol elimination in the three cases of GAC enhanced with O3, fresh GAC enhanced with O3and 1st reused GAC enhanced with O3, is the best and second best, respectively.
However, in actual wastewater treatment, using fresh activated carbon in each batch is economically impractical. The synergistic effect of adsorption with GAC and ozonation is an attractive alternative. Fortunately, the employment of used GAC with or without ozone shows small difference in adsorption rates after two regenerations.
3.6 Adsorption and ozonation kinetics
The ozone oxidation data were analyzed with the following second order rate equation for irreversible bimolecular reaction:

whereris reaction rate constant,AandBare phenol and ozone concentrations, respectively. In our experiments, the concentration of ozone was replenished continuously and was essentially constant, so the rate equation can be reduced to first-order.

With separation of variables and integration of both sides, Eq. (6) becomes

whereA0is the initial concentration of phenol.
The adsorption data could be analyzed with either pseudo first order or pseudo second order kinetics:
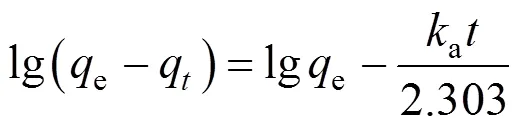

whereeandqare adsorption ability at equilibrium and at time(mg·g-1) respectively,a1anda2are the first and second order adsorption rate constants, respectively. Our conclusion in Section 3.3 indicates a pseudo second order kinetics. Differentiation of both sides of Eq. (9) gives

Based on the assumptions of complete mixing in the fluidized bed and simultaneous adsorption and ozonation in the reactor, the mass balance equation for phenol in the three phase fluidized bed reactor can be written as
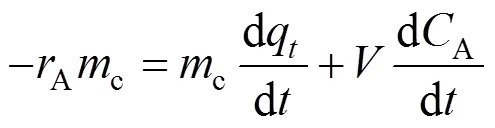
wherecis catalyst loading andis liquid volume in the reactor. Substitution of Eqs. (6) and (10) into Eq. (11) yields
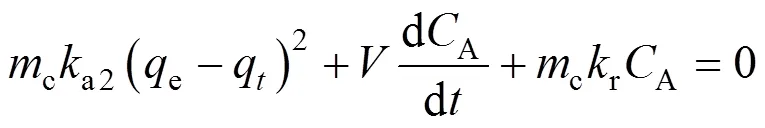
To integrate this equation, it is first written as an ordinary differential equation as follows
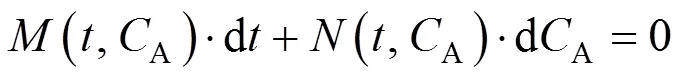
More specifically,

where
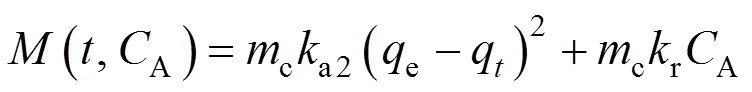
and

It should be noted that

Eq. (12) is not an exact ordinary differential equation, but it becomes an ODE after multiplication by an integrating factor,,

Then Eq. (12) becomes
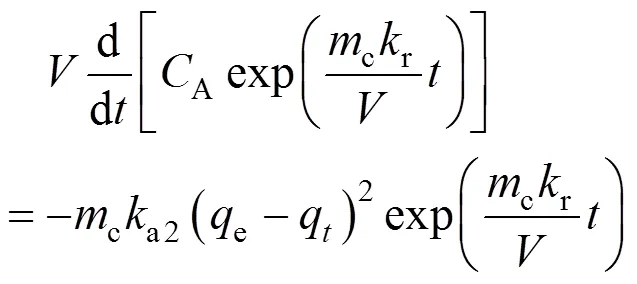
The integration of Eq. (19) gives

and Eq. (10) can be integrated to yield
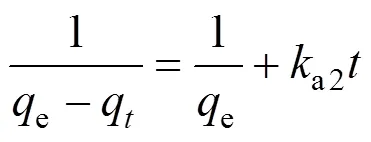

Substitution of Eqs. (21) and (22) in Eq. (20) gives
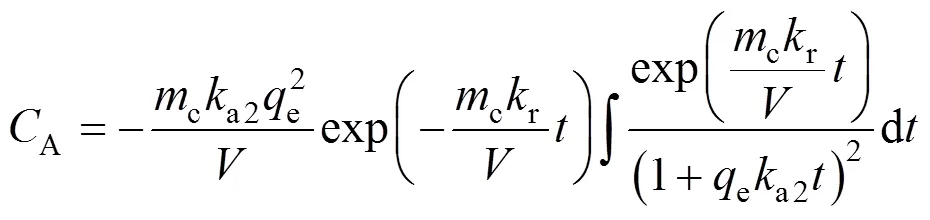
Since the integral in Eq. (23) can not be integrated analytically, it is integrated using Wolfram Mathematica Software. To obtain some approximate analytical solution, the following indefinite integral is utilized
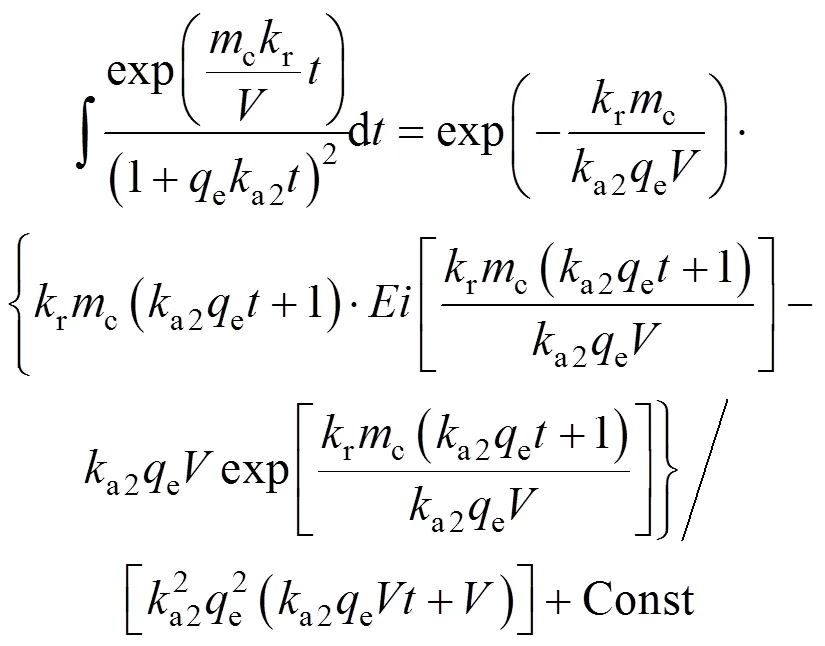
Application of the initial conditionAA0at0 allows the evaluation of the Const term.



Figure 9 shows the aqueous phenol concentration as a function of time for the three cases of GAC enhanced with O3. The comparison shows that the calculated values ofAfrom the differential equations agree reasonably well with the corresponding experimental results. Therefore, both the computational results and the approximate analytical solution can be used to predict the phenol concentration with respect to time and to design a three phase fluidized bed reactor for wastewater treatment.
4 CONCLUSIONS
Removal of phenol in a laboratory scale three phase fluidized bed reactor with activated carbon either without or enhanced with ozone was examined experimentally and theoretically. Phenolic degradation using only O3gave the worst result among the seven cases while using the coupling effect of GAC and O3provided the best result. As expected, the phenol removal performance of fresh and 1st reused GAC turned out best and second best, respectively. This holds true for the cases of GAC without and GAC enhanced with ozone. The oxidation of phenol in the case of only O3followed pseudo first order kinetics. Adsorption of phenol in all cases of GAC followed Langmuir isotherm and pseudo second order kinetics. Finally, the derived differential equations and their solutions for the three phase fluidized bed reactor yielded predicted results that agreed reasonably with the corresponding experimental results.
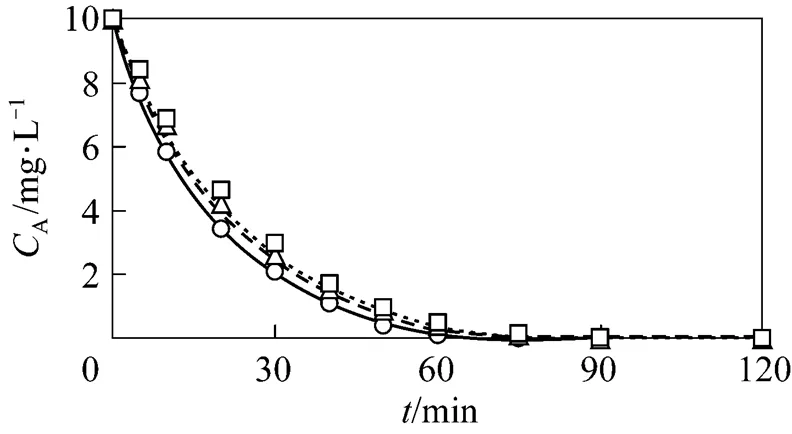
Figure 9 Comparison between the calculatedA(line) and experimentalA(dot) for GAC enhanced with O3
exp.:○?fresh GAC-O3;△?1st reused GAC-O3;□?2nd reused GAC-O3;

NOMENCLATURE
concentration, mg·L-1
Aphenol concentration, mg·L-1
Bozone concentration, mg·L-1
econcentration of phenol in the solution, mg·L-1
exponential integral
FFreundlich isotherm constants, L·g-1
LLangmuir isotherm constant, L·mg-1
a1first order adsorption rate constant, mg·g-1·min-1
a2second order adsorption rate constant, g·mg-1·min-1
rfirst order apparent kinetic rate constant, L·g-1·min-1
ccatalyst loading, g
degree of nonlinearity between solution concentration and adsorption
eadsorption ability at equilibrium, mg·g-1
maxmaximum monolayer adsorption capacity of adsorbent, mg·g-1
qadsorption ability at time, mg·g-1
Aoxidation reaction rate of phenol, mg·g-1·min-1
time, min
liquid volume, L
mathematical term
Euler gamma constant
integrating factor
Subscripts
A phenol
B ozone
r reaction
0 initial condition
1 Van Hulle, S.W.H., Audenaert, W., Decostere, B., Hogie, J., Dejans, P., “Sustainable wastewater treatment of temporary events: the Dranouter Music Festival case study”,.., 58 (8), 1653-1657 (2008).
2 Folchetti, N., 22 Chemistry: The Central Science, 9th ed., Pearson Education, 882-883 (2003).
3 Dohan, J.M., Masschelein, W.J., “Photochemical generation of ozone: Present state-of-the-art ozone”,.., 9, 315-334 (1987).
4 Zazo, J.A., Casas, J.A., Mohedano, A.F., Rodriguez, J.J., “Catalytic wet peroxide oxidation of phenol with a Fe/active carbon catalyst”,..:., 65, 261-268 (2006).
5 Khehra, M.S., Saini, H.S., Sharm, D.K., Chadha, B.S., Chimni, S.S., “Biodegradation of azo dye C.I. Acid Red 88 by an anoxic-aerobic sequential bioreactor”,, 70, 1-7 (2006).
6 Lucas, M.S., Dias, A.A., Sepia, A., Amaral, C., Peres, J.A., “Degradation of a textile reactive azo dye by a combined chemical-biological process: Fenton’s reagent yeast”,., 41, 1103-1109 (2007).
7 Right, S., Basha, A.C., “Chemical or electrochemical techniques followed by ion exchange for recycle of textile dye wastewater”,..., 149, 324-330 (2007).
8 Wang, S., Zhu, Z.H., “Effects of acidic treatment of activated carbons on dye adsorption”,, 75, 306-314 (2007).
9 Polaert, I., Wilhelm, A.M., Delmas, H., “Phenol wastewater treatment by a two-step adsorption-oxidation process on activated carbon”,..., 57, 1585-1590 (2002).
10 Alvarez, P.M., Beltran, F.J., Gomez-Serrano, V., Jaramillo, J., Rodriguez, E.M., “Comparison between thermal and ozone regenerations of spent activated carbon exhausted with phenol”,., 38, 2155-2165 (2004).
11 Lei, L., Gu, L., Zhang, X., Su, Y., “Catalytic oxidation of highly concentrated real industrial wastewater by integrated ozone and activated carbon”,..:, 327, 287-294 (2007).
12 Mowla, D., Ahmadi, M., “Theoretical and experiment investigation of biodegradation of hydrocarbon polluted water in a three phase fluidized bed bioreactor with PVC biofilm support”,..., 36, 147-156 (2007).
13 Alvarez, P.M., Beltran, F.J., Masa, F.J., Pocostales, J.P., “A comparison between catalytic ozonation and activated carbon adsorption/ozone-regeneration processes for wastewater treatment”,..:., 92, 393-400 (2009).
14 Langmuir, I., “The adsorption of gases on plane surfaces of glass, mica and platinum”,...., 40, 1361-1403 (1918).
15 Freundlich, H.M.F., “U ber die adsorption in losungen”,..., 57, 385-470 (1906).
16 Ho, Y.S., Ofomaja, A.E., “Kinetics and thermodynamics of lead ion sorption on palm kernel fibre from aqueous solution”,., 40 (11), 3455-3461 (2005).
17 Beolchini, F., Pagnanelli, F., Reverberi, A.P., Veglio, F., “Copper biosorption onto: pH-edge tests and related kinetic and equilibrium modeling”,...., 42, 4881-4887 (2003).
18 Allen, S.J., Mckay, G., Porter, J.F., “Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems”,..., 280, 322-333 (2004).
19 Wong, Y.C., Szeto, Y.S., Cheung, W.H., Mckay, G., “Adsorption of acid dyes on chitosan equilibrium isotherm analyses”,., 39 (6), 693-702 (2004).
20 Esposito, A., Pangnanelli, F., Lodi, A., Solisio, C., Veglio, F., “Biosorption of heavy metals by: An equilibrium study at different pH and biomass concentrations”,, 60, 129-141 (2001).
21 Matatov-Meytal, Y.I., Sheintuch, M., “Abatement of pollutants by adsorption and oxidative catalytic regeneration”,...., 36 (10), 4374-4380 (1997).
22 Matatov-Meytal, Y.I., Sheintuch, M., Shter, G.E., Grader, G.S., “Optimal temperatures for catalytic regeneration of activated carbons”,, 35 (10/11), 1527-1531 (1997).
23 Teng, H., Hsieh, C.T., “Influence of surface characteristics on liquid-phase adsorption of phenol by activated carbons prepared from bituminous coal”,...., 37 (9), 3618-3624 (1998).
** To whom correspondence should be addressed. E-mail: pratarn@nanotec.or.th
2010-06-02,
2010-12-27.
the National Nanotechnology Center (NANOTEC) (601003) and the National Science and Technology Development Agency (NSTDA).
 Chinese Journal of Chemical Engineering2011年1期
Chinese Journal of Chemical Engineering2011年1期
- Chinese Journal of Chemical Engineering的其它文章
- Dynamic Simulation and Analysis of Industrial Purified TerephthalicAcid Solvent Dehydration Process*
- Preparation of p-Hydroxybenzaldehyde by Hydrolysis of DiazoniumSalts Using Rotating Packed Bed*
- Liquid-solid Equilibria in Quinary System Na+, K+, Mg2+//Cl-,?at 25 °C*
- Pervaporation Separation of Butanol-Water Mixtures UsingPolydimethylsiloxane/Ceramic Composite Membrane*
- Reaction Kinetics of Biodiesel Synthesis from Waste Oil Using a Carbon-based Solid Acid Catalyst
- Enzyme-catalyzed Synthesis of Vitamin E Succinate Using aChemically Modified Novozym-435*
