電化學(xué)置換法制備高Pt利用率的類核殼型Ni-Pt電催化劑
孫 盾 何建平 周建華 王 濤 狄志勇 丁曉春
(南京航空航天大學(xué)材料科學(xué)與技術(shù)學(xué)院,南京 210016)
Fuel cells,especially proton exchange membrane fuel cells (PEMFCs)are expected to be a major energy conversion device in the hydrogen economy.But,the low catalytic activity of electrodes for the hydrogen electro-oxidation reaction and the high cost of Pt-based electrocatalysts still pose an obstacle to their commercialization[1-4].The most common solution to these problems is to employ bimetallic catalysts.Certain platinum-based alloys,PtM(M=Fe,Co,Ni,V,Cr,Mn,etc.)[5-9],exhibit somewhat enhanced catalytic activity in comparison with pure Pt metal.However,they commonly contain more than 80%(mass fraction,w)of Pt in a Pt-based alloy.Consequently,considerable effort should be spent on searching for alternative electrocatalysts with high activity and stability,as well as low Pt content.
Core-shell bimetallic nanoparticles have earned much attention because of their unique structure[10-12].The nanoparticles with non-noble metal as core and noble metal as shell can further reduce the content of Pt metal,moreover,the activity of a Pt shellcanbeenhancedthrough structural-and electronic-effects[13]. Another advantage of core-shell structure is that only surface atoms are catalytically active.
A number of techniques have been used to prepare Pt-based core-shell catalysts,such as electroless plating,microemulsion and polyhydric alcohol reduction[14-16].One versatile method for the bimetallic catalyst is the successive reduction technique, wherein the second metal is reduced by the preformed core seeds through adding another reducing agent.Compared to these conventional strategies,the facile replacement reaction is superior in the fabrication of core-shell structured nanoparticles[17], bearing several advantages listed as follows:(1)no additional reducing agent is needed,(2)spontaneous formation of the shells deposited on the surface of the initial cores,(3)self-nucleation of secondarily added metals can be avoided,(4)inhomogeneous growth of the shells on the surface of the initial cores can be prevented. Adzic et al.[18]first reported the synthesis of Pt-based core-shell catalyst for fuel cell reactions,combining the underpotential deposition of a Cu monolayer and subsequent displacement with Pt.However,the electrochemical method is not feasible from the viewpoint of mass production of for well-dispersed nanoparticles.
In this paper,we present an effective and facile approach to prepare the Pt-based core-shell like catalyst to achieve high catalytic performance with a lower amount of Pt.The morphology and electrocatalytic activity of Ptshell-Nicorecatalysts are investigated.It shows that Ptshell-Nicore/XC-72 catalyst exhibited a high catalytic activity for hydrogen electro-oxidation.
1 Experimental
All the reagents were of analytical purity and used without further purification.Trisodium citrate,acetone,cetyltrimethyl ammonium bromide,sodium borohydride,isopropanol,Ni(Ac)2, and NaH2PO2were purchased from Sinopharm Chemical Reagent Limited Corporation.
The carbon-supported core-shell Ni-Pt electrocatalyst was prepared through an electroless process to load the Ni-core seeds on the surface of commercial carbon black Vulcan XC-72 (Cabot),and a galvanic replacement method that made the Ptshell over the Ni-core.Trisodium citrate and cetyltrimethyl ammonium bromide were used as the stabilizer and dispersant,respectively.The theoretical total metal loadings are 20%(w)(8% (w)for Pt).
1.1 Pretreatment of Vulcan XC-72
1 g of carbon was suspended in 500 mL water,followed by adding 500 mg of sodium borohydride.The mixture was stirred overnight at 50℃,filtered after being sufficiently washed,and then vacuum-dried.The pretreatment was necessary for the removal of surface contaminants,especially for that of surface oxides;besides,it might be that impregnation of the high surface area carbon by small amounts of borohydride(residues of borohydride)later result in the formation of the Ni nuclei on the carbon surface when Ni acetate is added.These later grow further in size.
1.2 Synthesis of Ni core
Citrate-stabilized Ni-core nanoparticles(seed particles)were prepared via Ni(Ac)2being reduced by NaH2PO2.Briefly,40 mg of the pretreated carbon was mixed with 20 mL of water and 20 mL of isopropanol,then sonicated for 10 min.After the addition of 1.0 mL of 0.1 mol·L-1Ni(Ac)2aqueous solution,1.0 mL of 0.1 mol·L-1trisodium citrate aqueous solution and 0.01g of cetyltriethylammnonium bromide(CTAB),the mixture was vigorously stirred and then heated to 80℃.Finally,3.0 mL of 0.1 mol·L-1NaH2PO2was added slowly into the above-obtained solution under continuous stirring for 1 h..
1.3 Synthesis of Pt shell by replacement of Ni core
The suspension was filtered and washed with water and acetone to remove excess NaH2PO2,in this way the sequential galvanic replacement reaction would not be disturbed by the excess reducing agent.The product was re-dispersed in a mixture of water and isopropanol.After adjusting the pH of the suspension to 6 by addition of 0.2 mol·L-1HCl solution,0.5 mL of 0.038 mol·L-1H2PtCl6was added under vigorously stirring and heated to 60℃for 3 h.The suspension was then centrifuged to recover the solid product,which was re-dispersed and washed thoroughly with acetone,ethanol,and water for more than 6 times to remove the remnant CTAB.The final solid product was dried overnight in a vacuum oven at 60℃ and denoted as Ptshell-Nicore/XC-72.
1.4 Synthesis of comparison samples
For comparison,carbon-supported monometallic Ni samples and Pt samples(denoted as Ni/XC-72 and Pt/XC-72)with the same total metal loading(20%(w))were prepared by a similar procedures.
In order to check whether the excess NaH2PO2was thoroughly removed and prove that the Pt-shell was formed via replacement reaction,not reduced by the remnant reducing agent,carbon supported Ptshell-Nonecoresamples (denoted as Ptshell-Nonecore/XC-72) with the same amount of Pt but without Ni was prepared by a similar procedure.
1.5 Characterization
X-ray diffraction patterns were recorded by a Bruker D8 ADVANCE diffractometer using Cu Kαradiation(λ=0.154056 nm). Transmission electron microscopy(TEM,FEI Tecnai G2)operating at 200 kV was applied to characterize the morphology and particle size distribution.The UV-Vis spectra were measured with a Shimadzu UV2450 spectrometer equipped with quartz cells.
1.6 Electrochemical measurement
An electrochemical interface(CHI 660A)and a conventional three-electrode system were employed to conduct voltammetry experiments on the catalysts.Cyclic voltammetry(CV)test in 1 mol·L-1KOH solution with a scan rate of 20 mV·s-1was used to examine the coating quality of the nanoparticles,and to check whether there are pinholes on the shell;further in 0.5 mol·L-1NaClO4to study the electrochemical properties of the core-shell sample and the monometallic samples with the same scan rate. Electrochemical hydrogen absorption/desorption tests in 0.5 mol·L-1H2SO4with a scan rate of20 mV·s-1was used to evaluate catalytic activity of the samples.
The working electrode was prepared as follows:5 mg of the catalyst was mixed with 1 mL of ethanol and 50 μL of 5%(w) Nafion solution(Du Pont).The mixture was sonicated for 30 min to obtain inky slurry.Approximately 25 μL of the slurry was applied to the surface of the glassy carbon electrode to form a thin layer of ca 0.1256 cm2in geometrical area.A saturated calomel electrode(SCE)and a Pt foil were used as the reference electrode and the counter electrode,respectively.
2 Results and discussion
2.1 Physical characterization and structural studies
The structure and phase analysis of the catalyst samples were performed by XRD.Fig.1 shows the XRD patterns of Ptshell-Nicore/XC-72,Pt/C(JM)and Ni/XC-72 catalysts with a metal loading of 20%(w).The first peak located at about 24.8°in all the XRD patterns is associated with the Vulcan XC-72 carbon support,according to the peak of XC-72.Peaks suggestive of Nicore,located at 2θ of 44.5°and 51.7°correspondingly indexed to a relatively poorly-resolved(111)-plane and a well-resolved (200)-palne,are detected in the Pt-shell/Ni-core structure,which are consistent with Ni/XC-72.
There emerges a positive angle shift for the four characteristic peaks of Pt,which are located at 2θ of(111),(200),(220)and (311)planes,as shown in Table 1,indicating the bimetallic combination of Pt and Ni.The phenomenon can be assigned to the incorporation of Ni into the structure of Pt by the in situ galvanic replacement process.The broad diffraction peaks suggest that small Ptshell-Nicore/XC-72 and Pt/C(JM)particles are obtained with a narrow size distribution[19].The average size of the Ptshell-Nicoreand Pt particles estimated by Debye Scherrer formula[20]are 3.4 and 3.6 nm,respectively.
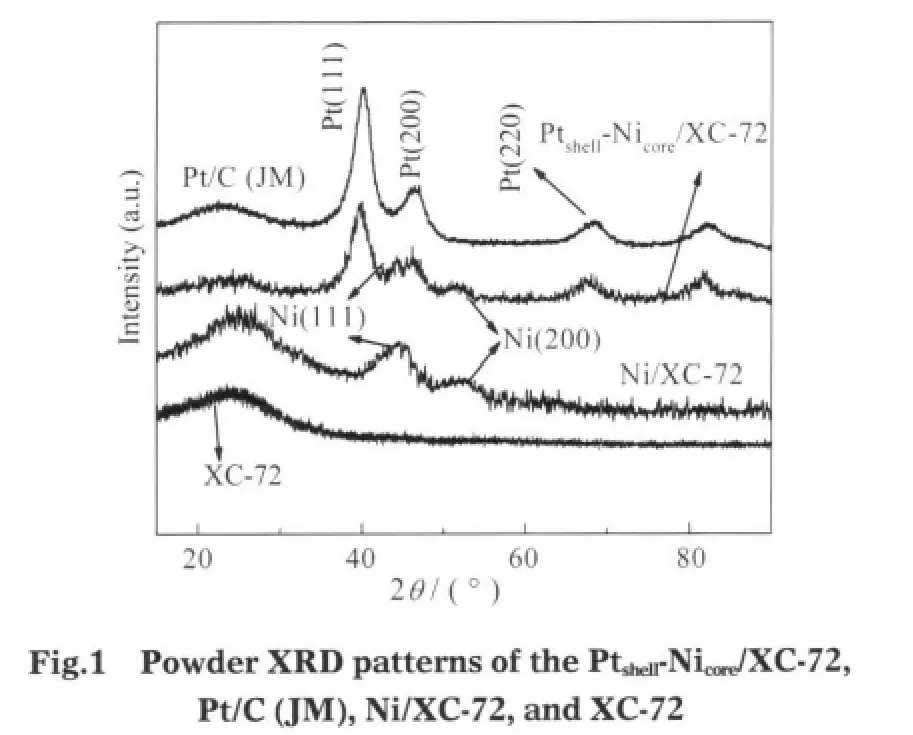
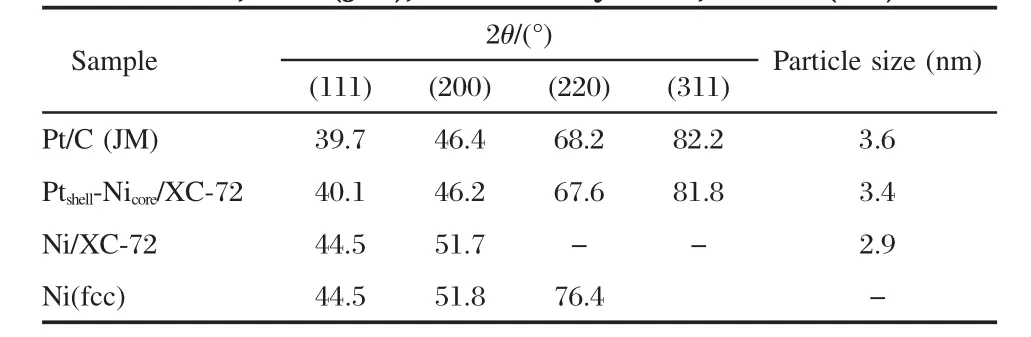
Table 1 Structure and mean particle size of the Ptshell-Nicore/ XC-72,Pt/C(JM),Ni/XC-72 by XRD,and Ni(fcc)
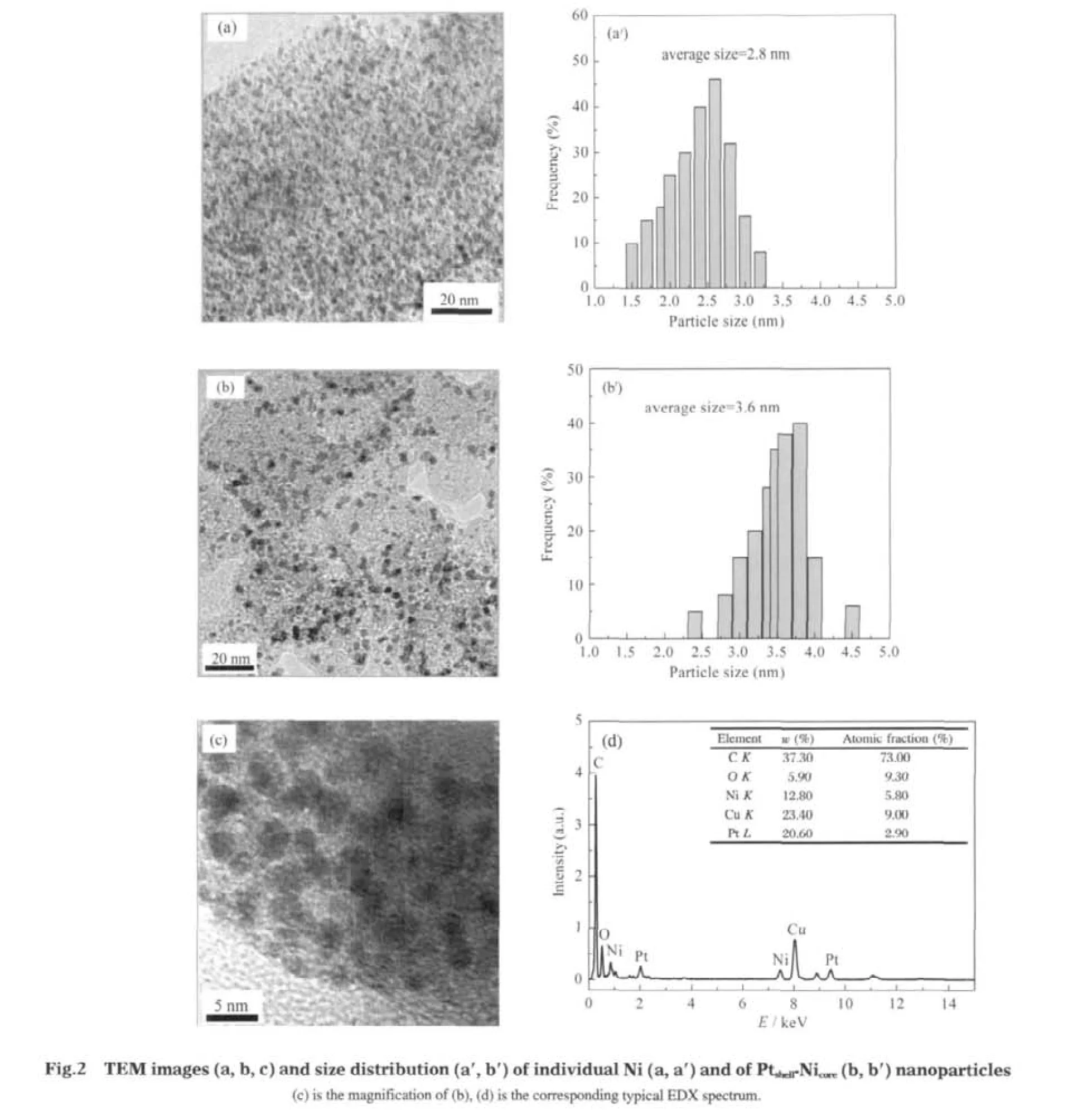
TEM was used to characterize the morphology of the synthesized catalysts.As shown in Fig.2,all the nanoparticles are well dispersed without agglomeration and present a narrow size distribution,as a result of the stabilizing effect of CTAB.Fig.2(a,a′) shows the images of individual Ni nanoparticles and the corresponding size histogram.The spherical particles are well-separated and monodisperse with a main size of 2.6 nm.Fig.2(b,b′) shows the same particles after replacement of Pt,leading to deposition of metallic Pt on the surface of the Ni seeds with a main size of 3.6 nm.The average particle size of Ptshell-Nicoreparticles is 3.6 nm,in good agreement with that from the above XRD measurement.The smaller average particle size and good dispersion of Ptshell-Nicore/XC-72 might be beneficial for electrocatalysis.Additionally,the presence of both Pt and Ni metals was qualitatively confirmed by energy-dispersive X-ray(EDX)spectrometric analysis.
The formation of Pt-shell via chemical replacement and the core-shell like structure before catalyst depositing on the carbon was investigated by UV-Vis spectroscopy.A certain molar quantity of NaH2PO2,equivalent to Ni(Ac)2,was introduced to prepare Ni-seed,which insufficiently reduced Ni(Ac)2.Therefore,it ensures there is no excess reducing remaining agent before adding H2PtCl6into the Ni-seed solution,to form the Ptshell through the replacement reaction.
As shown in Fig.3,a peak at 350 nm was assigned to the Ni(Ac)2solution,after which it was reduced by NaH2PO2,a peak at 400 nm detectable in curve(a)appears.As pure Ni is not UV-visible active[17],the peak at 400 nm is also an absorption band of the remnant Ni ions,so there is no excess reducing agent.This red shift behavior may be caused by the citrate complex of Ni(II). The curve of H2PtCl6solution shows a peak at 260 nm as a result of the ligand-to-metal charge-transfer transition in the PtCl2-6[21], and Pt is also not UV-visible active.After adding H2PtCl6to Nicore colloid,the peak at 260 nm was no longer visible from curve b(Ptshell-Nicore),suggesting that all PtCl2-6ions were completely reduced.Conclusively,a Pt-shell was formed by galvanic replacement,and then the Ni-Pt core-shell structure generated, for there is no excess reduction agent before adding the H2PtCl6.
This galvanic replacement mechanism can summarized as follows:the process of Pt shells forming on Ni cores is driven by an in situ replacement reaction between the Ni atoms and PtCl2-6ion without any additional reducing agent.When PtCl2-6ions in a positive metal oxidation state approach the surface of Ni nanoparticles,they can be directly reduced to Pt through the sacrificial oxidation of the nickel atoms and simultaneous deposition of Pt on the surface.Meanwhile,the Ni atoms are oxidized to Ni2+and the electron transfer between the two metals results in core-shell type nanoparticles.This redox reaction can spontaneously proceed under the favorable potential generated between the two metals,this method has been regarded as an efficient route for the selective formation of bimetallic structures[22]. The reaction mechanism of the replacement process can be ex-pressed as:
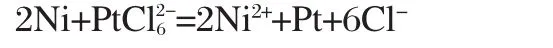
In order to examine the coating quality of the nanoparticles, CV,a surface sensitive technique,is used to further examine the core-shell structure.
CV curves of the Ni/XC-72 and Ptshell-Nicore/XC-72 in 1 mol·L-1KOH are compared in Fig.4.As shown in Fig.4,the curve of Ni/ XC-72 exhibits two redox peaks within the potential range of 0.2-0.4 V(vs SCE),which can be considered as the following redox reaction[23]:
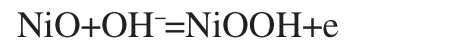
No redox peaks can be seen after forming the Ptshell-Nicorestructure,suggesting the absence of large and isolated Ni or nickel oxide particles on the surface of Ptshell-Nicore.Pt was therefore presented as a shell wrapping the Ni core via a galvanic replace-ment reaction.
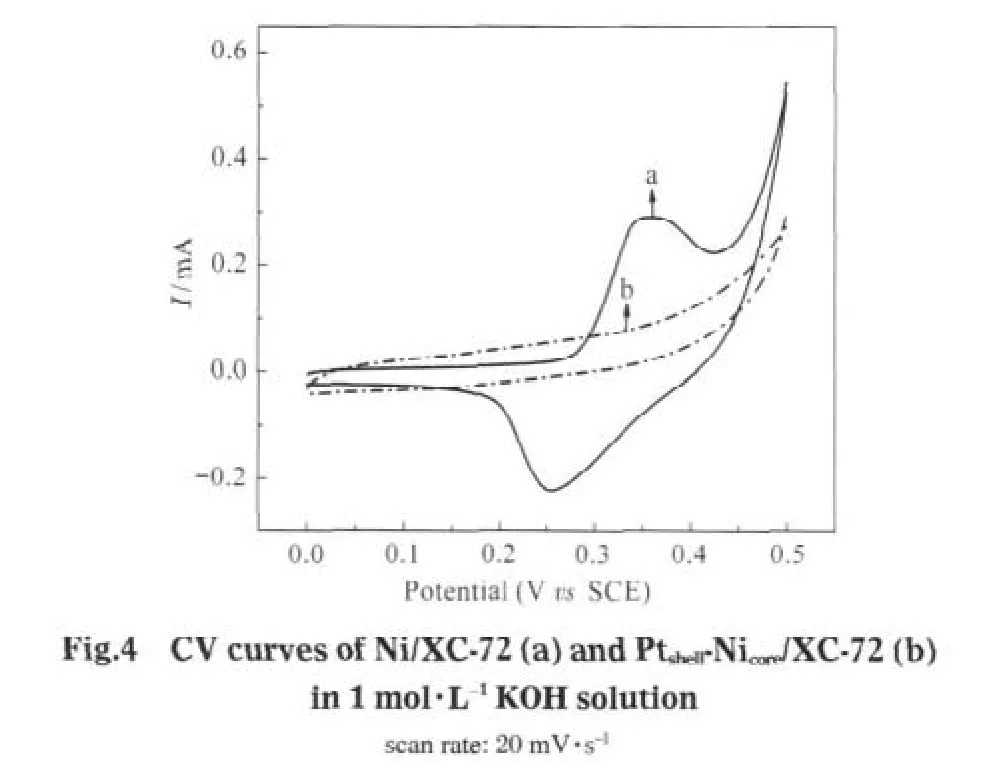
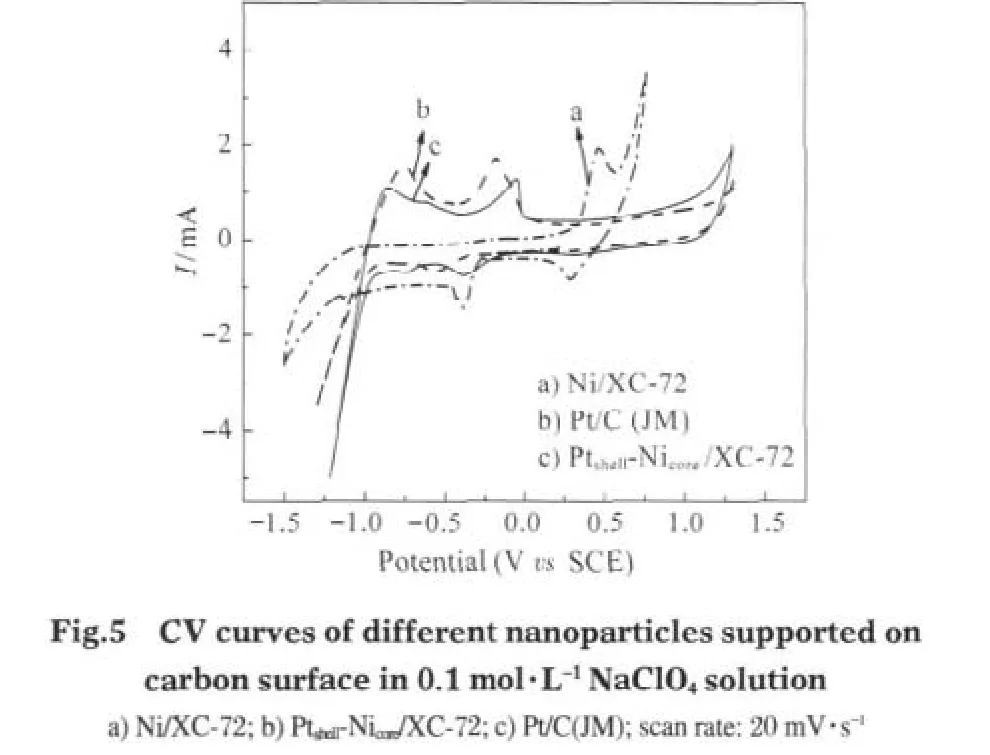
CV curvesofNi/XC-72,Pt/C(JM),and Ptshell-Nicore/XC-72 in 0.1 mol·L-1NaClO4are shown in Fig.5.The CV curve of Ni/XC-72 exhibits the oxidation peak and reduction peaks at about 0.50, 0.32,and-0.40 V,respectively,and an indistinct oxidation peak at-0.25V,whichmaybe explained asfollows:-0.25 and-0.40 V are two of the redox peaks,corresponding to the oxidation process of Ni to Ni2+.Likewise 0.50 and 0.32 V are similar to the negative couple,corresponding to the redox between Ni2+and Ni3+[24].
Compared to Ni/XC-72,the CV curve of Ptshell-Nicore/XC-72 shows only a couple of redox peaks similar to that of the Pt/C (JM),indicating that the Ptshell-Nicore/XC-72 is representative of the electrochemical properties of the pure Pt surface.The absence of redox peaks characteristic of Ni indicates that the Ni core is completely covered by the Pt shell and there are no discernible Ni sites,also in agreement with the analysis by CV in KOH electrolyte solution.However,The redox peak of Ptshell-Nicore/XC-72,shifting to the range of higher potential,still differs from that of Pt/C(JM),indicating that the Pt-shell is slightly different from that of pure Pt surface,which is likely due to the structural-and electronic-effects between Pt-shell and Ni-core. Conclusively,it′s suggested that the Pt-Ni particles have a Ptshell-Nicorestructure.
2.2 Electrocatalytic performance of catalysts
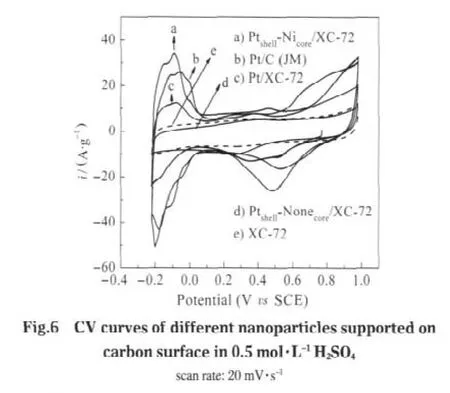
To learn more about the practical performance of the Ptshell-Nicore/XC-72 catalysts and to investigate the Pt utilization and activity with this core-shell structure,CV tests were performed in 0.5 mol·L-1H2SO4.From Fig.6,it can be clearly seen that Ptshell-Nicore/XC-72,Pt/C(JM),and Pt/XC-72 exhibit distinct hydrogen evolution peaks.Whereas for the Ptshell-Nonecore/XC-72,no hydrogen adsorption/desorption peaks but only a capacitance characteristic of XC-72 was seen,indicating the non-existent of Pt,this proved that the reducing agent was thoroughly removed before adding the H2PtCl6in our method,which can ensure that Pt shell was acquired via replacement reaction.For the catalysts,the hydrogen region from-0.22 to 0.20 V(vs SCE)corresponds to the reductive adsorption of protons in the cathodic scan and the subsequent oxidation of the hydrogen adatoms in the anodic scan[25]. A smaller oxidation charge in this region for Pt/C(JM),and especially for Pt/XC-72,implies a smaller electrochemical active area for these catalysts compared to Ptshell-Nicore/XC-72.
In general,the electrochemically active surface(SA)of Pt particles is used to reflect the intrinsic electrocatalytic activity[26].SAis calculated by the formula[27]:
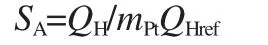
where Q is the total charge,mPtis the Pt loading on glassy carbon substrate,and QHrefis assumed to be 0.21 mC·cm-2corresponding to a surface density of 1.3×105atom·cm-2of Pt,the parameters of the electrochemical performance are listed in Table 2.Obviously,the Ptshell-Nicore/XC-72 shows excellent performance with the SAvalue as high as 100.1 m2·g-1,which is 1.2 times as large as that of Pt/C(JM)(84.7 m2·g-1)and 2.5 times as large as that of Pt/XC-72(40.3 m2·g-1),even though the content of Pt (8%(w))is 40%that of Pt/C(JM,20%(w)),indicating a higher Pt utilization and activity with the Ptshell-Nicorestructure.In ourprevious work,the SAof Pt/CMK-5 reaches a value of 100-138 m2·g-1(20%(w)Pt)[28].On the premise of reducing usage of Pt, loading Pt particles on commercial carbons with this core-shell structure obtain the performance level similar to Pt/CMK-5, which also indicates a high activity of core-shell nanopaticles in the electrocatalysis field.
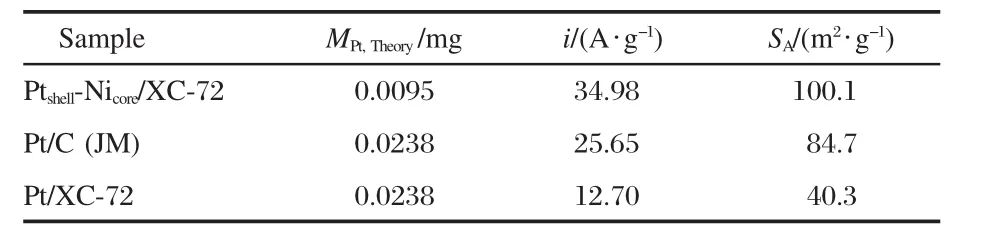
Table 2 Electrochemically active surface areas(SA)of Ptshell-Nicore/XC-72,Pt/C(JM),and Pt/XC-72
3 Conclusions
In summary,highly active and dispersed Ptshell-Nicore/XC-72 electrocatalyst was successfully synthesized by an in situ galvanic replacement strategy.The formation of core-shell like structure has been supported by various techniques including XRD, UV-Vis,and electrochemical techniques.Cyclic voltammetry showed that the Ptshell-Nicore/XC-72 had a higher activity with lower loadings of Pt compared to Pt/C(JM),which might have resulted from the enhanced Pt utilization by the core-shell like structure.Consequently,the carbon-supported core-shell nanoparticles have a better potential for application as catalysts for fuel cells.
1 Arico,A.S.;Srinivasan,S.;Antonucci,V.Fuel Cells,2001,1:133
2 Ye,J.S.;Cui,H.F.;Wen,Y.;Zhang,W.D.;Xu,G.Q.;Sheu,F.S. Microchim.Acta,2006,152:267
3 Ralph,T.R.;Hogarth,M.P.Platinum.Met.Rev.,2002,46:3
4 Gottesfeld,S.;Raistrick,I.D.;Srinivasan,S.J.Electrochem.Soc., 1987,134:1455
5 Jalan,V.;Taylor,E.J.J.Electrochem.Soc.,1983,130:2299
6 Mukerjee,S.;Srinivasan,S.;Soriaga,M.P.;McBreen,J.J.Phys. Chem.B,1995,99:4577
7 Min,M.K.;Cho,J.;Cho,K.;Kim,H.Electrochim.Acta,2000, 45:4211
8 Drillet,J.F.;Ee,A.;Friedemnan,J.;Kotz,R.;Schnyder,B.; Schmidt,V.M.Electrochim.Acta,2002,47:1983
9 Paffet,M.T.;Beery,J.G.;Gottesfeld,S.J.Electrochem.Soc., 1988,135:1431
10 Scott,R.W.J.;Wilson,O.M.;Oh,S.K.;Kenik,E.A.;Crooks,R. M.J.Am.Chem.Soc.,2004,126:15583
11 Toshima,N.;Yonezawa,T.New J.Chem.,1998,22:1179
12 Schmid,G.;West,H.;Mehles,H.;Lehnert,A.Inorg.Chem.,1997, 36:891
13 Rolison,D.R.Science,2003,299:1698
14 Ji,M.;Chen,X.Y.;Wai,C.M.;Fulton,J.L.J.Am.Chem.Soc., 1999,121:2631
15 Liu,C.;Wu,X.W.;Klemmer,T.;Shukla,N.;Yang,X.M.;Weller, D.;Roy,A.G.;Tanase,M.;Laughlin,D.J.Phys.Chem.B,2004, 108:6121
16 Liu,Z.;Guo,B.;Hong,L.;Lim,T.H.Electrochem.Commun., 2006,8:83
17 Chen,D.;Li,J.;Shi,C.;Du,X.;Zhao,N.;Sheng,J.;Liu,S.Chem. Mater.,2007,19:3399
18 Zhang,J.;Lima,F.H.B.;Shao,M.H.;Sasaki,K.;Wang,J.X.; Hanson,J.;Adzic,R.R.J.Phys.Chem.B,2005,109:22701
19 Antolini,E.;Salgado,J.R.C.;Gonzalez,E.R.J.Electroanal. Chem.,2005,580:145
20 Antolini,E.;Cardellini,F.J.Alloy.Compd.,2001,315:118
21 Teranishi,T.;Hosoe,M.;Tanaka,T.;Miyake,M.J.Phys.Chem.B, 1999,103:3818
22 Bosnich,B.Inorg.Chem.,1999,38:2554
23 Wang,Y,G.;Xia,Y.Y.Electrochim.Acta,2006,51:3223
24 Bao,F.;Li,J.F.;Ren,B.;Yao,J.L.;Gu,R.A.;Tian,Z.Q.J.Phys. Chem.C,2008,112:345
25 Papageorgopoulos,D.C.;Keijzer,M.;Veldhuis,J.B.J.;de Bruijn, F.A.J.Electrochem.Soc.,2002,149:A140
26 Wang,L.;Zhao,Y.;Lin,K.;Zhao,X.;Shan,Z.;Di,Y.;Sun,Z.; Cao,X.;Zou,Y.;Jiang,D.;Jiang,L.;Xiao,F.S.Carbon,2006, 44:1336
27 Kim,H.J.;Kim,D.Y.;Han,H.;Shul,Y.G.J.Power Sources, 2006,159:484
28 Zhou,J.H.;He,J.P.;Dang,W.J.;Zhao,G.W.;Zhang,C.X. Electrochemical and Solid-State Letters,2007,10:B191

