殼聚糖制備多孔炭及其在電化學(xué)超級(jí)電容器中的應(yīng)用
季倩倩 郭培志,* 趙修松,2,*
(1青島大學(xué)化學(xué)化工與環(huán)境學(xué)院,纖維新材料與現(xiàn)代紡織國(guó)家重點(diǎn)實(shí)驗(yàn)室培育基地,山東青島 266071;2Department of Chemical and Biomolecular Engineering,National University of Singapore,4 Engineering Drive 4,Singapore 117576)
Supercapacitors or electrochemical capacitors are electrochemical energy-storage devices suited ideally to rapid storage and release of energy,which can be divided into two main areas based primarily on their modes of energy storage,the redox supercapacitor and the electrochemical double layer capacitor (EDLC)[1-4].With the advantages of high power density,high efficiency,and long cycle life[5-6],supercapacitors have important applications in information technology,hybrid electric vehicle, and aerospace[7-8].
Much interest has focused on the application of carbon as electrode material because of its accessibility,chemical stability, and variety of structures[9-11].For activated carbons materials,the main pores are micropores,which are not easily accessible to large ions,and the specific capacitance of activated carbon electrodes generally limits to about 45 F·g-1in aqueous electrolyte solutions[12].The energy storage mechanism is mainly contributed to the formation of electric double layer at the electrode/electrolyte interface.The more the mesopore appears,the more specific capacitance may be achieved.Ordered mesoporous carbons that can be obtained based on mesoporous silica templates have been studied,however,these methods need somewhat complicated steps[13].Recently,porous carbon electrode materials obtained directly from the biomass have attracted great interest due to their widespread nature[14].For example,Béguin et al.[15]reported that porous carbon directly derived from the carbonization of sodium alginate has a capacitance as high as 200 F·g-1. Bamboo-based activated carbons after KOH activation at 800℃has capacitance less than 70 F·g-1[16].
Chitosan,poly(2-amino-2-deoxy-D-glucose),is the second most abundant biopolymer after cellulose[17]and has been selected for the preparation of porous carbon as supercapacitor electrode materials in this article.
1 Experimental
1.1 Materials
Chitosan(Mw=(1.5-3)×105)was received as a gift from Qingdao Jifa Co.Ltd.KOH(AR)was purchased from Sinopharm Chemical Reagent Company.Acetylene carbon black(99.99%(w))and polytetrafluorothylenelatex(PTFE 60%(w))were purchased form Strem Chemicals and Aldrich,respectively.
1.2 Preparation of porous carbon
Porous carbon samples are obtained directly from carbonization of chitosan in a tube furnace at 600,700,800,and 900℃for 90 min with N2as shielding gas,separatively.The heating rate was 10℃·min-1.The as-obtained black solid was then washed with HF(20%,w)solution for 48 h.Finally,the solid was filtrated with distilled water and dried at 60℃for 6 h.The samples obtained at 600,700,800,and 900℃are referred to as C-600, C-700,C-800,and C-900,respectively.
1.3 Characterization
Thermogravimetric analysis(TGA)was conducted on a thermogravimetric analyzer TGA 822e(Mettler Toledo,Switzerland) with an air flow rate of 100 mL·min-1and a temperature ramp of 10 K·min-1.The pore-structural properties of the samples were investigated using physical adsorption of nitrogen at the liquidnitrogen temperature(77 K)on an automatic volumetric sorption analyzer Tristar3000(Micromeritics,USA).Prior to measurements,the samples were vacuum-degassed at 473 K for 300 min.
1.4 Fabrication of porous carbon electrode
The electrochemical measurements were performed on a CHI760C electrochemical workstation(CH Instruments,USA) using a three-electrode cell with porous carbon as working electrode,platinum wire as counter electrode,and mercuric oxide electrode as reference electrode in 30%(w)KOH solutions.The working electrode was prepared by mixing the active materials with polytetrafluorethylene(PTFE),and acetylene carbon black with mass ratio of 80∶15∶5,and was blended to achieve a homogeneous mixture.The resulting slurry was then pressed onto a nickel foam substrate(1 cm×1 cm)at 1.5×107Pa.The typical mass load of each electrode material was about 5 mg.Then the electrodes were vacuum dried at 110℃.
2 Results and discussion

Fig.1 Nitrogen sorption isotherms of samples C-600(a), C-700(b),C-800(c),and C-900(d)adsorption isotherm:hollow symbol;desorption isotherm:solid symbol
Nitrogen sorption isotherms were recorded to access the pore properties of the porous carbon samples.Fig.1 shows the nitrogen sorption isotherms of four carbon samples after carbonation of chitosan at different temperatures.For samples of C-700,C-800,and C-900(Fig.1(b-d)),at low pressure the initial step region is ascending abruptly and then followed by a plateau indicating that the adsorption has virtually stopped because multilayer of the pore wall,and as can be seen there have two marked leaps. This can be clearly seen from the enlarged isotherm of C-800 in the inset in Fig.1.The N2uptake at high relative pressure is increased rapidly,which reveals a high development of mesoporosity.It can be seen that the isotherm is type IV isotherm curves with a marked leap in the adsorption at relative pressures p/p0of 0.45,which is typical for mesoporous samples.As there are many smaller size pores on the pore wall,after the marked leap there is a small plateau and then descending rapidly,all of these may result in the other marked leap.As can be seen in Fig. 1a,nitrogen sorption isotherm of C-600 is different evidently from that of C-700,C-800,and C-900,this probably because of the low temperature of carbonization.Table 1 lists the data of the pore volumes and pore size distribution of carbon samples. The surface area is increased from 278 m2·g-1of C-600 to 625 m2·g-1of C-900 with the pore size also decreased from 3.5 to 2.8 nm,and the pore volume increased from 0.17 cm3·g-1of C-600 to 0.27 cm3·g-1of C-800,but the pore volume of C-900 decreased to 0.25 cm3·g-1probably due to the collapse of sample pores.The average pore sizes of the samples lie in 2-5 nm,which were propitious to form electric double-layer capacitance[18-22], making for the ion migration of inorganic electrolyte and electronic adsorption because the diameters of K+and OH-in the KOH solutions are smaller than 0.4 nm[23].
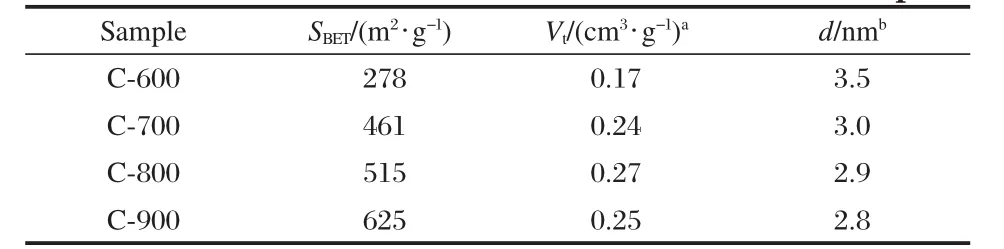
Table 1 Pore structures of chitosan-based carbon samples
Thermal gravity analysis(TGA)under air flow shows that the degradation temperature of porous carbons is somewhat relevant to the precursor carbonization temperature(Fig.2).The mass loss below 80℃,ascribed to the desorption of adsorptive water,in TGA curve of C-600 is 4%which is the lowest in the four samples probably due to the smallest surface area of C-600.It can be seen that the mass is drastically decreased when the temperature lies in the range of 450-500℃.The temperature that the most mass loss occurs is about 575℃for C-900,however,the temperatures are in the range of 595-600℃for the last three samples.This indicates that the pore structure of C-900 may be collapsed as discussed in the above.
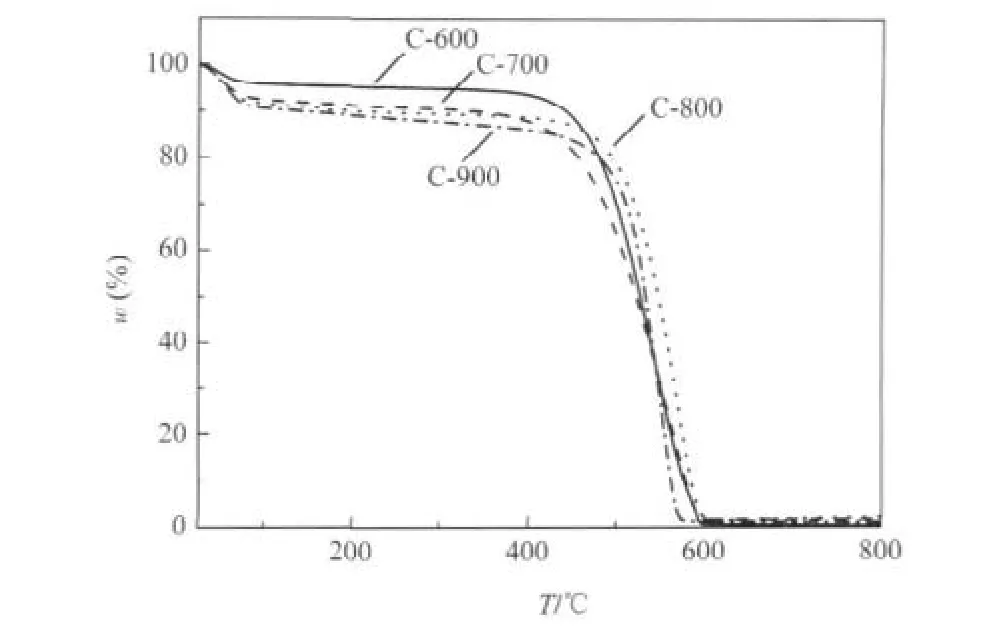
Fig.2 TGA curves of C-600,C-700,C-800,and C-900
Fig.3 shows the CV curves of carbon samples at scan rates of 5 and 50 mV·s-1.The CV curves of four samples are clearly different as shown in Fig.3.As the pore of C-900 collapsed,this may prevent ions from entering into porous carbon,so the CV curve of C-900 (Fig.3 curves d)has an anomalous shape and its specific current is also very small.In Fig.3 curves b,the CV curves of C-700 have a relative regular shape than C-600 and C-900,but not as well as C-800.It can be observed that the CV curve of C-800 displays rectangular shape even at a high scan rate(Fig.3(B))while those of other samples are not.It is easy to see that the specific current of C-800 is higher than that of any other samples,indicating that C-800 has the lowest resistance and the highest capacitance among the samples.The shape of the CV curves of C-600 and C-900 is far from rectangular, which proves that the pore structures of the chitosan-based canbon samples have a strong effect on their electrochemical properties.For C-800 sample,however,the CV curve also shows almost symmetrical anode process,indicating that electrode containing C-800 has good ability of capacitance[24-25].The CV curves are not nearly rectangles at the scan rate of 50 mV·s-1,indicating thattheohmic resistance is large at a high scan rate[26].
Galvanostatic charge-discharge is a commonly used way in electrochemical capacitor study,information such as capacitance and long cycle capability of electrode materials can be obtained based on these experiments[27].In Fig.4,charge-discharge curves of C-800 are shown at the current density of 50 mA·g-1. It has to be pointed out that the charge-discharge curves are not exactly linear,probably because of the randomness of the pore[28].The specific capacitance C can be calculated from galvanostatic charge-discharge curves with the equation,C=itd/(m·ΔV)[29-30], whereiisthecurrentdensityinthegalvanostaticcharge-discharge measurement,m is the mass of the active materials,tdis the variance metric of charge or discharge time and ΔV is the voltage interval of charge or discharge.The capacitances of the samples calculated from galvanostatic charge-discharge curves are listed in Table 2.It can be observed that the capacitances of C-600 and C-700 are 96 and 120 F·g-1at 50 mA·g-1,respectively,and decreased rapidly with the increase of the current density.Interestingly,C-800 shows the highest capacitance of 154 F·g-1at 50 mA·g-1and the capacitances decrease to 125 F· g-1at 100 mA·g-1.Further changing the current density from 500 to 1000 mA·g-1gives a weak effect on the capacitance changed from 94 to 78 F·g-1.This is probably because that the porous of C-800 is randomness and it did not allow a rapid diffusion of ions[31].However,the capacitance of C-900 electrode is very small,only 28 F·g-1at 50 mA·g-1,as discussed in the above,this may because of the collapse of the pores.
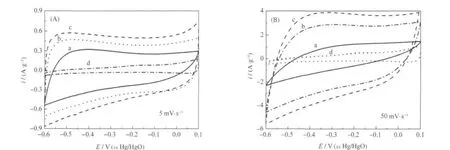
Fig.3 CV curves of C-600(a),C-700(b),C-800(c),and C-900(d)samples at different scan rates
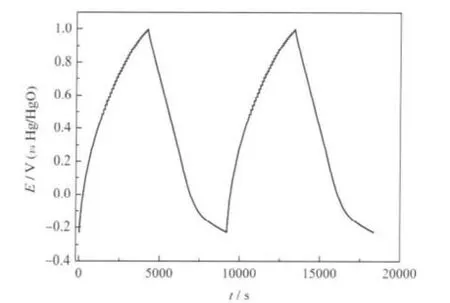
Fig.4 Galvanostatic charge-discharge curve of C-800 based electrodes in 30%KOH solutions at a current density of 50 mA·g-1
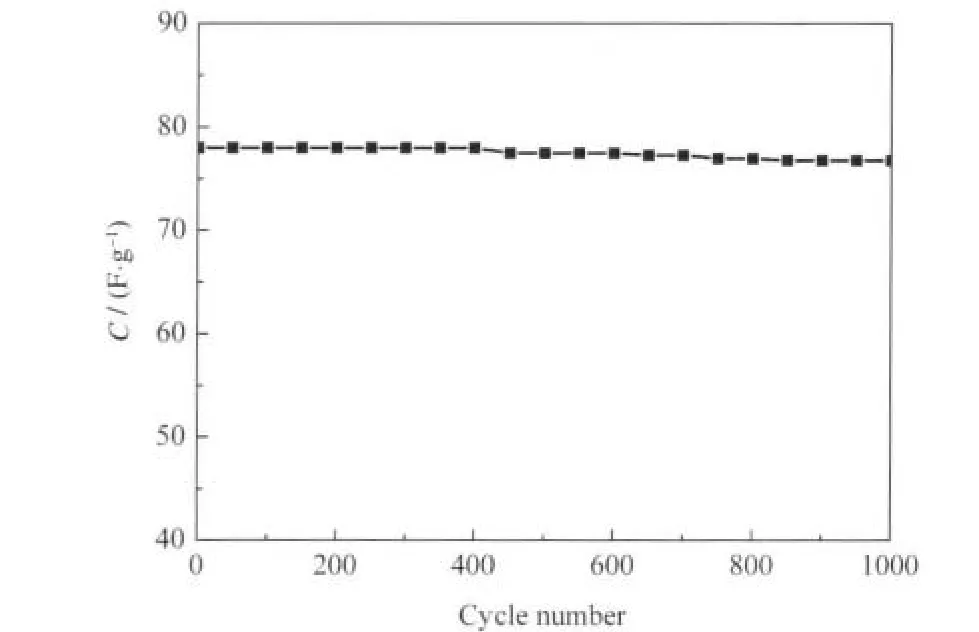
Fig.5 Specific capacitance of the C-800 electrode at a current density of 1000 mA·g-1
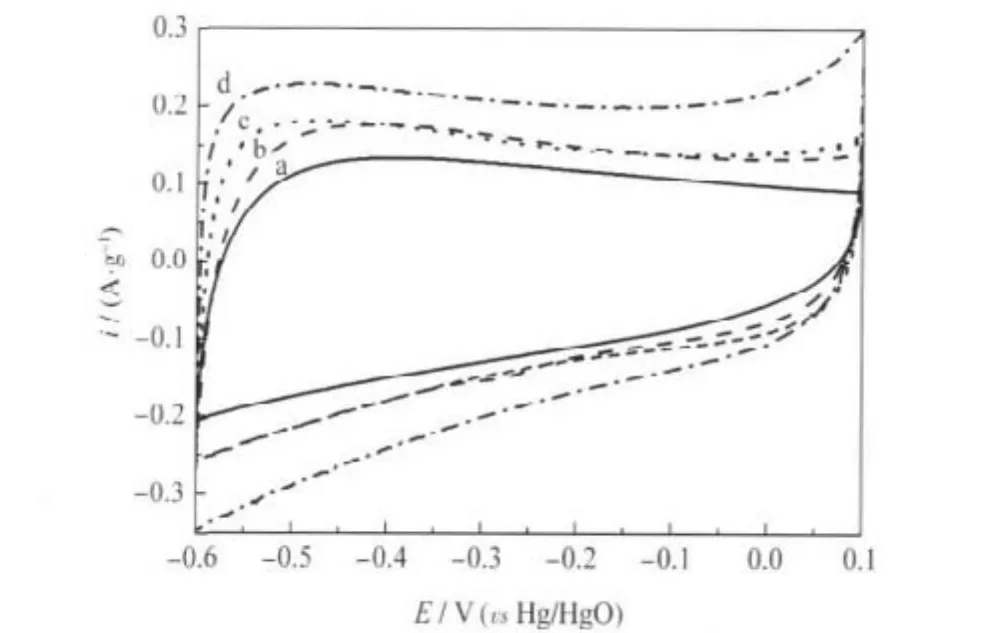
Fig.6 CV curves of C-800 in KOH solutions with different mass fraction at a scan rate of 2 mV·s-1(a)1%,(b)10%,(c)20%,(d)30%

Table 2 Capacitances of C-600,C-700,C-800,and C-900 electrode at different current densities
The electrochemical stability of the active material and its repeatability were investigated by galvanostatic charge-discharge measurement.Fig.5 shows the relationship between cycle number and the capacitance of the C-800 sample at a current density of 1000 mA·g-1.After 1000 cycle test,the specific capacitance of the C-800 decreased slightly from 78 to 76.8 F·g-1,indicating that 98.5%of the original value was maintained.This result reveals that the electrode of C-800 had high stability and capacitanceretainabilityevenatalargecurrentdensityof1000mA·g-1.
The effect of electrolyte concentrations on the electrochemical properties of C-800 has also been investigated.The CV curves of C-800 at the scan rate of 2 mV·s-1in different KOH solutions are shown in Fig.6.It can be seen that the CV curve in the KOH solution with a lowest concentration of 1%is far away from rectangular and its specific current is very small(Fig.6 curve a).With the electrolyte concentration up to 10%(Fig.6 curve b),the shape of the CV curve become more rectangular and similar results can be observed when the concentration is 20%(Fig.6 curve c).In our experiments,the CV curve obtained from the 30%KOH solution shown in Fig.6 curve d displays almost rectangular shape and symmetrical electrode process,indicating that electrode using C-800 had good ability of capacitance.These changes indicated that the concentration of electrolyte has an obvious effect on the electrochemical properties of porous carbon due to the variation of the sizes of solvated K+in the different concentration electrolyte solutions[32].
3 Conclusions
Porous carbon materials are fabricated through the carbonization of commercial chitosan at different temperatures.With the temperature increased from 600 to 900℃,the surface areas show consecutively increase.However,the capacitances of the samples increase from C-600 to C-800 and then decreases drastically for C-900 which is ascribed to the characteristics of pore structure of the samples.The highest capacitance in the experiments is 154 F·g-1obtained from C-800 electrode under the current density of 50 mA·g-1and the electrode show excellent cycle performance even at high current densities.It is suggest that the environmentally friendly porous carbon electrode made by chitosan has potential applications as electrochemical capacitor electrode.
1 Bard,A.J.;Faulkner,L.R.Principle and application of electrochemical method.Trans.Shao,Y.H.;Zhu,G.Y.;Dong,X. D.;Zhang,B.L.Beijing:Chemical Industry Press,2005:373-395 [Bard,A.J.;Faulkner,L.R.電化學(xué)方法原理和應(yīng)用.邵元華,朱果逸,董獻(xiàn)堆,張柏林譯.北京:化學(xué)工業(yè)出版社,2005:373-395]
2 Conway,B.E.Electronic supercapacitor:scientific fundamental and technological and applications.New York:Kluwer Academic/ Plenum Publishers,1999:19-55
3 Frackowiak,E.;Béguin,F.Carbon,2001,39:937
4 Brodd,R.J.;Bullock,K.R.;Leising,R.A.;Middaugh,R.L.; Miller,J.R.;Takeuchi,E.J.Electrochem.Soc.,2004,151:K1
5 Conway,B.E.J.Electrochem.Soc.,1991,138:1539
6 Arbizzani,C.;Mastragostino,M.;Soavi,F.J.Power Sources, 2001,100:164
7 K?tz,R.;Carlen,M.Electrochim.Acta,2000,45:2483
8 Burke,A.J.Power Sources,2000,91:37
9 Winter,M.;Brodd,R.J.Chem.Rev.,2004,104:4245
10 Chmiola,J.;Yushin,G.;Gogotsi,Y.;Portet,C.;Simon,P.; Taberna,P.L.Science,2006,313:1760
11 Lee,J.;Kim,J.;Hyeon,T.Adv.Mater.,2006,18:2073
12 Jiang,Q.;Zhao,X.F.;Huang,B.;Du,B.;Zhao,Y.Acta Phys.-Chim.Sin.,2009,25:757 [江 奇,趙曉峰,黃 彬,杜 冰,趙 勇.物理化學(xué)學(xué)報(bào),2009,25:757]
13 Lee,G.J.;Pyun,S.Langmuir,2006,22:10659
14 Alejandro,A.;Natalia,M.;Néstor,T.;Hugo,S.;Cristina,D. Boiresource Technoligy,2007,98:1635
15 Raymundo-Pi?ero,E.;Leroux,F.;Béguin,F.Adv.Mater.,2006, 18:1877
16 Kim,Y.J.;Lee,B.J.;Suezaki,H.;Chino,T.;Abe,Y.;Yanagiura, T.;Park,K.Y.;Endo,M.Carbon,2006,46:1592
17 Zeng,X.F.;Ruckenstein,E.Ind.Eng.Res.,1996,35:4169
18 Jurewicz,K.;Vix-Guterl,C.;Frackowiak,E.;Saadallah,S.;Reda, M.;Parmentier,J.;Patarin,J.;Béguin,F.J.Phys.Chem.Solids, 2004,65:287
19 Lee,J.;Yoon,S.;Hyeon,T.;Oh,S.M.;Kim,K.B.Chem. Commun.,1999:2177
20 Fuertes,A.B.;Pico,F.;Rojo,J.M.J.Power Sources,2004,133: 329
21 Vix-Guterl,C.;Frackowiak,E.;Jurewicz,K.;Friebe,M.; Parmentier,J.;Béguin,F.Carbon,2005,43:1293
22 Fuertes,A.B.;Lota,G.;Centeno,T.;Frackowiak,E.Electrochim. Acta,2005,50:2799
23 Wang,D.W.;Li,F.;Zhao,J.P.;Ren,W.C.;Chen,Z.G.;Tan,J.; Wu,Z.S.;Gentle,L.;Lu,G.Q.;Cheng,H.M.ACS Nano,2009,3: 1745
24 Dong,X.P.;Shen,W.H.;Gu,L.Z.;Xiong,L.M.;Zhu,Y.F.;Li, H.;Shi,J.L.J.Phys.Chem.B,2006,110:6015
25 Brousse,T.;Toupin,M.;Bélanger,D.J.Electrochem.Soc.,2004, 151:A614
26 Zheng,J.P.J.Electrochem.Soc.,2003,150:A484
27 Eliad,L.;Salitra,G.;Soffer,A.;Aurbach,D.J.Phys.Chem.B, 2002,106:10128
28 Morishita,T.;Soneda,Y.;Hatori,H.;Inagaki,M.Electrochim. Acta,2007,52:2478
29 Conway,B.E.;Pell,W,G.J.Power Sources,2002,105:169
30 Pell,W.G.;Conway,B.E.J.Electroanal.Chem.,2001,500:121
31 Masarapu,C.;Zeng,H.F.;Wei,B.Q.ACS Nano,2009,3:2199
32 Li,H.Q.;Luo,J.Y.;Zhou,X.F.;Yu,C.Z.;Xia,Y.Y. J.Electrochem.Soc.,2007,154:A731

