Recent progresses of exosome-liposome fusions in drug delivery
Lulu Zheng ,Bo Hu ,Deyo Zho ,Wenjing Liu ,Qing Liu ,Yunyu Hung,* ,Shoo Run,*
a School of Life Science,Advanced Research Institute of Multidisciplinary Science,School of Medical Technology,Key Laboratory of Molecular Medicine and Biotherapy,Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering,Beijing Institute of Technology,Beijing 100081,China
b Department of Radiation Oncology,The First Affiliated Hospital of Zhengzhou University,Zhengzhou 450000,China
c Beijing Key Laboratory of Drug Resistance Tuberculosis Research,Beijing Tuberculosis and Thoracic Tumor Research Institute,and Beijing Chest Hospital,Capital Medical University,Beijing 101149,China
Keywords: Exosome Liposome Membrane fusion Drug delivery Disease treatment
ABSTRACT Exosomes are membrane-bound nanoscale extracellular vesicles,which produced by almost all organisms.Due to the excellent biocompatibility,long circulation time as well as low immunogenicity,exosomes as naturally-derived drug delivery carriers have experienced explosive growth over the past decades.However,issues such as insufficient loading efficiency,heterogeneous delivery efficiency,uncontrollable targeting ability,and low production limit their wide application.Recently,the emerging exosome-liposome fusion strategy has become a potential approach to solve such issues.Thus,this review mainly focuses on the currently developed exosome-liposome fusion strategy and their application in drug delivery as well as disease treatment.This review aims to shed light on the advantages of fusion strategy in drug delivery and provides a better understanding for more rational design.The current challenge and future perspective regarding their clinical translation and application will also be discussed.
1.Introduction
Exosomes are membranous vesicles with 30-150 nm in diameter,which can be produced by almost all types of cells [1].Exosomes were first discovered in sheep reticulum cells in 1983[2] and the terminology of exosomes was first proposed by Johnstone in 1987 [3].Although accumulating studies have been documented,the mechanisms involved in exosome biogenesis remain largely unknown.It is now widely believed that the biogenesis of exosomes is mainly originated from the endocytic pathway.Briefly,lipid raft domains of the plasma membrane sag inward to form early endosomes.Then,they mature and form late endosomes with the assistance of the Golgi complex.Next,the endosomal membrane buds inward to generate intraluminal vesicles (ILVs) and further aggregates to form multivesicular bodies (MVBs).Finally,the ILVs are released into extracellular milieu as exosomes once MVBs fuse with the plasma membrane [4].Exosomes contain a variety of bioactive components,including proteins,lipids,nuclear acids and metabolites [5].Despite that different cell-derived exosomes exhibit a high degree of heterogeneity and diversity,most of them share similar surface membrane components such as cholesterol,sphingomyelin,tetraspanins (CD9,CD63,CD81),integrins,as well as intraluminal components,such as heat shock proteins (HSP60,HSP70,HSP90),Alg2-interacting protein X (ALIX),and tumor susceptibility gene 101 (TSG101) (Fig.1) [6,7].Because of the ability to transfer these bioactive components between cells,exosomes are now considered as novel intercellular messengers.Given their important role in intercellular communication,they have been confirmed to be involved in a variety of biological or pathological processes [8] such as immune surveillance [9],tumor microenvironment formation [10],regulation of inflammation [11],viral infection [12].Moreover,owing to their abundant and informative components,exosomes have been widely used for disease treatment[13],liquid biopsy [14],prognosis prediction [15].

Fig.1.Biogenesis and composition of exosomes.
Recently,using exosomes as drug delivery system has attracted increasing attention due to their intrinsic advantages,such as good biocompatibility [16],low toxicity [17] and immunogenicity [18],prolonged circulation time [19],tissue-or cell-homing tendency[20] and biological barrier penetration ability [21-23].Unfortunately,the wide application is hindered by their insufficient loading efficiency [24],limited targeting specificity [25],heterogeneous delivery efficiency [26,27] and low production [28].To solve these problems,various strategies have been proposed,such as engineering exosomes using genetic editing [29],membrane surface modification [30],physical pathway-mediated membrane disruption [31-34].More recently,the fusion of exosomes with liposomes has emerged as a promising strategy.Liposomes are selected because they have the characteristics including flexible modification,sufficient loading and high delivery efficiency [35].More importantly,they share similar structure and composition with exosomes.However,issues such as rapid clearance [36],limited targeting ability[37] remain the major challenges in drug delivery.Therefore,the fusion strategy may take advantages of both and achieve desirable delivery performance and therapeutic effect [38].Given that these exosome-liposome fusion nanoparticles (EL-FNP) are composed of naturally-occurring and the Food and Drug Administration (FDA)-approved components,they may have the chance to move from bench work to clinical application.
Currently,a growing number of reviews have been well documented regarding exosome or liposomes,such as treatment [4],drug delivery [39] and diagnosis [40].However,few works have attempted to discuss the applications of EL-FNP in drug delivery and disease treatment.This review started with a brief summary of exosome biogenesis.Then,the applications of exosomes and liposomes alone in drug delivery were reviewed.Last,we focused on the currently developed strategy for EL-FNP and highlighted their applications in drug delivery and disease treatment.The challenge and future perspective regarding the clinical translation and application will also be discussed.
2.Exosomes for drug delivery
The natural vesicle structure,excellent biocompatibility and low immunogenicity endow exosomes the ability to serve as drug delivery carriers.Exosomes derived from normal cell could avoid the clearance by reticuloendothelial system (RES) and prolong blood circulation time [41].In addition,the specific components also improve their pharmacokinetic profiles and targeting ability [42-44].Compared with synthesized nanocarriers,exosomes possess a variety of advantages as drug delivery carriers and have been widely investigated in clinical studies (Table S1 in Supporting information).
2.1. Intrinsic targeting
Exosomes are important tools for remoting intercellular communication [1].Accumulating studies have revealed that various molecules docked on the exosome membrane are involved in these processes,such as tetraspanins [45],integrin [46,47],lactadherin[48],phosphatidylserine (PtdSer) [49],MHC class I (MHC-I) [50] or MHC class II (MHC-II) [51].These membrane-bound molecules can be identified as ligands to recognize a specific target on the recipient cells and thus mediate active targeting delivery or antigen presentation,which highly relies on the cell origin [52,53].For example,PtdSer can specifically recognize and bind to PtdSer receptors(Tim1 or Tim4) that are expressed on recipient cells [54].Moreover,integrinα4β1specifically recognizes and binds to vascular cell adhesion molecules 1 (VCAM-1) which overexpressed on tumor cells [55].Given the natural targeting ability,many researchers have developed exosomes as active targeting drug delivery carriers.For instance,Yuanetal.collected exosomes from macrophages and then used for the delivery of brain-derived neurotrophic factor (BDNF) to inflammation sites in the brain.The abundant integrin lymphocyte function-associated antigen 1 (LFA-1) docked on macrophage exosomes interacts with the intercellular adhesion molecule 1 (ICAM-1) expressed on brain microvascular endothelial cells,thereby mediating the transcytosis across the blood-brain barrier.They also demonstrated that ICAM-1 expression was upregulated in areas of brain inflammation,resulting in increased delivery efficiency [56].Similarly,Wuetal.collected exosomes derived from M2 macrophages and loaded hexyl 5-aminolevulinate hydrochloride (HAL) into exosomes by electroporation to obtain HAL-containing M2 exosomes (HAL@M2 Exo).Compared with free HAL,HAL@M2 Exo could target chemokines in atherosclerotic sitesviaexosomal chemokine receptors.Following intravenous injection,HAL@M2 Exo exhibited an excellent anti-atherosclerotic treatment outcome,with a 75.2% reduction in inflammation-induced aortic lesion area and a 73.9% reduction in aortic valve lesions [57].
2.2. Prolonged circulation time
The exosome surface proteins,such as CD55 and CD59,play a critical role in increasing the duration of exosomes by avoiding the absorption of opsonins and coagulation factors [24].The reduced opsonization and opsonization-mediated RES capture contribute to lower non-specific distribution and longer blood circulation time.In addition,CD47,an integrin-associated transmembrane protein expressed on the surface of exosomes,can bind to signal regulatory proteinα(SIRPα) on the surface of macrophages [58].The CD47-SIRPαreleases “don’t eat me” signal that avoids the phagocytosis of exosomes by macrophages,further extending the circulation time of exosomes.To prove this,Kamerkaretal.collected exosomes from normal human foreskin fibroblasts and prepared liposomes for comparison.To achieve tumor suppression,the exosome and liposome were engineered to carry siRNA or shRNA targeting KRASG12D.KRAS is known as a membrane-bound GTPase which is widely involved in cell growth,migration,and survival[59],KRASG12Dis the most common mutation of KRAS in pancreatic cancer inducing tumor progression and metastasis [60].After intraperitoneal injection (24 h),exosomes,but not liposomes were detected in plasma,demonstrating that exosomes exhibited increased blood circulation time compared to liposomes [46].
2.3. Flexible engineering
To further improve delivery efficiency,engineering exosomes with desirable properties or functions have attracted increasing attention [22,61-63].Currently,two main ways have been developed: Genetic engineering [64,65] and surface modification [66].Genetic engineering can alter the membranous and intraluminal components of exosomesviamanipulating donor cells.Targeted peptides or proteins can be artificially expressed on exosome surfaces by transfecting intended gene sequences into donor cells.For example,Tianetal.designed a vector which expressed the fusion protein of theαv integrin-specific internalizing RGD (iRGD) sequence and lysosome-associated membrane protein 2b (Lamp2b).The murine immature dendritic cells (imDCs) were then transfected with the vector.Invitroandinvivostudies demonstrated that the isolated exosomes carried iRGD on their surface and showed active targeting toαv integrin-positive breast cancer cells[67].Similarly,various intended peptides or proteins can be engineered onto the surface of exosomesviathis genetic approach,exhibiting the controllability and flexibility.
Surface modification allows for more kinds of functional ligands docking on exosomesviabiological,physical and chemical methods.Biological approaches take advantage of the existence of membrane-bound proteins on exosomes.These proteins can be used as a linking medium or an affinity tag to introduce exogenous functional moieties onto exosome surface [24].For instance,tetraspanins (CD9,CD63,CD81) are typical transmembrane proteins consisting of four transmembrane domains.They consist of an extracellular amino tail,a cytoplasmic carboxylic,and two loops[68].It was proven that peptide CP05 can specifically bind with the second extracellular loop of CD63 [69].Gaoetal.conjugated CP05 with a phosphorodiamidate morpholino oligomer (PMO) and then bonded the complex to exosomes to form EXOPMOfor treating Duchenne muscular dystrophy.Tetraspanins tend to interact with other exosomal membrane proteins,such as MHC proteins or integrins,to form a tetraspanin-enriched microdomain (TEM).TEM not only maintains the stability of tetraspanin but also serves as a binding site for different ligands [70].Compared to naked PMO,EXOPMOshowed an 18-fold increase of dystrophin expression in the quadriceps [71].
In addition,functional moieties can also be physically embedded in the exosome membrane.For example,Kooijmansetal.conjugated an epidermal growth factor receptor (EGFR)-targeting ligand with 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine(DMPE)-polyethylene glycol (PEG) to create a lipid composition with EGFR-targeting capability.After incubation,this lipid composition spontaneously embeds into the exosomal membrane.The results showed that modified exosomes exhibit significantly higher affinity to tumor cells with high EGFR expression.The physical modification does not affect the morphology,size,and surface protein of the exosomes,However,since the process is autonomous,the number and efficiency of embedding ligands cannot be easily controlled [72].
Moreover,covalent binding functional groups onto exosomes surface can be achieved by chemical methods.Tianetal.conjugated exosomes with c(RGDyK) peptide by bio-orthogonal copperfree azide alkyne cycloaddition.The modified exosomes (cRGDExo) showed higher affinity to integrinαvβ3.After intravenous injection,cRGD-Exo was more effectively enriched in cerebral vascular endothelial cells and resulted in stronger suppression of the inflammatory response and cellular apoptosis in the lesion region[73].Nieetal.successfully modified macromolecules,antibodies of CD47 and SIRPα,onto M1 macrophage exosome membranes using click chemistry.After systemic administration,ligands on the surface of engineered exosomes specifically recognized receptors on the surface of tumor cells and resulted in remarkably greater tumor accumulation.So far,click chemistry is the most popular chemical method since it takes advantages of good biocompatibility and high reaction efficiency [74].However,the click reaction requires azide and alkyne groups,additional metabolic engineering treatments need to be introduced to make exosomes with azide groups on their surface.
2.4. Versatile drug loading
To encapsulate intended drug into exosomes,a variety of endogenous or exogenous drug loading methods for exosomes have been developed (Fig.2).The endogenous loading strategy is mainly achieved by hijacking the biogenesis process of exosomes to package drugs into exosomes [75].During exosomes biogenesis,drugs are required to be pre-loaded into donor cells so that they can be packaged into intraluminal space or onto the membrane of exosomes [76,77].This approach avoids the waste of drugs and the possibility of damaging exosomes by omitting the step of wrapping drugs.By contrast,the exogenous loading strategy refers to the directly incubate drugs with exosomes after their biogenesis[78,79].These methods are simple to operate and therefore more popular than endogenous loading methods.Electroporation is the most common approach because it possesses a relatively higher drug loading efficiency [80].However,it is necessary to choose the appropriate loading method based on the experimental setting,exosome source,and physical or chemical properties of drugs [81].
Theoretically,the exosome bilayer and compartment structures allow them to carry a variety of drugs,including proteins [13],small molecules [82] and nuclear acids [83].
Nucleic acids drugs,including siRNA,miRNA,mRNA,and DNA are often used in the treatment of tumors [84-88] or other diseases [89-92].Nucleic acid drugs loaded by exosomes can achieve better efficacy.For example,Didiotetal.co-incubated siRNA with exosomes derived from U87,a glioblastoma cell line.Compared with naked siRNA,siRNA loaded in U87-derived exosomes were effectively internalized by mouse primary cortical neurons and caused effective silencing of target genes.The physicochemical characteristics of exosomes are not altered by siRNA loading.However,the loaded siRNA affected the surface charge of exosomes,which disturbs the intrinsic exosome uptake mechanism [93].Besides,Squadritoetal.controlled the expression of miRNAs or targeted transcripts promoting miRNA relocation from the cell cytoplasm or P bodies to MVBs.Using endothelial cells as a cellular model,the researchers proved that exosomes could transfer miRNA from macrophages to endothelial cells.More importantly,miRNAloaded exosomes inhibited the expression of target sequences in endothelial cells.In addition,miRNA enrichment in exosomes can be achieved by stimulating and activating the cellular production of miRNA-related pathways or by artificially overexpressing miRNAs in cells,all of which increases the potential for miRNA applications [94].
In addition,proteins can also be loaded into exosomes by both exogenous and endogenous ways.Haney and colleagues first transfected macrophages with plasmids expressing catalase,and the intended enzyme was detected in exosomes.After systemic administration,such genetically modified exosomes produced catalase in brain for at least a month,and resulted in significant improvements in motor functions in mice with Parkinson’s disease (PD)[95].Subsequently,they tried to exogenously load catalase into monocyte/macrophage-derived exosomes by several ways.The obtained exosomes showed desired size in a range of 100-200 nm.After intranasal administration,a significant number of exosomes was detected in PD mouse brain.Notably,those exogenous loading methods could also achieve high catalase loading efficiency [13].
Besides,exosomes are effective carriers of a variety of small molecule drugs.For example,exosomes had been shown to deliver curcumin,an anti-inflammatory molecule,to the target site.The curcumin delivered by exosomes was more stable in the blood and was efficiently enriched in the lesion [96].Compared with free drugs,exosome-loaded drugs have higher bioavailability and lower side effects,which is beneficial for clinical translation and application.
3.Liposomes for drug delivery
Liposomes are a class of well-researched drug delivery vesicles with multifunction.Compared to other synthetic drug delivery systems,liposomes are featured by low immunogenicity,excellent biocompatibility,easy preparation and modification [97-99].Given these advantages,numerous liposome-based nanomedicines have undergone clinical investigations and some of them are already approved for clinical applications (Table S2 in Supporting information).
3.1. Composition and preparation of liposomes
Liposomes share a similar bilayer membrane structure with cell,which are mainly composed of phospholipids and cholesterol[100].Phospholipids are amphiphilic molecules and tend to form a lipid bilayer structure in the water environment,which thus serves as the molecular skeleton of liposomes.Cholesterol is another commonly used ingredient of liposomes,it improves the fluidity of the bilayer and reduces the permeability of water-soluble molecules through the membrane.The inclusion of cholesterol also contributes to the greater stability of liposomesinvivo[101].Liposomes can spontaneously form in aqueous solution once phospholipid components are added.The interactions between hydrophobic groups form a single or multilayer vesicle with a hydrophilic head on the outside and a hydrophobic tail on the inside [102].Therefore,synthesized liposomes are now considered to have similar structure to exosomes.Despite of similar structure,they have own characters in physiochemical properties and composition (Table 1).

Table 1Comparison of physiochemical and structural properties between exosomes and liposomes.
3.2. The advantages of liposomes in drug delivery
3.2.1.Variousdrugloadingtypes
The hydrophobic drugs can be inserted into the bilayer structure of the phospholipid membrane while hydrophilic drugs can be encapsulated into the compartments inside the liposomes [105-108].In addition,the introduction of lipids with different charge alters the surface charge of the liposomes,providing an opportunity for loading charged drugsviaelectrostatic adsorption [109].Therefore,liposomes have become the most popular delivery carrier for a variety of drugs,including nucleic acid drugs (e.g.,siRNA[110,111],miRNA [112],mRNA [113],clustered regularly spaced short palindromic repeats-associated protein (CRISPR/Cas) [114]),protein drugs [115-117],polypeptide drugs [118],small molecule drugs [119-121].Apart from these conventional drugs,liposomes are also used for the delivery of metallic and alloy materials [122],rare gases [123],bone-related materials [124] and so forth.For example,Dandekaretal.prepared xenon gas (Xe) encapsulated liposomes (Xe-liposomes),which led antidepressant effects in clinical studies.The analysis results of the frontal cortex showed that Xeliposomes quickly mediated antidepressant-like effects,effectively overcoming the shortcomings of clinical oral therapeutic drugs,such as short half-life,multiple medications,and long medication cycles [123].Currently,liposomes are extensively used as carriers in the cosmetic.For example,Lietal.used liposomes loaded with astaxanthin to enhance the antioxidant capacity of skin.The thermal stability of astaxanthin was significantly improved after the encapsulation,with the initial decomposition temperature of astaxanthin increasing from 220 °C to 315 °C according to thermogravimetric analysis.In addition,a 17-time higher solubility of liposome-encapsulated astaxanthin in water was determined by dissolution experiments [125].In addition,liposomes also have a wide range of applications in pharmaceutical,food and farming industries [126-128].
3.2.2.Easymodification
Owing to the unique composition and existence of functional group,liposomes modification can be pursed.At present,modification of liposomes has two main aspects: surface modification and composition optimization.Surface modification refers to the binding of functional molecules to the surface of liposomes through various reactions without changing the original structure of liposomes [129].For example,modification of PEG prolonged the circulation time by forming a “cloud” layer of spatial hydration on the surface of nanoparticles that prevents absorption of plasma proteins,recognition by RES,and phagocytosis [130,131].In addition,functional groups,such as targeting motif,can be modified onto the surface of liposome too.Wangetal.functionalized liposome with mannose endowing the engineered nanoparticle (MANLP) macrophage targeting ability.Invitrotest proved that MAN-LPs were significantly higher enriched in RAW264.7 cells than unmodified liposome.Notably,this work also reported that the size of liposome can be controlled by adjusting the stirring rate alone,and the larger MAN-LP leaded to higher intestinal enrichment [132]
In addition to the basic composition,other materials,such as temperature-sensitive [133,134],photosensitive [135,136],magnetic sensitive [137] materials,can also be introduced into liposomes.For example,Yamazakietal.used methoxy diethyleneglycol methacrylate (MD),methacrylic acid (MAA) and lauroxy tetraethyleneglycol methacrylate (LT) as temperature-sensitivity,pH-sensitivity and anchoring materials,respectively,to form a random copolymer (poly(MD-MAA-LT)).The water solubility of the copolymer varies with the temperature and pH changes,thus endowing the modified liposome with both temperature and pH sensitivity.When circumstance is acidic and temperatures is above 35°C,the carboxyl on the polymer chain formed hydrogen bonds with the phosphate on the liposome membrane,which caused membrane cleavage or destabilization.Based on this,the drug was effectively released in tumor microenvironment [138].
4.Exosome-liposome fusion strategy
4.1. The advantages of EL-FNP
The EL-FNP retain almost components of exosomes and liposomes (Fig.3).Since the hybrids retain the advantages of both and to some extent address their own disadvantages,the exosome and liposome fusion strategy is receiving increasing attention.Compared with exosomes alone,EL-FNP have the following advantages:(1) Increased drug loading efficiency: typically,the drug loading efficiency of exosomes is less than 30% [13].EL-FNP are expected to provide higher drug loading efficiency.Huetal.compared the encapsulation efficiency of exosome and EL-FNP to antagomir-188,and found that the EL-FNP achieved a significantly higher encapsulation efficiency than exosome alone [139].(2) Improved yield efficiency: although a huge number of studies on exosomes are currently ongoing,very limited cases can actually enter into clinical application [140].Insufficient exosomes production is one of the major obstacles [141].Alternatively,the yield can be increased to great extent by replacing part of exosomes with easily prepared liposomesviathe fusion strategy [142].

Fig.3.Fusion of liposomes and exosomes.liposomes (upper left),and exosomes(upper right) from complexes through membrane fusion.
Compared with conventional liposomes,EL-FNP have the following advantages: (1) Active targeting ability: EL-FNP inherit the natural targeting motifs of exosomes which can direct the biodistribution of nanoparticles [143].Sunetal.demonstrated that fibroblast-derived EL-FNP showed fibroblast homing properties.Researchers found that EL-FNP preferentially enriched in fibrotic lung and significantly increased infiltration in pulmonary fibrotic tissue [38].(2) Increased circulation time: as mentioned above,exosomal surface molecules CD47,CD55 and CD49 can avoid the clearance by MPS [24].As expected,EL-FNP also possess CD47,CD55 and CD49 on their surface,which may also reduce MPS recognition and phagocytosis.(3) The ability to cross the biological barrier: previous studies have shown that exosomes were able to cross the blood-brain barrier and placental barrier through receptor-mediated endocytosis [21,23].Despite of the lack of crossphysiological barrier studies,we infer that the EL-FNP retain the ability to cross physiological barriers.
4.2. Fusion methods
4.2.1.Directincubation
The simplest method to form EL-FNP is direct incubation through hydrophobic interactions under temperature-dependent conditions.By direct incubation at 37 °C for 12 h,exosomes and liposomes can fuse together to form hybrid nanoparticles with high fusion efficiency and strong stability.For example,Linetal.used the above conditions fused exosomes and liposomes.To further confirm the generation of EL-FNP,they analyzed the size distribution of liposomes,exosomes,and the hybrid nanoparticles.The results showed that the hybrid nanoparticles were slightly larger than exosomes or liposomes in size and had a vesicle-like morphology.Western blot analysis confirmed that marker proteins for exosomes were also detected on the hybrid nanoparticles,which was solid evidence of the successful fusion [144].The direct incubation method can easily induce the fusion of exosomes and liposomes without additional operation steps and special incubation conditions.However,the EL-FNP obtained by this method may have an increased size and heterogenous size distribution [145].
4.2.2.Freeze-thaw
The freeze-thaw process can disrupt the original structure of liposomes and exosomes during the formation of ice crystal[146].Subsequently,components of both tend to recombine to form new membrane fragments.In one study,Satoetal.isolated exosomes from human epidermal growth factor receptor-2(HER2)-expressed CMS7 cells.The HER2-expressed exosomes and fluorescence-labeled liposomes were mixed at a volume ratio of 1:1.To induce membrane fusion,they froze the mixture in liquid nitrogen,and then thawed it at room temperature for 15 min.To demonstrate the success of the fusion,the researchers examined the morphology,particle size and polydispersity index (PDI)of the nanoparticles,respectively.The results showed no significant change of morphology of the resulting EL-FNP,but size increased with the increase of lipid fraction.Furthermore,HER2 and phosphorylated HER2 were detected in EL-FNP after freeze-thaw cycles.The above results turned out that the freeze-thaw method can obtain EL-FNP [147].However,the disadvantage of freeze-thaw method may cause the leaky of intraluminal contents of exosomes[148].
4.2.3.Continuousextrusion
The extrusion method uses physical and mechanical force to break the vesicles into membrane fragments.Subsequently,the resulting membrane fragments tend to self-assemble into new spherical vesicles to reduce the free energy [149].As an example,Jhanet al.prepared liposomes and isolated exosomes from lung adenocarcinoma (A549) cells,then mixed them in different volume ratios(9:1,4:1,1:1).Subsequently,the mixtures were continuously extruded through an extruder with different apertures of 400,200,and 100 nm,respectively.After fusion,the authors characterized the morphology,size and charge of the forming vesicles.The results demonstrated that the EL-FNP had a uniform particle size of approximately 100 nm and the number of vesicles increased by a factor of 6 to 43.Furthermore,siRNA was successfully loaded into the EL-FNPviaelectroporation with an encapsulation efficiency of 15%-20%.After the transfection with EL-FNP,A549 cells showed a 14.2-fold higher uptake efficiency than that of CCL-210 cells.Considering that EL-FNP contained the components of A549-derived exosome,EL-FNP might still possess the targeting ability of original exosomes.This result supported the conclusion that EL-FNP retained the targeting capacity of the exosomes [142].Although the continuous extrusion method is able to produce uniform EL-FNP,physical and mechanical forces may affect the activity and content of membrane proteins [150].
4.2.4.Sonicdegradation
The cavitation and mechanical action generated by ultrasound waves can destroy the original structure of exosomes and liposomes.During the following incubation process,the membrane fragments interact with each other and integrate to form intact EL-FNP [151].To investigate the fusion efficiency of exosomes and liposomes,Lietal.mixed two fluorescent molecules,fluorescein isothiocyanate (FITC) and rhodamine B (RB),with lipid molecules in a 1:1 molar ratio to form fluorescence resonance energy transfer (FRET) liposomes.Then exosomes and liposomes were mixed and ultrasonically agitated for 5 min by switching on and off at 2 s pulsed intervals.The organic phase was then completely removed by vacuum vortexing for 15 min and finally the mixture was extruded through a 200 nm polycarbonate membrane filter to obtain EL-FNP.The results of the fluorescence spectrophotometry confirmed that the peak emission of FITC was increased,but the peak emission of RB was decreased.This was caused by the increase in distance between the original lipid molecules of the liposome after the fusion.Therefore,the result also proved the successful fusion of exosomes and liposomes.In addition,the morphology of EL-FNP was uniform and the particle size was 125 ±6 nm [152].Unfortunately,although transmembrane their intraluminal content is lost to some extent [153].Therefore,this method requires suitable optimization to be widely applied.
4.2.5.Viralfusogenicproteins
Fusogenic proteins,such as the fusion subunit of the human immunodeficiency virus (HIV) Env glycoprotein (gp41),influenza hemagglutinin (HA),vesicular stomatitis virus G (VSV G),are characterized by speedy,environment-responsive fusion activities,which play a critical role in mediating infection of envelope viruses to host cells [154].They can induce membrane fusion by generating the structure of the trimer-of-hairpins and changing membrane morphologyviaa membrane dynamics pathway.The baculoviral envelope protein gp64 is known to possess membrane fusion function and thus is essential for baculovirus infection and budding,especially under acidic circumstances [155].Ragaetal.transfected the parent cells with a baculovirus-expression plasmid to obtain gp64-expressed exosomes.Then,the gp64-expressed exosomes were incubated with liposomes which labeled with 7-nitro-2-1,3-benzoxadiazol-4-yl (NBD) and rhodamine (Rho) for 30 min at 27 °C under acidic (pH 4.5) and neutral (pH 7.5) conditions,respectively.The result of FRET test showed that the NBD signal rebounded and ultimately reached around 17% of the maximum fluorescence value when liposomes and exosomes were combined under acidic circumstances (pH 4.5).The recovery of fluorescence signal indicated an increase in the spacing of NBD and Rho,demonstrating successful fusion of exosomes and liposomes.In contrast,NBD fluorescence did not exhibit any recovery under pH 7.4 condition.Meanwhile,the addition of gp64 antibody inhibited the recovery of NBD signal.All these results demonstrated that fusogenic protein gp64 mediated the fusion between liposomes and exosomes [156].In general,the most prominent advantage of this method is that it does not compromise the integrity of exosomes(Fig.4).
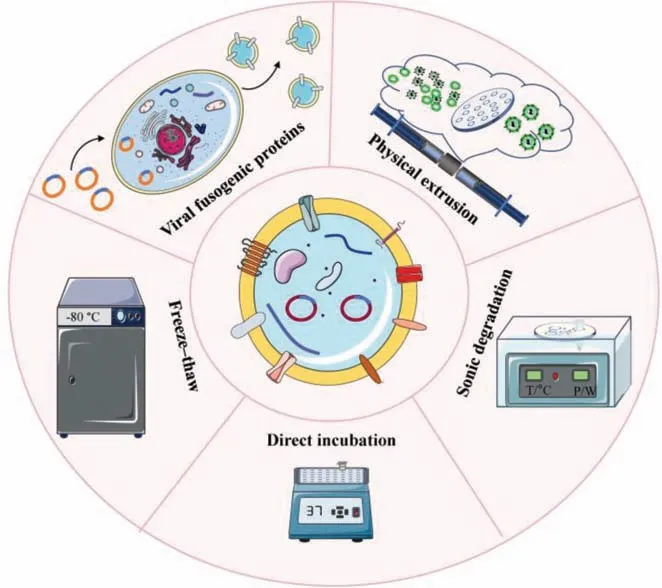
Fig.4.Methods to realize membrane fusion between exosomes and liposomes.
4.3. The applications of EL-FNP in drug delivery and disease treatment
4.3.1.Smallmoleculedrugsdelivery
With the development of EL-FNP,they have been used as drug carriers for the treatment of diseases.It is well known that pulmonary fibrosis is characterized by excessive deposition of extracellular matrix (ECM) in the interstitium,destruction of normal lung parenchymal structure and progressive loss of lung function.The median survival of pulmonary fibrosis is 2-5 years[157].In recent years,interstitial pulmonary fibrosis has also been characterized in coronavirus disease 2019 (COVID-19) patients[158].Sunetal.developed a mixed drug delivery system of clodronate (CLD)-loaded liposomes and exosomes (EL-CLD) to treat pulmonary fibrosis.When clodronate reaches a certain concentration in cells,EL-CLD eliminated macrophages by initiating apoptosis in macrophages and reduced the non-specific phagocytosis.Meanwhile,the targeted characteristics of exosomes derived from fibroblasts made it possible to precisely target the tissue of the pulmonary fibrosis system.The accumulation and infiltration of ELCLD drug delivery system were significant in pulmonary fibrosis,and the system had a greater co-location with fibrotic tissues as well.EL-CLD enhanced the inhibitory effect of drugs,thus improving the therapeutic effect of pulmonary fibrosis [38].The drug delivery system demonstrates good selectivity and ideal drug delivery efficiency in the treatment of pulmonary fibrosis and may be a promising fiber-specific drug delivery system for anti-fibrosis therapy in lung tissues.
Breast cancer is one of the most common malignant tumors in women,and its incidence and case fatality rate are high,which seriously threatens women’s physical and mental health [159].Sagaretal.combined exosomes derived from mouse macrophages with liposomes to form EL-FNP.By using doxorubicin (DOX) as a model drug,the drug-loading potential and anti-tumor effect of hybrid nanoparticles were analyzed.The results showed that,the EL-FNP could form particles with uniform particle size of about 177 nm.When the DOX concentration was 100 μg/mL,the drug loading of EL-FNP was up to 99%.Notably,the drug release efficiency was up to 83% under acidic conditions.In addition,invitroexperiments,EL-FNP showed enhanced internalization and cytotoxicity on tumor cells K7M2 and 4T1 compared to the normal fibroblasts NIH/3T3,indicating that EL-FNP had obvious cytotoxic selectivity for tumor cells [145].In summary,the application of EL-FNP has broadened the field of drug carriers and will promote the development of drugs that are limited due to delivery problems (Fig.5).

Fig.5.EL-FNP delivered DOX for the treatment of breast cancer.(A) Comparison between nanovesicles in terms of size,PDI,and surface charge.(B) DOX loading efficiency and drug content by weight with respect to carrier in different initial concentration of DOX (μg/mL).(C) Percentage release of DOX from HE-DOX and control liposome-DOX in normal physiological condition (phosphate buffer solution,PBS,pH 7.4) and acidic condition (acetate buffer,pH 5.5).(D) Ratio of median fluorescence of HE internalization to liposome internalization in different cell.(E) Comparative toxicity of HE-DOX on K7M2,4T1,and NIH/3T3 showing IC50 value of Free DOX and HE-DOX treatment.(F)Hybridization of immune cell derived sEVs with synthetic liposome using membrane extrusion.Reproduced with permission [145].Copyright 2019,Elsevier.
4.3.2.Nucleicaciddrugsdelivery
Conventional therapies usually achieve only short-lived results because they target the protein rather than the true underlying cause [160].In contrast to conventional approaches,nucleic acid therapies can achieve lasting or even curative effects by precisely targeting disease-causing gene sites through gene re-editing[161,162].However,a current challenge for the application of nucleic acid drugs is the lack of a safe and effective delivery system[163].As described above,EL-FNP also have the potential to deliver nucleic acid drugs.For example,Huetal.constructed NIH-3T3 cells with high expression of C-X-C motif chemokine receptor 4 (CXCR4) using genetic engineering techniques,then collected exosomes with the same high expression of CXCR4.Finally,exosomes were fused with liposomes carrying antagomir-188 to form the ELFNP.Stromal cell-derived factor 1 (SDF1),also known as C-X-C motif chemokine 12,is mainly expressed by bone marrow mesenchymal stem cells (BMSCs) and specifically binds to CXCR4,so EL-FNP could aggregate in the bone marrow with exosomes/liposomes ratios of 1:1 and 4:1.No significant pathological changes were observed in all organs of the mice.Further,the authors tested the effect of EL-FNP on an age-related osteoporosis mouse model.The results showed that the relative expression of mir-188 was significantly reduced.The specific aggregation and release of antagomir-188 by EL-FNP in mouse bone marrow promoted bone formation and inhibited adipogenesis of bone marrow mesenchymal stem cells,thereby reversing age-related bone loss and reducing cortical bone porosity in mice.In summary,it provides a promising anabolic therapy for age-related bone loss [139].
CRISPR/Cas9 is an adaptive immune defense developed by bacteria and archaea to combat invading viruses and exogenous DNA[164].The gene editing technology based on CRISPR/Cas9 system consists of two important components: a guide RNA (gRNA) that has a guide function and a Cas9 protein that performs a DNA double-stranded cleavage function.Thus,the system can utilize gRNA complementary to the target sequence to guide Cas9 nucleases for recognition and cleavage of specific target DNA [165].The successful delivery of CRISPR/Cas9 to recipient cells is essential for gene editinginvivo.However,safety and effectiveness are still remained challenging.To solve these problems,Linetal.promoted the fusion of exosomes and liposomes by incubating at 37°C for 12 h,finally preparing the EL-FNP.The fluorescence activated cell sorter (FACS) results showed that the synthesized EL-FNP successfully delivered CRISPR/Cas9 into mesenchymal stem cells(MSCs),compared to liposomes and exosomes.In addition,the authors treated MSCs with DNase before incubating with EL-FNP,and quantitative real-time PCR (qRT-PCR) analysis showed that EGFP mRNA could be expressed in MSCs.This was further evidence that the plasmid was mainly encapsulated in the EL-FNP and could be transfected into the cell as well as stably expressed [144].This study proved that EL-FNP can effectively improve the current situation that exosomes are difficult to wrap large nucleic acids and provide a new strategy for CRISPR/Cas9 delivery (Fig.6).

Fig.6.EL-FNP delivered CRISPR/Cas9 into the mesenchymal stem cells.(A) Representative electron microscopy image of exosomes after incubation with liposomes.(B) FACS analysis of green fluorescence protein expression of MSCs with incubation of the nanoparticle.(C) qRT-PCR analysis of EGFP mRNA level.(D) Cell viability of MSCs with incubation of the nanoparticles.Reproduced with permission [144].Copyright 2018,Wiley Online Library.
4.3.3.Synergisticdrugsdelivery
Combination therapy has become a major treatment modality because it can combine therapeutic effects of different drugs[7,166-170].There is no denying that the delivery of drugs plays a crucial role.Chengetal.used lentiviral vector-mediated plasmid transfection into CT26 cells to obtain CD47-overexpressing exosomes.Exosomes were used to fuse with thermosensitive liposomes to form EL-FNP (hGLV).Numerous studies have shown that CD47 competitively bound to SIRPα,which inhibits the immune escape of tumor cells [171-173].To investigate whether CD47-overexpressed hGLV affects M1 macrophage-mediated phagocytosis of tumor cells,mCherry-labeled CT26 cells were constructed by gene transfection as model cells.CT26 cells and macrophages were co-cultured at a certain ratio for 4 h.The phagocytosis efficiency of macrophages was determined and the results showed that the phagocytosis efficiency of macrophages in the hGLV group was 56.4%,which was much higher than other control groups.This result effectively proved the above conclusion.In addition,the authors used hGLV loaded with the photothermal agent ICG and the immune adjuvant R837 (I/R@hGLV) and explored its anti-tumor efficiency.The results showed that intravenous injection of I/R@hGLV inhibited tumor growth to a certain extent compared with the PBS group;laser irradiation significantly inhibited tumor growth and almost completely eliminated tumors in the ICG@hGLV group.This was due to immunogenic cell death (ICD) induced by light and heat could produce tumor-associated antigens [174,175] as well as cooperate with R387 to activate the maturation of DCs,finally enhanced immune efficacy [176].By blocking the CD47 immune checkpoint,the author combined photothermal therapy with immunotherapy for cancer treatment and achieved better curative effects.
EL-FNP are used to treat metastatic peritoneal carcinoma (mPC).mPC is a fatal disease without effective treatment [177].HIPEC is currently a common clinical treatment method [178].However,HIPEC cannot effectively penetrate large tumor tissues [179].To overcome transmission limitations and enhance the effect of HIPEC treatment,Lvetal.used CD47-expressing exosomes from fibroblasts,fused with thermosensitive liposomes,to form EL-FNP (gETL NPs).Further,the authors utilized EL-FNP loaded with granulocyte macrophage-colony stimulating factor (GM-CSF) and docetaxel(DTX) to obtain G/D-gETL NPs.The results ofinvitrotoxicity assays showed that G/D-gETL NPs exhibited significantly higher cytotoxicity than DTX-loaded liposomes and free DTX at HIPEC-related temperatures.To investigate macrophage polarization,the authors incubated macrophages with G/D-gETL NPs or free GM-CSF with the M2 phenotype for 24 h.Next,macrophage surface proteins CD86 (M1 phenotype surface marker) and CD206 (a M2 phenotype surface marker) were used as markers to distinguish M1 and M2 phenotypes.Flow cytometry quantification of the ratio of M1 and M2 macrophages showed that G/D-gETL NPs converted macrophage phenotypes with comparable efficiency to free DTX.In addition,to verify whether CD47 plays a role in gETL NPs,the authors pretreated M1 macrophages with gETL NPs,and then incubated them with human colon cancer HCT116 cells.Results revealed that the gETL NP-treated macrophages’phagocytosis effi-ciency was 32.5%,which was comparable to that of CD47 proteintreated macrophages (37.2%) and about 6-fold higher than that of PBS-treated macrophages (5.4%).In antitumor experiments,under the low-temperature conditions of HIPEC,the nanocomposite preferentially accumulated in the tumor after intravenous administration and accelerated the release of the payload.When treated with EL-FNP loaded with CM-CSF and/or DTX,tumor development was significantly inhibited,and the antitumor effect was enhanced(Fig.7) [180].

Fig.7.EL-FNP delivered GM-CSF and DTX for mPC treatment.(A) Schematic diagram of the synthesis and application gETL NPs.(B) Quantitative analysis of apoptosis based on flow cytometry.(C) Flow cytometry analysis of the expression of M2 macrophages markers (CD68+CD206+).(D) Flow cytometry analysis of the expression of M1 macrophages markers (CD68+CD86+).(E) Flow cytometry analysis of phagocytosis of tumor cells by M1 macrophages.(F) Quantification of the accumulation of NPs in major organs and tumors at 48 h post injection based on DIR fluorescence.(G) Change of tumor volume with time.(H) Quantitative analysis of the ratio of M1 macrophages(CD86+)/M2 macrophages (CD206+) by immunofluorescence staining of tumor sections.Reproduced with permission [180].Copyright 2020,Wiley Online Library.
EL-FNP are also being used to solve women’s health issues.Ovarian cancer (OC) is a malignant tumor occurring on the ovary,which has the highest mortality rate of gynecological malignant tumor.Since the early symptoms are not obvious,most patients with ovarian cancer are found to be in the middle and late stages [181].Lietal.fused tumor exosomes expressing CD47 and cRGD-modified liposomes to form EL-FNP (HENPs).Triptolide(TP) could be loaded as a therapeutic drug within HENPs.In the meantime,calcium phosphate (CaP) was used as the medium to adsorb miR497 on the surface of nanoparticles through electrostatic adsorption.Final bioinspired hybrid nanoplatform was named miR497/TP-HENPs.The results showed that,due to the natural targeting of exosomes,HENPs enhanced drug entry into the cell compared to liposomes alone.To investigate the potential anticancer effects of miR497/TP-HENPs,OC cells were treated with miR497-HENPs,free TP,TP-HENPs,and miR497/TP-HENPs.The results showed that the miR497/TP-HENPs group could synergistically overcome OC resistance and had the strongest cell-killing ability.In animal imaging experiments,compared to the free Dir group and the Dir Liposomes group,the miR497/TP-HENPs group was observed stronger Dir fluorescence in the tumor sites but less Dir fluorescence in the normal sites.In addition,the miR497/TPHENPs group had the same anticancer effectinvivoas it didin vitro.The above results all proved miR497/TP-HENPs effectively improved the shortcomings of the above two drugs and could be effectively absorbed by tumor cells.OC increased sensitivity to chemotherapy drugs and promoted tumor cell apoptosis by promoting the dephosphorylation of the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway [152].
5.Summary and outlook
Exosomes are originally served as long-distance communication between cells.Due to the active targeting ability and good biocompatibility,exosomes are now widely used for drug delivery.By employing exosomes as carrier,the overall performance of medications can be greatly improved.However,due to the complex purification process and low drug loading efficiency,it is not yet suitable to use exosomes in therapeutic industry.Liposomes are a kind of well-researched drug delivery carrier,which are simple in synthesis and efficient in delivery.Researchers have found that the EL-FNP not only inherited the active targeting ability of exosomes,but also maintained satisfactory drug loading efficiency,cell delivery efficiency,and safety.More importantly,the fusion strategy significantly reduces the need for exosomes in drug production,and improved controllability of vesicles.So far,the EL-FNP have already been used in cancer treatment and gene editing.It can be easily imaged that with the continuous maturation of exosome separation and modification techniques,as well as the emergence of new methods to stimulate exosome generation and new liposomes,EL-FNP will gradually develop into mature delivery systems.After that,EL-FNP will be poised to shine in the field of disease-targeted drug delivery,enabling effective translation from the laboratory to the clinic.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We appreciate Servier Medical Art for providing relevant graphic material,Figs.1 to 4 partly generated by using these materials.The license is under a Creative Commons Attribution 3.0 unported license.This work was supported by the National Key Research & Development Program of China (Nos.2021YFC2302400,2021YFA1201000,2021YFE0106900),the National Natural Science Foundation of China (Nos.32171394,32101148,82202338),the Beijing Nova Program (Interdisciplinary Cooperation Project) from Beijing Municipal Science & Technology Commission (No.20220484207),the Beijing Natural Science Foundation (No.L222128),and the Fundamental Research Funds for the Central Universities (No.2022CX01013).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108647.
 Chinese Chemical Letters2024年2期
Chinese Chemical Letters2024年2期
- Chinese Chemical Letters的其它文章
- Hybrid ionic/electronic interphase enabling uniform nucleation and fast diffusion kinetics for stable lithium metal anode
- Multifunctional properties of a polar spin chain compound[N(C3H7)4][Cu(C8H4NO4)]·H2O exhibiting both one-dimensional magnetism and nonlinear optical activity
- Ti3C2Tx MXene wrapped,carbon-coated porous Si sheets for improved lithium storage performance
- Interfacial charge redistribution to promote the catalytic activity of Vs-CoP-CoS2/C n-n heterojunction for oxygen evolution
- Synergy of phosphorus vacancies and build-in electric field into NiCo/NiCoP Mott-Schottky integrated electrode for enhanced water splitting performance
- Ultrathin ternary PtNiGa nanowires for enhanced oxygen reduction reaction
