Photothermal Catalytic Selective Oxidation of Isobutane to Methacrylic Acid over Keggin-Type Heteropolyacid
Yichuan Wang,Xiao Sun,Zeyue Wei,Xuanyu Zhang,Weixin Huang
Key Laboratory of Precision and Intelligent Chemistry,iChEM,Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes and Department of Chemical Physics,University of Science and Technology of China,Hefei 230026,China
Thermal and photothermal catalytic selective oxidation of isobutane to methacrylic acid (MAA) are comparatively studied over a keggin-type Cs2.9Cu0.34V0.49PMo12O40 heteropolyacid acid.An introduction of light was observed to enhance both the i-C4H10 conversion and the MAA selectivity,and consequently the MAA formate rate,particularly at low temperatures.Characterization results show that oxidation of methacrolein (MAL) to MAA is the rate-limiting step while UV light illumination promotes the oxidation of σ-bonded MAL with OH groups to σ-bonded MAA on the catalyst surface.These results demonstrate a synergistic effect of thermal catalysis and photocatalysis in selective oxidation of isobutane to MAA,which suggests photothermal catalysis as a promising strategy to catalyze the selective oxidation of higher hydrocarbons at relative mild reaction conditions.
Key words: Photothermal catalytic reaction,Thermal catalytic reaction,Selection oxidation,Reaction mechanism,In situ characterization
Methacrylic acid (MAA) is an important chemical raw material for the industrial manufactures of rubber,adhesives,polymer additives,etc.[1,2].Presently,MAA was synthesized mainly by acetone cyanohydrin method,ethylene carbonylation,and isobutylene oxidation method [2],which involve toxic or expensive raw materials or complex multi-step synthesis processes,whereas one-step selective isobutane (i-C4H10) oxidation to MAA is an attractive low-cost and environmental-friendly route [3].Recently,photothermal catalysis has emerged as a novel type of catalysis which exhibits a synergistic effect of using thermocatalysis and photocatalysis on catalyzing chemical reactions [4].Particularly,photocatalysis has been demonstrated very effective in activating the C-H bond of hydrocarbons at mild conditions [5].
Heteropolyacid catalysts with a keggin structure were used to catalyze the selective oxidation ofi-C4H10to MAA [1-3];meanwhile,they were reported as photocatalysts [6-13].We were thus motivated to study the photothermal catalytic performance of a keggin-type Cs2.9Cu0.34V0.49PMo12O40heteropolyacid catalyst in the selectivei-C4H10oxidation to MAA.
The Cs2.9Cu0.34V0.49PMo12O40heteropolyacid was synthesized following a previous report [14].Structural characterizations,including FT-IR spectrum (FIG.S1(a)in Supplementary materials (SM)) [1],Raman spectrum (FIG.S1(b) in SM) [15,16],and XRD pattern(FIG.S1(c) in SM) [17],confirmed the keggin structure of as-synthesized Cs2.9Cu0.34V0.49PMo12O40heteropolyacid.SEM image and corresponding elemental mapping images (FIG.S2 in SM) show that the Cs2.9Cu0.34V0.49PMo12O40heteropolyacid consists of spherical nanoparticles with a uniform distribution of all elements.
Both thermal and photothermal catalytic reactions were evaluated in a CEL-GPPCM photothermocatalytic micro-reactor system (Beijing China Education Aulight Technology Co.,Ltd.).A thermal couple directly contacted the catalyst bed to reliably control the reaction temperature,and a 300 W CEL-HXF300-T3 Xe light (Beijing China Education Au-light Technology Co.,Ltd.) was used during the photothermal catalytic reactions.FIG.1compares the thermal and photothermal catalytic performance of Cs2.9Cu0.34V0.49PMo12O40heteropolyacid in the selectivei-C4H10oxidation.As shown inFIG.1(a),thei-C4H10conversion gradually increases from 0.44% at 300 ℃ to 1.90% at 350 ℃ during the thermal catalytic reaction while increases from 2.51% at 300 ℃ to 5.24% at 335 ℃ but then slightly decreases to 4.91% at 350 ℃ during the photothermal catalytic reaction.Therefore,the photothermal catalysis is more capable of catalyzing the selectivei-C4H10oxidation over Cs2.9Cu0.34V0.49PMo12O40than the thermal catalysis,indicating more efficient activation of reactants and surface intermediates by light than by heat.
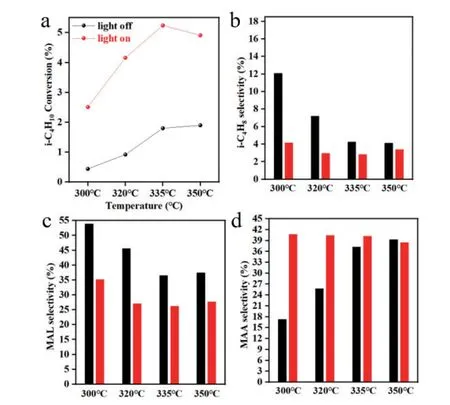
FIG.1 (a) i-C4H10 conversion rate,(b) selectivity of i-C4H8,(c) MAL selectivity,and (d) MAA selectivity of i-C4H10 oxidation reaction thermally (red) or photothermally (black) catalyzed by Cs2.9Cu0.34V0.49PMo12O40 heteropolyacid at various temperatures (reaction condition: 100 mg of 20-40 mesh catalyst,flow rate ratio of i-C4H10:O2:N2=5:5:10).
The major detected products were isobutene(i-C4H8),methacrolein (MAL),MAA and COx(Table S1 in SM).As shown inFIG.1(b-d),during the thermal catalytic reaction,thei-C4H8 selectivity is 12.05%at 300 ℃,7.20% at 320 ℃,4.23% at 335 ℃,and 1.90%at 350 ℃,the MAL selectivity is 53.91% at 300 ℃,45.58% at 320 ℃,36.53% at 335 ℃ and 37.44% at 350 ℃,and the MAA selectivity is 17.22% at 300 ℃,25.73% at 320 ℃,37.21% at 335 ℃ and 39.23% at 350 ℃;during the photothermal catalytic reaction,thei-C4H8 selectivity is 4.14% at 300 ℃,2.94% at 320 ℃,2.81% at 335 ℃ and 3.36% at 350 ℃,the MAL selectivity is 35.14% at 300 ℃,27.04% at 320 ℃,26.21% at 335 ℃ and 27.63% at 350 ℃,and the MAA selectivity is 40.70% at 300 ℃,40.40% at 320 ℃,40.18% at 335 ℃ and 38.43% at 350 ℃.Therefore,the thermal catalysis facilitates the MAL production at low temperatures,and as the reaction temperature increases,the MAA production increases at the expense ofi-C4H8and MAL.This demonstrates a sequential oxidation ofi-C4H10toi-C4H8,MAL and MAA,in which the oxidation of MAL to MAA exhibites a larger barrier than the oxidation ofi-C4H8 to MAA.Compared with the thermal catalysis,photothermal catalysis enhances the MAA production significantly at 300 and 320 ℃,but not at 335 and 350 ℃;meanwhile,photothermal catalysis enhances the COxselectivity at all studied temperatures.These observations suggestes that the introduction of light promotes the oxidation ofi-C4H8 and MAL to MAA more than to COxduring the selectivei-C4H10oxidation at 300 and 320 ℃,but to COxmore than to MAA at 335 and 350 ℃.
The above results demonstrates that the introduction of light enhances both thei-C4H10conversion and the MAA selectivity,and consequently the MAA formatation rate of selectivei-C4H10oxidation reaction catalyzed by the Cs2.9Cu0.34V0.49PMo12O40heteropolyacid.Under the studied conditions,the highest MAA formation rate is 1.00 mmolMAA·h-1·gcatalyst-1 at 350 ℃ for thermal catalysis and 2.82 mmolMAA·h-1·gcatalyst-1 at 335 ℃ for photothermal catalysis.Thus,thermal catalysis and photocatalysis exerts an obvious synergistic effect on selectively catalyzingi-C4H10to MAA,particularly at low temperatures.
In the UV-Vis diffuse reflectance spectrum(FIG.2(a)),the Cs2.9Cu0.34V0.49PMo12O40heteropolyacid shows obvious absorptions for the light with wavelengths smaller than 539 nm,corresponding to a bandgap of 2.3 eV.The electrochemical impedance spectroscopy (EIS) of Cs2.9Cu0.34V0.49PMo12O40(FIG.2(b)) gives a typical arc characteristic of semiconductors [18].The photocurrent measurements of Cs2.9Cu0.34V0.49PMo12O40(FIG.2(c)) show reproducible photocurrent generation with light illumination and photocurrent disappearance without light illumination,suggesting an efficient photoexcited charge separation process.In the photoluminance spectrum excited by a laser of 300 nm (PL) (FIG.2(d)),Cs2.9Cu0.34V0.49PMo12O40exhibites a major peak at 460 nm and a shoulder peak at 539 nm.The shoulder peak at 539 nm results from the recombination of photoexcited electrons at the band edge of conduction band and photoexcited holes at the band edge of valence band,while the major peak at 460 nm should result either from the recombination of photoexcited electrons at the band edge of higher conduction band and photoexcited holes at the band edge of valence band,or from the recombination of photoexcited electrons at the band edge of conduction band and photoexcited holes at the band edge of lower valence band.This indicates that the gaps between various energy bands of the Cs2.9Cu0.34V0.49PMo12O40heteropolyacid should be rather narrow,which might be related to the complex composition [18].XPS valence-band spectrum of the Cs2.9Cu0.34V0.49PMo12O40heteropolyacid (FIG.2(e))was measured to give the valence edge of 1.86 eV below the Fermi level.According to the UV-Vis DRS and XPS valence-band spectra,the band structure of the Cs2.9Cu0.34V0.49PMo12O40heteropolyacid was plotted(FIG.2(f)),showing the edges of valence band and conduction band located at 1.86 and-0.44 eV relative to RHE,respectively.The reduction potential of O2to·O2-radical was-0.33 eV relative to RHE [19].We failed to found the oxidation potential ofi-C4H10toi-·C4H9radical,but a valence band edge of 1.86 eV relative to RHE is capable of oxidizing CH4to ·CH3radical[20,21].Thus,upon light illumination,the photoexcited electrons in the conduction band are capable of activating O2and the photoexcited holes in the valence band can activatei-C4H10.
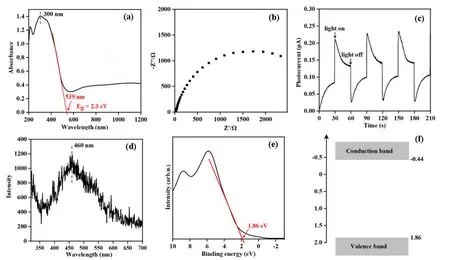
FIG.2 (a) UV-Vis spectrum,(b) electrochemical impedance,(c) photocurrent,(d) PL spectrum,(e) valence-band XPS spectrum,and (f) band structures of Cs2.9Cu0.34V0.49PMo12O40 heteropolyacid.
Diffuse-reflectance infrared spectroscopy (DRIFTS)was used to study the thermal and photothermal catalytic reaction mechanisms.MAL and MAA adsorptions without and with light illumination were firstly examined.As shown inFIG.3(a),MAL adsorption at 25 ℃ gives vibrational features of C=C bond at 1601 cm-1[22],C=O bond at 1760 cm-1[23],various C-H bonds at 2392,2772,and 3034 cm-1[24-26],and various O-H bonds at 3636,3610 and 3570 cm-1[23],suggesting an dominant π-bonded molecular adsorption of MAL.MAA also dominantly forms the π-bonded molecularly-adsorbed species at 25 ℃,giving vibrational features of C=C bond at 1617 cm-1,C=O bond at 1760 cm-1,various C-H bonds at 2392,2830,and 3076 cm-1,and various O-H bonds at 3625,3598,and 3567 cm-1.When the temperature is increased to 335 ℃ (FIG.3(b)),the features of π-bonded MAL and MAA molecules disappeares,and in addition to the negative vibrational features of O-H bonds,weak vibrational features at 1912,1891,1838,1435,1375,1349,and 1316 cm-1emerge for MAL adsorption while those at 1912,1361,and 1339 cm-1for MAA adsorption.This indicates the formation of σ-bonded MAL and MAA species with the rupture of C=C bond into C-C bond and the formation of C-O bonds with Cs2.9Cu0.34V0.49PMo12O40.The C-O bonds of the adsorbed species gives the vibrational feature between 1435 and 1316 cm-1[27],while the C=O bonds of the adsorbed species with similar structures to oxiranone[28,29] gives the vibrational features at 1912,1891,and 1838 cm-1.Upon UV light illumination (FIG.3(a) and(b)),the DRIFTS spectra for MAL and MAA adsorption at 25 ℃ and for MAA adsorption at 335 ℃ barely change,suggesting that UV light does not promote the desorption or surface reactions of π-bonded MAL and MAA molecules and σ-bonded MAA species on Cs2.9Cu0.34V0.49PMo12O40;however,the DRIFTS spectrum for MAA adsorption at 335 ℃ shows slight increase of the σ-bonded MAA features at the expense of the OH features,indicating that UV light promotes the oxidation of σ-bonded MAL on Cs2.9Cu0.34V0.49PMo12O40with OH groups to σ-bonded MAA.
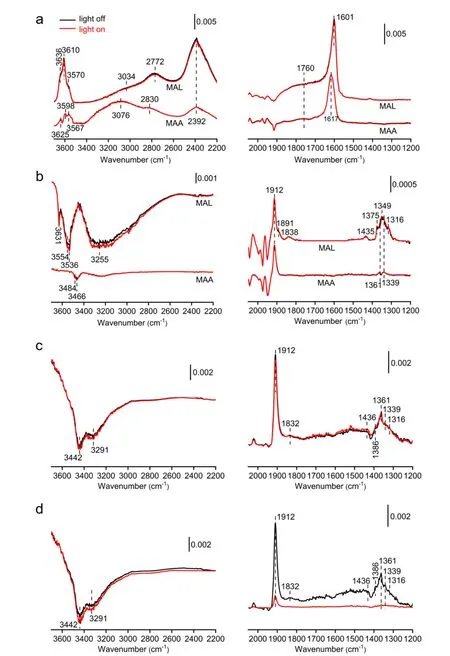
FIG.3 DRIFTS spectra of MAL and MAA adsorption on Cs2.9Cu0.34V0.49PMo12O40 at 25 ℃ (a) and 335 ℃ (b) followed by evacuation without (black line) and with (red line) UV light illumination.(c) In situ DRIFTS spectra of i-C4H10 oxidation on Cs2.9Cu0.34V0.49PMo12O40 at 335 ℃ without and with UV light illumination.(d) DRIFTS spectra of i-C4H10 oxidation on Cs2.9Cu0.34V0.49PMo12O40 at 335 ℃ followed by evacuation without and with UV light illumination.
FIG.3(c) compares thein situDFIFTS spectra ofi-C4H10oxidation without and with UV light illumination at 335 ℃.The spectrum without UV light illumination shows the vibrational features at 1912,1832,1436,1386,1361,1339,and 1316 cm-1and the loss features at 3442 and 3291 cm-1,demonstrating the formation of dominant σ-bonded MAL and MAA species at the expense of OH groups.Upon UV light illumination,the vibrational feature at 1361 cm-1grows slightly at the expense of those at 1912,3442,and 3291 cm-1.As shown inFIG.3(d),the DRIFTS spectrum ofi-C4H10oxidation at 335 ℃ without UV illumination does not vary much upon the subsequent evacuation,indicating that the observed species irreversibly adsorbs on the catalyst surface.But with UV light illumination,the vibrational feature at 1912 cm-1greatly weakens and those at 1832,1436,1386 and 1316 cm-1almost varnishes,and the loss features of OH groups grow;meanwhile,the features at 1361 and 1339 cm-1remain.Thus,i-C4H10oxidation at 335 ℃ forms major σ-bonded MAA and minor σ-bonded MAL on Cs2.9Cu0.34V0.49PMo12O40,consistent with the above experiment result that the oxidation of MAL to MAA is the rate-limiting step of catalytic oxidation ofi-C4H10to MAA;meanwhile,UV light illumination is capable of oxidizing σ-bonded MAL with OH groups to σ-bonded MAA,consequently enhancing both thei-C4H10conversion and MAA selectivity,particularly at temperatures where the over-oxidation reactions are suppressed.
In summary,via a comparative study of thermal and photothermal catalytic selective oxidation of isobutane over Cs2.9Cu0.34V0.49PMo12O40,we demonstrate the synergistic effect of thermal catalysis and photocatalysis in selective oxidation of isobutane to MAA.The oxidation of MAL to MAA is the rate-limiting step.UV light illumination promotes the oxidation of σ-bonded MAL on Cs2.9Cu0.34V0.49PMo12O40with OH groups to σ-bonded MAA to enhance both thei-C4H10conversion and MAA selectivity,particularly at low temperatures.These results demonstrate that photothermal catalysis is promising in catalyzing the selective oxidation of hydrocarbons at relative mild reaction conditions.
Supplementary materials:Catalyst synthesis,catalyst characterizations of Cs2.9Cu0.34V0.49PMo12O40heteropolyacid,and catalytic reaction are shown in FIG.S1-S3 and Table S1.
ACKNOWLEDGEMENTS
This work was financially supported by the Shaanxi Yancheng Petroleum (Group) Co.,Ltd.,the National Natural Science Foundation of China (No.22202189),and the Changjiang Scholars Program of the Ministry of Education of China.
 CHINESE JOURNAL OF CHEMICAL PHYSICS2023年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2023年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Atomistic Modeling of Lithium Materials from Deep Learning Potential with Ab Initio Accuracy
- On-the-Fly Nonadiabatic Dynamics of Caffeic Acid Sunscreen Compound
- Minimum-Modified Debye-Hückel Theory for Size-Asymmetric Electrolyte Solutions with Moderate Concentrations
- Design Strategy of Infrared 4-Hydroxybenzylidene-imidazolinone-Type Chromophores based on Intramolecular Charge Transfer: a Theoretical Perspective
- Quantum Dynamics Calculations on Isotope Effects of Hydrogen Transfer Isomerization in Formic Acid Dimer
- Controllable Modulation of Morphology and Property of CsPbCl3 Perovskite Microcrystals by Vapor Deposition Method
