Controllable Modulation of Morphology and Property of CsPbCl3 Perovskite Microcrystals by Vapor Deposition Method
Na Dong,Fangfang You,Ting He,Yi Yao,Faqiang Xu
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei 230029, China
As a direct wide bandgap semiconductor,CsPbCl3 has great potential applications in the field of near-ultraviolet photodetectors,lasers and higher-order multiphoton fluorescent detectors.In this work,we synthesized CsPbCl3 micro/nanocrystals by vapor deposition method with CsCl and PbCl2 powders as the source materials.It was confirmed that the formation of CsPbCl3 perovskite through the chemical reaction of CsCl with PbCl2 occurred in the quartz boat before the source evaporation,not in vapor or on substrate surface.The evaporated CsPbCl3 can form micro/nanocrystals on substrate surfaces under appropriate conditions.Various morphologies including irregular polyhedrons,rods and pyramids could be observed at lower temperature,while stable and uniform CsPbCl3 single crystal microplatelets were controllably synthesized at 450 ?C.Prolonging the growth time could modulate the size and density of the microcrystals,but could not change the morphology.Substrate types made little difference to the morphology of CsPbCl3 crystals.The photoluminescence spectra indicated that the crystallinity and morphology of CsPbCl3 micro/nanocrystals have significant effects on their optical properties.The work is expected to be helpful to the development of optoelectronic devices based on individual CsPbCl3 microcrystal.
Key words: Vapor deposition method,CsPbCl3 micro/nanocrystal,Controlled synthesis,Photoluminescence
I.INTRODUCTION
Lead halide perovskites have been extensively studied in the past decade since Kojimaet al.used CH3NH3PbBr3and CH3NH3PbI3as the visible-light sensitizer in photoelectrochemical cells [1].Owing to their unique optical and electric properties,a series of lead halide perovskite materials have become research hotspots in the field of optoelectronic devices such as solar cells,photodetectors,and lasers [2-7].Considering the fact that organic-inorganic hybrid lead halide perovskites are unstable under ambient conditions because of the presence of organic cation [8],more studies have focused on the all inorganic perovskites such as cesium lead halide (CsPbX3) perovskites which display improved thermal stability while reserving intriguing photoelectronic properties [9].
As a typical direct and wide bandgap semiconductor,CsPbCl3perovskite has been paid much attention and found to have wide applications.The usage in fast scintillators makes CsPbCl3a promising material in high-energy physics and for near-ultraviolet optoelectronic detectors [10,11].Siuet al.studied the nonlinear absorption and emission characteristics of single crystalline CsPbCl3perovskite microcavities under multiphoton excitation,and revealed the prospect in high-performance upconversion lasing devices[12].Sumet al.prepared single crystalline CsPbCl3whisperinggallery-mode microcavities with low threshold and high spectral coherence by van der Waals epitaxy method[13].In addition,Shi and co-workers reported a photodetector based on high quality horizontal CsPbCl3microwire networks with high on/offphotocurrent ratio of 2×103and a photoresponsivity of 14.3 mA/W,as well as a fast response speed [14].Anyway,it is expected that some types of morphologies of CsPbCl3would be promising candidates for new kinds of optoelectronic applications.Therefore,the morphologycontrolled synthesis of CsPbCl3microcrystals is important for exploring the potentials of CsPbCl3.
In this work,we systematically investigated the modulation effect of some factors including evaporation temperature of source materials,growth temperature,growth time,and substrate type on the morphologies and optical properties of CsPbCl3microcrystals prepared using one-step vapor deposition method.It is found that the growth temperature plays a crucial role in modulating the morphology and size of CsPbCl3microcrystals.And the room temperature photoluminescence property of CsPbCl3microcrystals grown at different temperatures significantly depends on the crystallinity and the morphology of CsPbCl3crystals.
II.EXPERIMENTS
The source materials including cesium chloride(CsCl,>99.9%) and lead chloride (PbCl2,>99.9%) were purchased from Alfa Aesar and used without any further purification.The Si(100) wafers with an oxide overlayer about 300 nm were bought from Suzhou Research Materials Micro-nano Technology Co.,Ltd.and were cleaved to 10 mm×10 mm slices for using as substrate.At first,the SiO2/Si(100) substrate slices were ultrasonically cleaned in acetone,ethanol,and deionized water for 15 min,respectively.Next,they were immersed in a fresh piranha solution (concentrated sulfuric acid:hydrogen peroxide solution=3:1) for 1 h at 90?C in order to remove the residual acetone and other impurities on the surface.Finally,the substrates were rinsed with ultrapure water repeatedly and dried in a stream of N2gas.
The CsPbCl3microcrystals were prepared in a homebuilt vapor deposition system,a quartz tube furnace(inner diameter=6 cm,length=100 cm) with two temperature zones,equipped with a mass flow controller and pressure monitor.A quartz boat filled by the mixture of CsCl and PbCl2powders with a molar ratio of 1:1 was placed in the center of upstream zone of the furnace.The cleaned SiO2/Si(100)substrate slices were placed in the center of downstream zone of the furnace.Prior to heating,the quartz tube was pumped down with high-purity Ar gas at a flow rate of 100 sccm for 30 min to purge out any residual air in the tube.Afterwards,the upstream zone was heated to 600?C at a rate of 10?C/min,and the downstream zone was heated to 390-470?C in 45 min.The inner pressure was maintained at about 380 torr.The growth lasted for a duration of 5-70 min since the target temperatures were reached at almost the same time and then the furnace was naturally cooled to room temperature.
The crystal structure was characterized by X-ray diffraction (XRD) technique using the TTR-III diffractometer with Cu-Kαradiation.The morphology of assynthesized samples was obtained by the SU8220 cold field emission scanning electron microscope (FESEM).The photoluminescence(PL)spectra were measured on a Jobin Yvon-LabRamHR Evolution spectrometer with the excitation wavelengthλex=325 nm.And,the X-ray diffraction(XRD)spectra and photoluminescence(PL)spectra all were obtained under ambient conditions.
III.RESULTS AND DISCUSSION
A.Solid-state reaction of the source materials
The formation route of CsPbCl3perovskite from the sources of CsCl and PbCl2was firstly considered,because it is imperative for us to determine the evaporation mode and appropriate temperature.Thus,a series of parallel experiments were performed to make sure where the reaction of CsCl and PbCl2takes place,in the quartz boat,in vapor or on the substrate surface.The mixed powders of CsCl and PbCl2in a quartz boat were heated in the upstream zone from room temperature (25?C) to 310,420,435,550 and 600?C,respectively.As the temperature increased,the color and state of source materials in the quartz boat gradually changed from white solid to yellow solid,and finally to colorless liquid.This interesting phenomenon indicated that the chemical reaction of CsCl with PbCl2might take place and new substances have been formed in the quartz boat.So,the XRD measurements were carried out to identify the crystal structure of the reaction products in the quartz boat and the results are demonstrated inFIG.1.

FIG.1 The XRD patterns of the reaction products in the quartz boat under a series of heating temperatures.
As shown inFIG.1,the black line corresponding to the RT XRD pattern of the original powders shows several broad diffraction peaks which match very well with planes of orthorhombic phase PbCl2(JCPDS No.26-1150) and cubic phase CsCl (JCPDS No.73-0390).When the source powders are heated to 310?C,partial diffraction peaks are well assigned to monoclinic phase Cs4PbCl6reported in JCPDS card (No.78-1685),and the diffraction peaks at 15.9?and 22.6?can be indexed to the (100) and (101) planes of tetragonal phase CsPbCl3(JCPDS No.18-0366),suggesting the formation of Cs4PbCl6and CsPbCl3in the quartz boat.It can be clearly seen that the intensity of the characteristic peaks of tetragonal phase CsPbCl3increases gradually and the location of the peaks shifts to the left slowly,but the numbers and intensities of the diffraction peaks of Cs4PbCl6,PbCl2and CsCl decrease significantly when the source powders are heated to 420?C,435?C,and 550?C,respectively.Finally,when the heating temperature goes up to 600?C,sharp and intense diffraction peaks of the products match well with tetragonal phase CsPbCl3and no impurityrelated peaks are observed,confirming that high purity of CsPbCl3perovskite is formed in the quartz boat.Referring to the melting points of these chemicals (CsCl:615?C,CsPbCl3: 552?C,Cs4PbCl6: 493?C,PbCl2:421?C) [15],we can deduce that the source components in the quartz boat experience not only evaporation but also solid-state chemical reactions in the heating process.The by-product Cs4PbCl6is almost completely converted into CsPbCl3or evaporated when the temperature is increased to 600?C.Therefore,it is concluded that the formation of CsPbCl3perovskite through the chemical reaction of CsCl with PbCl2occurred in the quartz boat before the source evaporation,instead of in vapor or on substrate surface.
B.Structure modulation of CsPbCl3 microcrystals
Now that the formation of CsPbCl3perovskite through the solid-state chemical reaction of CsCl and PbCl2is verified to occur in the quartz boat,the CsPbCl3molecules can be directly and easily evaporated to the surface of the substrate in the downstream region at an appropriate temperature.Therefore,the evaporation temperature is significant for the formation of high quality CsPbCl3on the substrate surface for vapor deposition method.FIG.2 exhibits the SEM images of the as-prepared samples under different evaporation temperatures and the corresponding XRD curves.According toFIG.2(a-c),the particle density gradually increases with increasing evaporation temperature from 550?C to 650?C.Almost no product is deposited on SiO2/Si(100) substrate at 550?C,but at 600?C lots of frustums of pyramids with clear edge and smooth surface are observed which are in the size range of about 2 μm to 12 μm.When the evaporation temperature is up to 650?C,the deposited products become the mixture of quasi-square platelets and frustum of pyramids which are in the range of microscale.The XRD patterns inFIG.2(d) demonstrate that there is no diffraction peak for the sample deposited at 550?C,while sharp diffraction peaks appear for the samples prepared at 600?C and 650?C,which are consistent with the standard XRD pattern of tetragonal phase CsPbCl3.The above results indicate that CsPbCl3microcrystals with high crystallinity could be synthesized at 600?C and 650?C.Considering the higher morphology uniformity of the CsPbCl3microcrystals prepared at 600?C than that at 650?C,we choose 600?C as the appropriate evaporation temperature in subsequent experiments.

FIG.2 The SEM images of CsPbCl3 samples prepared under the evaporation temperature of (a) 550 ?C,(b) 600 ?C,(c)650 ?C,(d) corresponding XRD patterns.During the growth,the substrate temperature was 430 ?C and the growth time was 20 min.
To explore the influence of growth temperature on CsPbCl3crystals,the SiO2/Si(100) substrates were placed in the center of downstream zone of the furnace with the temperature being controlled in the range of 390?C to 470?C,and the morphology and size evolution of the produced CsPbCl3microcrystals are shown inFIG.3.As shown inFIG.3(a,b),irregular polyhedrons with an average size of 3 μm are obtained at 390?C.When the growth temperature is increased to 410?C,the SEM images ofFIG.3(c,d) show diverse morphologies including rods,pyramids and frustums of pyramids with relatively sharp edges.When the growth temperature is up to 430?C (FIG.3(e,f)),the microcrystals of frustums of pyramids and square platelets are found to co-exist on the surface with the size distribution range of 2-30 μm.The critical change happens at 450?C,at which only square platelets can be observed as shown inFIG.3(g,h).Although some of the square platelets are conglutinated with each other,the smooth surface and sharp edge with 30-80 μm in width and length suggest that they have high crystalline quality.However,when the temperature is further increased to 470?C,distorted and wrinkled platelets are stuck together tightly to form a dendritic shape with 500 μm in length,which may be due to the poor tolerance of perovskite materials when the temperature is out of an appropriate range [17].
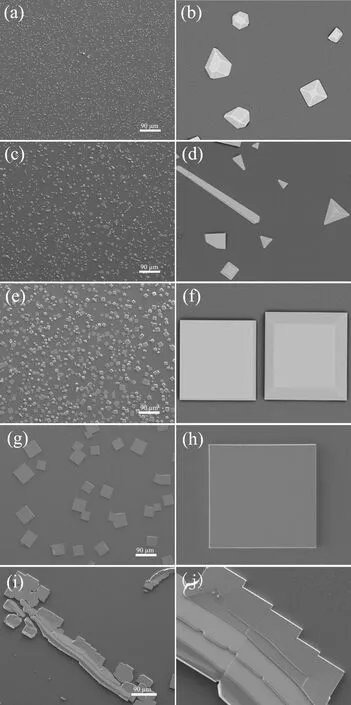
FIG.3 The SEM images of the as-prepared CsPbCl3 samples under growth temperatures of (a,b) 390 ?C,(c,d)410 ?C,(e,f) 430 ?C,(g,h) 450 ?C,(i,j) 470 ?C.The source evaporation temperature was kept at 600 ?C and the growth time was 70 min.
The morphology evolution of the samples could be explained by the Gibbs-Curie-Wulfftheorem.It is generally accepted that the surface free energy plays a vital part in nucleation as well as crystal growth,and determines the equilibrium crystal shape at a certain temperature.The facets with higher surface energy usually make up a very small fraction of the surface or even disappear in the final crystals due to instability,but the facets with lower surface energy will be preserved,determining the shape of the final crystals[18].Hence,the morphology of CsPbCl3microcrystals is irregular polyhedrons at low temperature but regular square platelets at high temperature.High temperature might promote crystal growth but inhibit crystal nucleation,so the size of CsPbCl3microcrystals increases by 20 times and the density of single crystal decreases when the growth temperature rises from 390?C to 450?C.And the nucleation is almost out of control when the temperature is up to 470?C as shown inFIG.3(i,j).
The XRD measurements were carried out in order to further confirm the crystal structure of the samples prepared at different growth temperatures and the results are shown inFIG.4.The positions of the diffraction peaks are in good agreement with tetragonal phase CsPbCl3.The diffraction intensity increases initially with the increase of temperature and reaches the maximum at 450?C,then decreases afterwards.Remarkably,the dominant diffraction peaks assigned to (100)and(200)lattice planes at 16?and 32.2?imply that the CsPbCl3microcrystals deposited on SiO2/Si(100) substrates possess strong (200) preferred orientation and even the nature of single crystals at 430?C and 450?C.It’s noticeable that the diffraction intensity is related to not only the density but also the uniformity of the microcrystals.As can be seen,the samples at 390?C and 410?C show high concentration of microcrystals(FIG.3(a,c)) but much weaker diffraction intensities(FIG.4),this behavior might be attributed to the multiple morphology and heterogeneity of the microcrystals.Although the tetragonal phase single crystal is still the main component at 390?C and 410?C,its diffraction peaks are not enhanced due to the existence of other crystal phases.The similar phenomenon also happens between the samples at 430?C and 450?C,the former has larger microcrystal concentration but lower diffraction intensity than the latter.In addition,it is interesting that the change trend in diffraction intensity may remain consistent with the evolution in crystal morphology and crystal quality of the CsPbCl3microcrystals,which is similar to the CH3NH3PbBr3crystals as Zhanget al.reported previously [19].The single crystal morphology and excellent crystallinity lead to strong XRD diffraction peaks.
Based on the above optimization of temperature on the morphology,uniformity and crystal quality of CsPbCl3microcrystals,the effect of growth time was further explored with the source evaporation at 600?C and the substrate temperature at 430?C,respectively,and the SEM images are shown inFIG.5(a-c).Obviously,the shapes of the CsPbCl3microcrystals are kept almost unchanged in the form of frustums of pyramids and platelets,while the size and density show a lift trend with the extension of the growth time from 5 min to 20 min to 70 min.It means that increasing growth time is beneficial for synthesizing large-sized CsPbCl3single crystals as expected but cannot change the morphology of the microcrystal.

FIG.5 The SEM images of CsPbCl3 microcrystals prepared at the growth time of (a) 5 min,(b) 20 min,(c) 70 min.The source evaporation and substrate temperatures were 600 ?C and 430 ?C,respectively.
At last,the influence of substrate types on the morphology of products was also investigated by SEM technique.As demonstrated inFIG.6,the shapes of the CsPbCl3microcrystals prepared on three different substrates are similar.This result may further verify that the perovskite molecules are formed in the source boat,and nucleate in the same mode on the surface of different kind of substrates,as a result,the produced microcrystals exhibit the same morphology under the same temperature conditions.Combined with the above results,it is suggested that the growth time has an imperative effect on the size and particle density of CsPbCl3microcrystals,while the substrate type makes little difference to the morphology of CsPbCl3crystals.

FIG.6 The SEM images of CsPbCl3 microcrystals prepared on the substrate of (a) SiO2/Si(100),(b) Si(100),and (c)Al2O3(0001).The source evaporation and substrate temperatures were 600 ?C and 430 ?C,respectively,the growth time was 70 min.
C.Optical properties of the CsPbCl3 microcrystals
The CsPbCl3has a wide bandgap with a large exciton binding energy about 75 meV [20],which suggests that stable excitons may exist in interior region of the crystal and this material is suitable for near-ultraviolet emission,so it is necessary to estimate the optical properties of the CsPbCl3microcrystals.
The PL measurements were conducted at 300 K for the samples prepared under various growth temperatures,and the spectra are shown inFIG.7.The results imply that the PL emissions at 390?C and 410?C have the same broad feature which shows the dominant peak at~414.5 nm and the shoulder at~420.0 nm.The intensity of emission peaks increases with increasing temperature from 390?C to 450?C,but decreases from 450?C to 470?C.The change trend of PL emission intensity could be explained by the XRD results and morphologies at different temperatures shown inFIG.3andFIG.4,which indicate that the samples with higher crystalline and homogeneity have stronger PL emission intensity.Importantly,the CsPbCl3microplatelets grown at 450?C exhibit the highest PL intensity and several sub-peaks in long-wavelength region,the shape of PL spectrum differs from those grown at 390?C and 410?C.At 430?C,the PL emission peak has obvious sharp peaks.The peak shape is more remarkable and representative with increased temperature to 450?C.However,when the temperature reaches 470?C,the peak intensity decreases with the decrease of peak shape characteristics.All these results are consistent with the variation of XRD intensity inFIG.4.It is demonstrated that the PL intensity of the characteristic peak gets increased due to the improvement of crystal quality.Additionally,the main emission peaks have obvious blueshift with lifted temperature.So it is proposed that the difference of spectrum shape and blueshift of the emission peak may be due to the difference in the crystallinity and morphology of CsPbCl3microcrystals grown at different temperatures.The nature of the optical emission features and transition mechanism needs further investigation and analysis in more detail,which have been under progress.
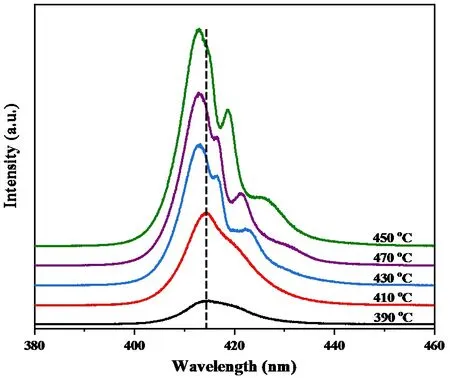
FIG.7 Steady-state PL spectra of the CsPbCl3 samples prepared at various growth temperatures measured using a 325 nm excitation wavelength at 300 K.
IV.CONCLUSION
In summary,controllable growth of microcrystals were systematically studied through vapor deposition method.At first,the CsPbCl3perovskite phase is proved to form in the source boat before the source evaporation via the reaction of CsCl with PbCl2on heating,not on the substrate surface.Through tuning the growth temperature,the micro-sized CsPbCl3crystals with different morphologies including rods,frustums of pyramids,square platelets could be obtained.Additionally,the growth time and temperature have great effects on the size and particle density of CsPbCl3microcrystals.The photoluminescence intensity is remarkably consistent with the crystal quality and uniformity.Importantly,the CsPbCl3microplatelets grown at 450?C show excellent luminous performance and the main photoluminescence characteristic peak has blue shift with increasing temperature.This work can offer pivotal parameters for controlling the growth of CsPbCl3microcrystals with specific morphology and size,and may provide new potential applications in the field of photoelectric devices.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No.11575187) and the National Key Research and Development Program(No.2016YFB0700205).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2023年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2023年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Atomistic Modeling of Lithium Materials from Deep Learning Potential with Ab Initio Accuracy
- On-the-Fly Nonadiabatic Dynamics of Caffeic Acid Sunscreen Compound
- Minimum-Modified Debye-Hückel Theory for Size-Asymmetric Electrolyte Solutions with Moderate Concentrations
- Photothermal Catalytic Selective Oxidation of Isobutane to Methacrylic Acid over Keggin-Type Heteropolyacid
- Design Strategy of Infrared 4-Hydroxybenzylidene-imidazolinone-Type Chromophores based on Intramolecular Charge Transfer: a Theoretical Perspective
- Quantum Dynamics Calculations on Isotope Effects of Hydrogen Transfer Isomerization in Formic Acid Dimer
