Functional Connectivity of Electroencephalographic Signals in Migraine Under Somatosensory Stimulation*
ZHONG Shu-Jun ,LIU Si-Tong ,QIU Meng-Yuan ,WANG Jing ,GUO Huai-Lian ,LIU Zun-Jing**
(1)Department of Neurology,People’s Hospital,Peking University,Beijing 100044,China;2)Department of Neurology,Beijing Friendship Hospital,Capital Medical University,Beijing 100050,China;3)Department of Neurobiology,School of Basic Medical Sciences,Capital Medical University,Beijing100069,China;4)Key Laboratory for Neuroscience,Ministry of Education,National Health and Family Planning Commission,Peking University,Beijing 100191,China)
Abstract Objective Migraine is a complex brain dysfunction disease with a prevalence of 14.4% worldwide.Functional connectivity measures the statistical interdependences between two neural signals,and different functional connectivity patterns reflect different models of how brain regions work together.Therefore,investigating functional connectivity between different brain regions is important for understanding the pathophysiological mechanisms of migraine.Previous electroencephalogram-based functional connectivity analyses in migraine patients have mainly focused on visual and painful stimulation.We sought to investigate cortical responses to somatosensory stimulation in migraine patients during the interictal period,with the aims of better understanding the neurological dysfunction in migraine and providing clues for the prevention and treatment of migraine.Methods Twenty-three patients with migraine without aura,10 patients with migraine with aura,and 28 healthy controls were recruited.Detailed basic data and medical history were collected from all participants,and the scale assessment was completed.All participants underwent electroencephalogram recording under median nerve somatosensory stimulation.The coherence of 68 brain regions was calculated as functional connectivity and correlations with clinical parameters were evaluated.Results Functional connectivity in migraine without aura and migraine with aura patients is atypical compared to controls under median nerve somatosensory stimulation,and the abnormal functional connectivity mainly involves areas of sensory discrimination,pain modulation,emotional cognition,and visual processing.The cerebral cortex in migraine without aura and migraine with aura patients may possess a common way of responding to somatosensory stimulation.The functional connectivity abnormalities in migraine patients have correlations with clinical features and may partly reflect the severity of migraine.Conclusion Our results provide evidence of altered functional connectivity in migraine patients under somatosensory stimulation,and suggest that the dysfunction in the brain network may be involved in the pathological process of migraine.
Key words migraine,electroencephalogram (EEG),functional connectivity,somatosensory stimulation,coherence
Migraine is a common neurological disorder characterized by episodic,usually lateral,moderate or severe pulsating headaches,often accompanied by nausea,vomiting,photophobia,and phonophobia[1-2].About 20% of migraine patients experience a brief and completely reversible neurological aura before or during the headache[3],including visual,sensory or other central nervous system symptoms.The global prevalence of migraine is 14.4%[4],with the prevalence in women two to three times higher than in men[5].Migraine is considered the second most disabling disease in the world[6],seriously affecting the quality of life in patients.
The pathophysiological mechanisms of migraine are not fully understood.It is now generally accepted that migraine is a complex brain dysfunction disease with a genetic basis[7-10].Functional connectivity measures the statistical interdependencies between two neural signals[11].The brain’s advanced cognitive function depends on the interaction between different brain regions,and different functional connectivity patterns reflect different models of how brain regions work together[12].Therefore,investigating functional connectivity between different brain regions is important for understanding the pathophysiological mechanisms of migraine.Electroencephalogram(EEG) is the electrical activity produced by neurons in the brain,recorded by electrodes placed on the head,and reflects the summation of postsynaptic potentials from cortical pyramidal neurons[13-14].The temporal precision and non-invasive nature of EEG make it particularly well suited to studying the brain function changes associated with migraine[15].And changes in brain function during the interictal period in migraine patients can reveal the underlying neurological dysfunction in migraine.
In addition to the headache,migraine attacks are characterized by hypersensitivity to visual,auditory,somatosensory,and olfactory stimulation[16-17].Therefore,the multisensory integration of somatosensory,visual,auditory,and olfactory stimulation may play an important role in the pathophysiology of migraine.Functional imaging studies have shown that migraine patients lack normal habituation to repetitive stimulation[18-20].In migraine patients,functional magnetic resonance imaging(fMRI)[21]revealed abnormal pain processing during the interictal period compared to controls,with impaired habituation to repetitive painful stimulation found in the bilateral anterior insula,middle cingulate cortex,and thalamus.An EEG study[22]showed that alpha-band phase synchronization was enhanced in migraine patients in the presence of visual stimulation.Another EEG study[23]using CO2laser stimulation on the right hand to produce painful stimulation showed that in migraine patients,more information was transferred from the right centralparietal-temporal regions to bilateral frontal regions,possibly as a sign of sustained activation of cortical functional networks.
Previous EEG-based functional connectivity analyses in migraine patients have mainly focused on visual[22]and painful stimulation[23],while EEG functional connectivity in migraine patients under somatosensory stimulation needs further investigation.In contrast to classical somatosensory evoked potentials,we applied functional connectivity analysis to somatosensory stimulation states to detect differences in sensory information processing between migraine patients and healthy controls.Somatosensory stimulation does not elicit painful perception,is gentler than visual and painful stimulation,and can reduce migraine attacks induced by experimental stimulation.According to the previous study[24],theta activity was increased in migraine patients during the interictal period compared to controls.In this study,we focused on analyzing the functional connectivity of the theta band.Considering the differences in clinical and physiological characteristics between migraine without aura and migraine with aura[25],migraine patients were divided into migraine without aura(MO) and migraine with aura (MA) groups.To our knowledge,the current study is the first to analyze EEG functional connectivity in migraine patients during the interictal period under somatosensory stimulation,intending to provide clues for the prevention and treatment of migraine.
1 Methods
1.1 Participants
Migraine patients were consecutively recruited from the Headache Clinic of the Department of Neurology,Peking University People’s Hospital,and divided into migraine without aura (MO) and migraine with aura (MA) groups.The diagnosis of migraine was based on the International Classification of Headache Disorders,3rd edition (ICHD-3)[1].The inclusion criteria were age 18-70 years,age less than 50 years at migraine onset,and headache for more than 1 year.The healthy controls enrolled were matched for age and gender and had never reported a history of migraine or other types of headaches.Anyone with a history of brain damage,severe mental disease,or an inability to stay still was excluded.
All participants provided informed consent before participating in the study.This study was approved by the Medical Ethics Committee of the Peking University People’s Hospital.
Detailed basic data and medical history were collected from all participants,including the age of migraine onset,presence or absence of aura,headache location,type,duration,intensity,attack frequency,associated symptoms,precipitating and relieving factors,current medication,and family history.And the Hospital Anxiety and Depression Scale (HADS)[26]was also completed.Migraine patients also completed a Visual Analogue Scale (VAS)[27]and a Migraine Disability Assessment (MIDAS) questionnaire[28]to assess headache intensity and headache-related disability.
1.2 Somatosensory stimulation
Median nerve somatosensory stimulation was performed using an electrical stimulator (Han’s Acupoint Neural Stimulator,China),which generates a constant current square-wave pulse to stimulate the median nerve at the right wrist with a frequency of 2 Hz,pulse width of 0.6 ms,and current of 3-20 mA.The stimulation intensity was just above the motor threshold (thumb movement) without eliciting painful perception.
1.3 EEG recording
The EEG recordings were obtained from an EEG cap containing a 64-electrode system (BrainAmp,Brain Products GmbH,Germany) that covered the whole brain according to the extended international 10-20 system[29],with FCz as the reference electrode point and a sampling rate of 1 000 Hz.The electrode impedance was kept below 5 kΩ.All participants underwent 5 min of EEG recordings while awake and with eyes closed under median nerve somatosensory stimulation.In addition,participants were informed in advance to avoid unnecessary movements,such as body actions and blinking,during the EEG recording.Migraine patients were in the interictal phase at the time of EEG recording and had no migraine attack for at least 48 h before or after the recording (ascertained by telephone interview).
1.4 EEG pre-processing
EEG data were pre-processed using the EEGLAB toolboxes[30]in MATLAB 2017a (The Mathworks,Natick,MA,USA).The raw data were filtered (1-45 Hz),the sampling rate was reduced to 500 Hz,and the bad channels were interpolated by the surrounding channels.Obvious limb movement artifacts were manually removed from the EEG signal by visual inspection.Independent Component Analysis (ICA) was used to remove eye movement artifacts (blinks and horizontal eye movements).The data were re-referenced to a common average reference and then divided into 10-second segments.Finally,18 validated segments with a total of 180 s were selected for subsequent analysis.
1.5 EEG source reconstruction
Neural electrical signals need to pass through volume conduction (consisting of the cortex,cerebrospinal fluid,meninges,skull,and scalp) before reaching the recording electrodes.Due to the presence of volume conduction,EEG functional connectivity measurements may produce spurious components and confusion[31].EEG source reconstruction is an inverse solution that can locate and reconstruct cortical activity based on scalp EEG data,which can not only reduce the effects of volume conduction but also compensate for the low spatial resolution of EEG[32].Based on the Desikan-Killiany atlas[33],the source reconstruction time series were retrospectively mapped to the 68 regions of interest (ROIs) defined by the Desikan-Killiany atlas using Brainstorm[34],with each hemisphere having 34 identical ROIs (Table S1).Specifically,the EEG electrodes were first aligned to the public MRI template by identifying anatomical landmarks (left and right ear lobes and nasal bone),the number of cortical dipole sources was set to 15 000,and the head model was obtained based on openMEEG plug-in calculations.The noise and data covariance matrices were calculated using the EEG data,and the signal sources were obtained using the minimum norm estimation method,after which the source reconstruction time series were mapped to the 68 ROIs.
1.6 EEG functional connectivity
In this study,we use coherence as the measure of the functional connectivity between brain regions.Coherence measures the linear relationship of two brain regions in the frequency domain,which is widely used to reveal the interrelationship of EEG signals[35].Mathematically,the coherence functionCxy(f)at frequencyfof signalsxandyis obtained from the normalization of the power spectral densitiesPxx(f)andPyy(f) and the cross power spectral densityPxy(f)of “x” and “y” as follows[36]:
Where the power spectral density is estimated using pwelch’s[37]overlapped averaged periodogram method.The value of coherence ranges from 0 to 1,where 0 indicates that the frequency components of the two signals are linearly independent and 1 indicates the greatest linear dependence.Higher coherence indicates more cooperation and information transfer between brain regions.Therefore,coherence is an important metric for quantifying the synchronization properties of two EEG signals[38].In this study,coherence was averaged at 4-7.5 Hz to produce the average functional connectivity matrix in the theta band.
1.7 Statistical analysis
Statistical analysis was performed using SPSS 27.0 (IBM Corp.,Armonk,NY,USA).Categorical data were presented as frequencies (percentages).and compared between groups using the chi-squared test or Fisher’s exact test.Normally distributed measures were presented as mean±standard deviation (mean±SD),using the independent samplest-test for comparisons between two groups and one-way analysis of variance for comparisons between three groups.Non-normally distributed measures were presented as median (interquartile range) and compared between groups using non-parametric tests.A value ofP<0.05 was considered statistically significant.
Statistical analyses of EEG functional connectivity were performed in MATLAB.Functional connectivity was defined as the coherence between each pair of ROIs.Functional connectivity data from three groups of participants formed the following comparison groups: migraine without aura (MO)versus controls,migraine with aura (MA) versus controls,and migraine without aura (MO) versus migraine with aura (MA).The independent samplesttest was used for comparisons between groups.To minimize type I errors and improve the accuracy of statistical analysis,the Bonferroni correction[39]was applied for multiple comparisons,andP<0.01 was considered significant for functional connectivity differences.
To assess the association between functional connectivity and clinical features,we evaluated the correlations between functional connectivity and clinical parameters in migraine patients using Pearson correlation analysis,including migraine duration(years),attack frequency (d/month),VAS score,MIDAS score,and HADS score.The significance level was set at 0.05.
2 Results
2.1 Demographic and clinical data of participants
A total of 62 participants were recruited for this study,excluding one migraine patient who had a headache on the day of the EEG recording.Therefore,a total of 61 participants were included in the analysis,including 23 MO patients,10 MA patients,and 28 controls.All participants were right-handed.Demographic and clinical data are shown in Table 1.There were no statistical differences in age,gender,HADS scores and stimulation intensity among the three groups.Headache duration,attack frequency,VAS scores,MIDAS scores,and family history of headache were not significantly different between MO and MA groups.
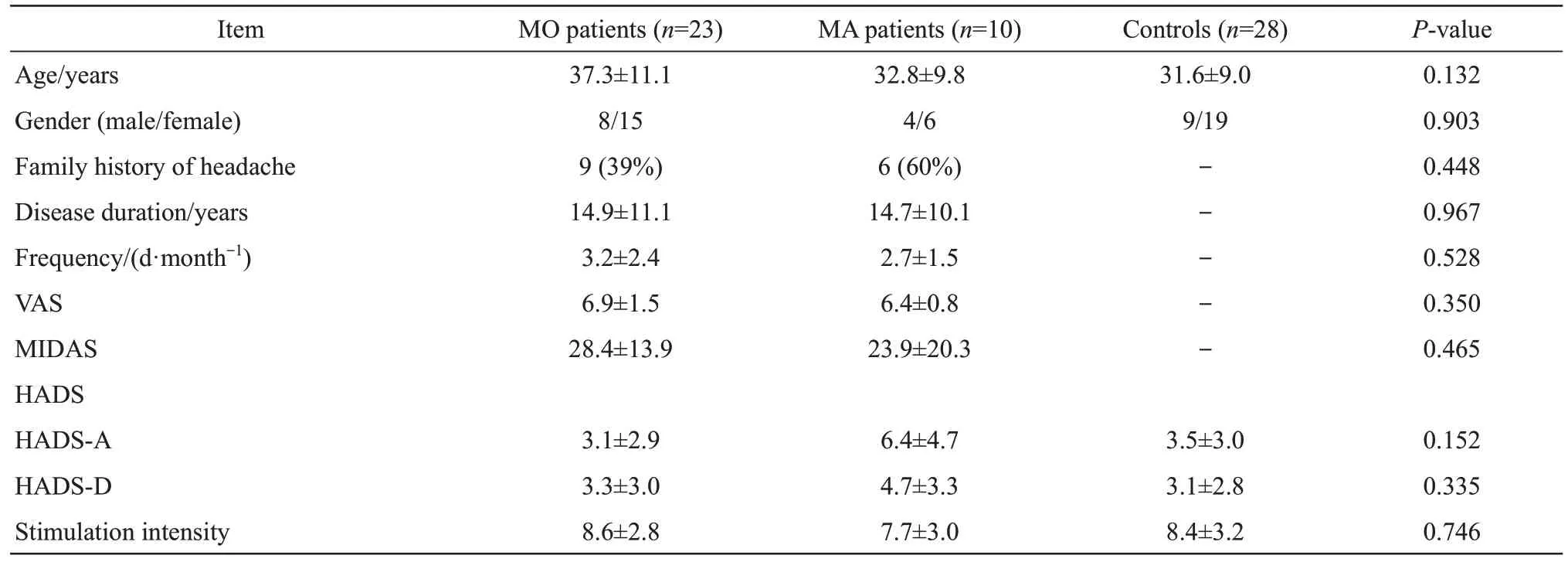
Table 1 Demographic and clinical data of MO patients,MA patients,and controls
2.2 EEG functional connectivity analysis
In this study,we calculated coherence as functional connectivity in the theta band of the EEG signals.For each subject,we obtained a symmetric coherence functional connectivity matrix of 68×68.The vector dimension of the functional connectivity features was 68×(68-1)/2=2 278.
The results of the differences in coherence between MO patients,MA patients and controls are shown in Figure 1,where Figure 1a shows the mean coherence matrix in the theta band for MO patients,MA patients and controls.And Figure 1b shows paired comparisons,thresholded to obtain a significant difference.The Bonferroni correction threshold was set at 0.01 (a pre-correctionP-value<0.01/2 278=4.39×10-6was considered statistically significant).The coherence matrix with significant differences after Bonferroni correction was presented using the BrainNet Viewer[40],a tool for visualizing brain network connectivity,as shown in Figure 2.
The results showed that in the state of median nerve somatosensory stimulation,functional connectivity was significantly altered in both MO and MA patients compared to the controls (Bonferroni correction,P<0.01).No significant differences in functional connectivity were found between MO and MA patients.
Table 2 lists the ROIs for which coherence was either increased or decreased in the theta band in both MO and MA patients compared to the controls,and the results of all statistical analyses of differential brain areas are tabulated separately in Table S2.
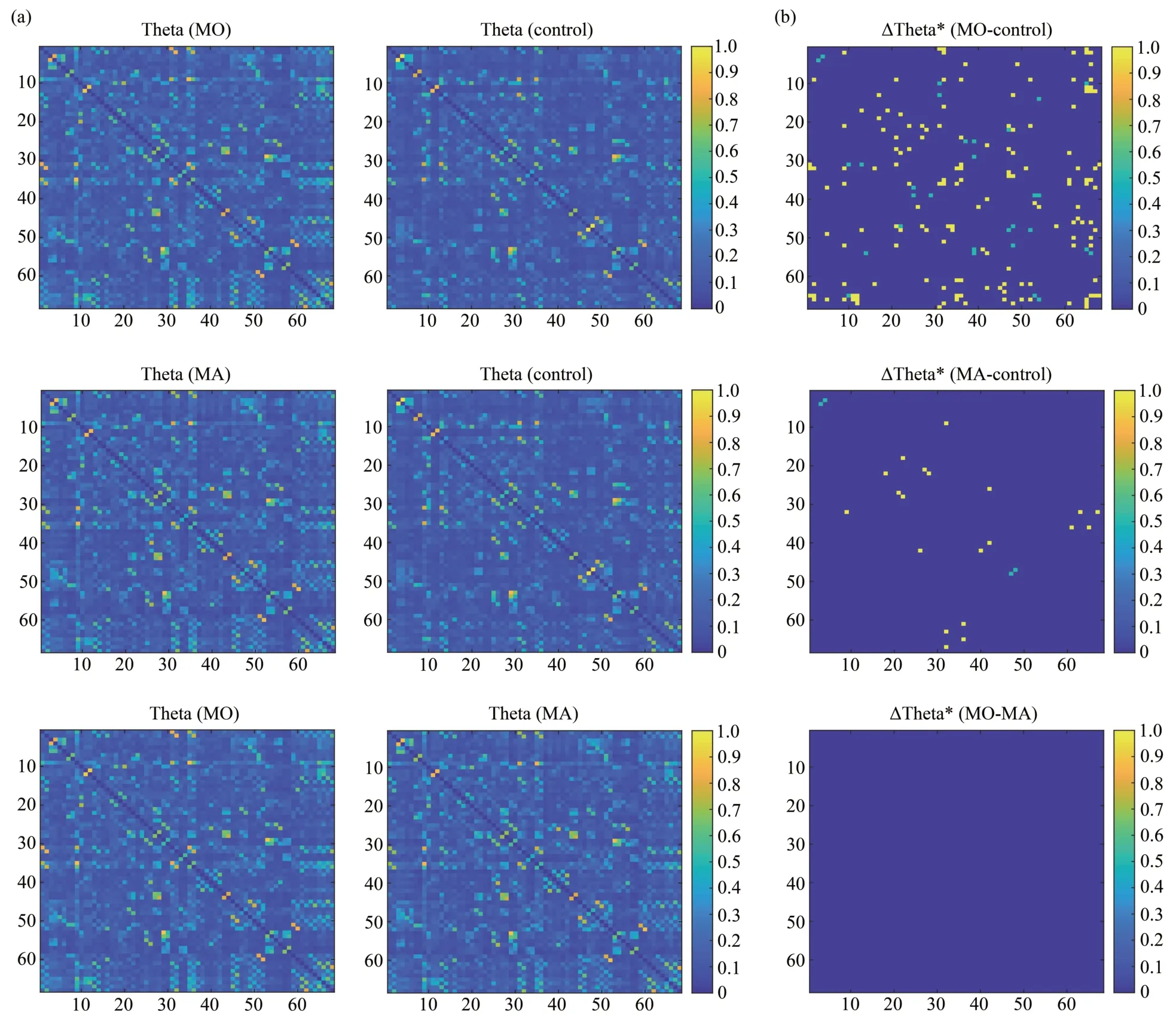
Fig.1 Comparison of coherence in the theta band between MO patients,MA patients and controls
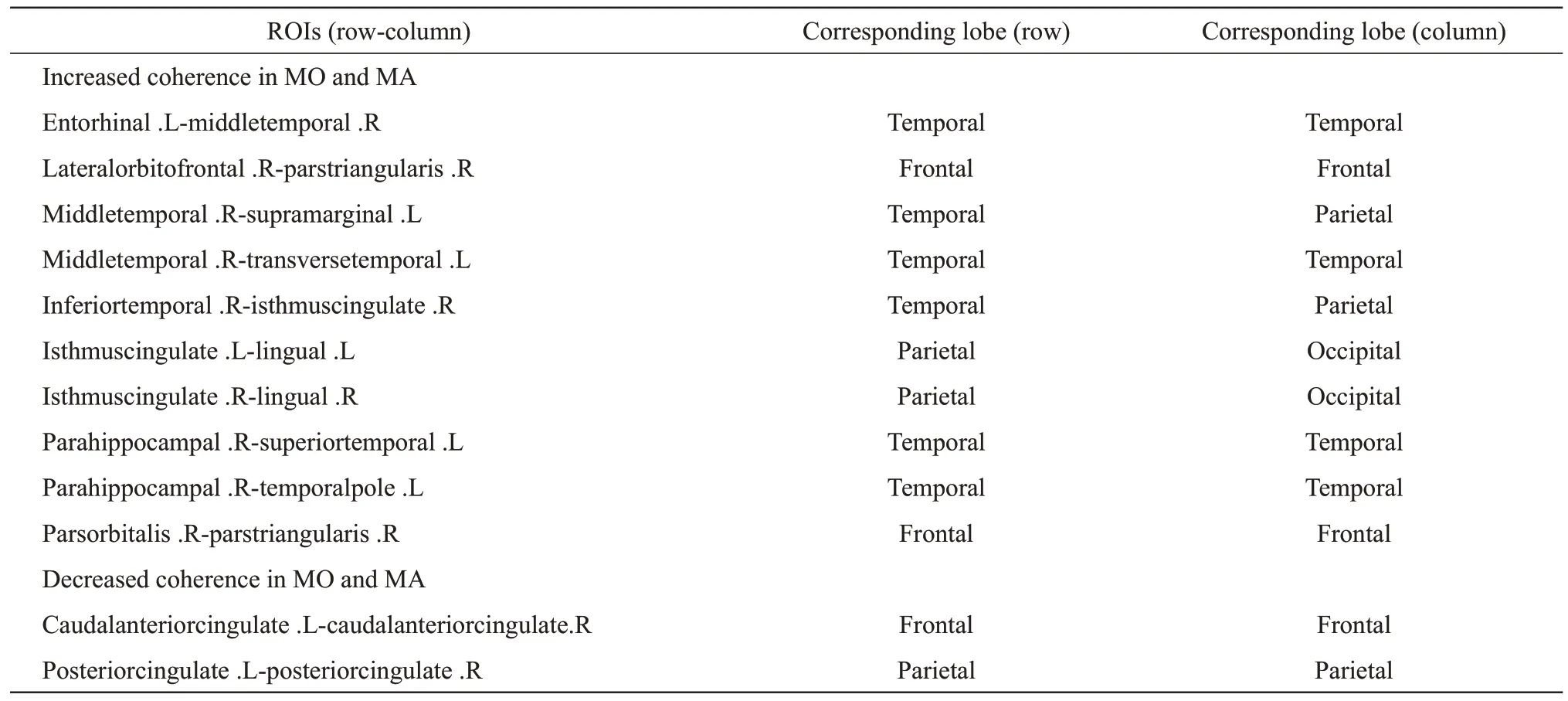
Table 2 ROIs for both MO and MA with increased or decreased coherence in the theta band compared to controls
The functional connectivity that was significantly different in MA patients was also significantly different in MO patients,and functional connectivity abnormalities were more prevalent in MO patients.Abnormal functional connectivity was found in the frontal,temporal,parietal,and occipital lobes,with enhanced functional connectivity mainly in the frontal lobe (parsorbitalis,lateralorbitofrontal,parstriangularis),temporal lobe (parahippocampal,transversetemporal,temporalpole,superiortemporal,middletemporal,inferiortemporal,entorhinal),parietal lobe (isthmuscingulate,supramarginal),and occipital lobe (lingual).The areas with reduced functional connectivity are mainly located in the frontal lobe(caudalanteriorcingulate) and the parietal lobe(posteriorcingulate).
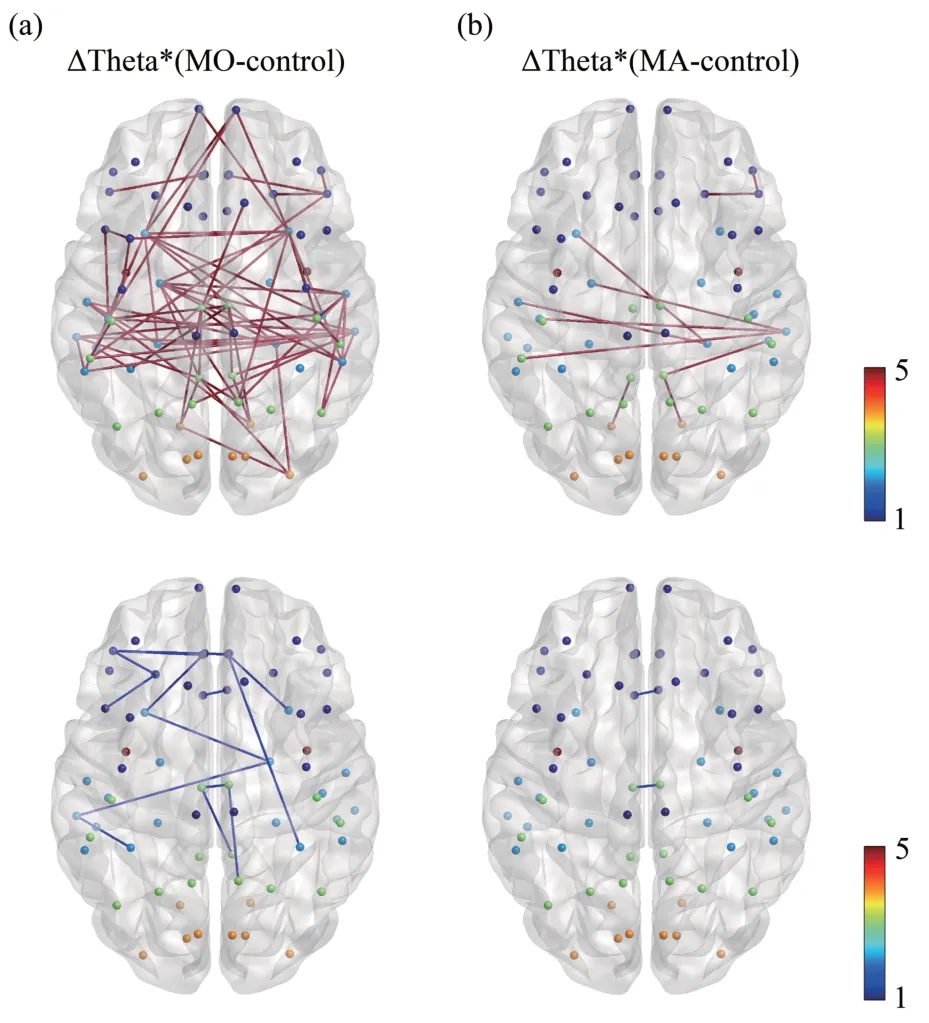
Fig.2 Coherence differences in the theta band between MO patients,MA patients and controls
2.3 Correlation between EEG functional connectivity and clinical features in migraine patients
We evaluated the correlation between significantly different functional connectivity and clinical data in migraine patients (MO and MA)compared to controls using Pearson correlation analysis.These included migraine duration (years),attack frequency (days/month),VAS score,MIDAS score,and HADS score.The significance level was 0.05.
Pearson correlation analysis showed that coherence with significant differences in the theta band in migraine patients correlated with clinical parameters (P<0.05;Table S3 and Figure 3).Anxiety score on the HADS showed a positive correlation with the coherence in caudalanteriorcingulate(left)-caudalanteriorcingulate(right) (r=0.471,P=0.006),posteriorcingulate(left)-posteriorcingulate(right) (r=0.453,P=0.008),whereas it showed a negative correlation with the coherence in isthmuscingulate (left)-lingual (left) (r=-0.383,P=0.028).Migraine duration showed a positive correlation with the coherence in inferiortemporal(right)-isthmuscingulate (right) (r=0.403,P=0.020),isthmuscingulate (left)-lingual (left) (r=0.364,P=0.038),parsorbitalis (right)-parstriangularis (right)(r=0.371,P=0.034),whereas it showed a negative correlation with the coherence in caudalanteriorcingulate (left)-caudalanteriorcingulate(right) (r=-0.415,P=0.016) and posteriorcingulate(left)-posteriorcingulate (right) (r=-0.404,P=0.020).
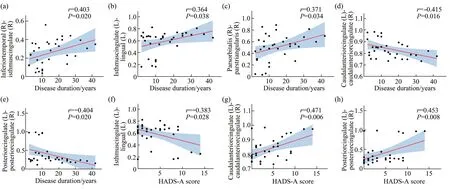
Fig.3 Correlations between coherence with significant differences in the theta band and clinical parameters in migraine patients (Pearson correlation analysis)
3 Discussion
3.1 Comparison of EEG functional connectivity between migraine patients and controls
The aim of this study was to investigate cortical responses to somatosensory stimulation in MO patients,MA patients,and controls during the interictal period.The results showed that in the state of median nerve somatosensory stimulation,EEG functional connectivity was significantly altered in both MO and MA patients compared to the controls.Abnormal functional connectivity was found in the frontal,temporal,parietal,and occipital lobes,with enhanced functional connectivity mainly in the frontal lobe (parsorbitalis,lateralorbitofrontal,parstriangularis),temporal lobe (parahippocampal,transversetemporal,temporalpole,superiortemporal,middletemporal,inferiortemporal,entorhinal),parietal lobe (isthmuscingulate,supramarginal),and occipital lobe (lingual).The areas with reduced functional connectivity are mainly located in the frontal lobe(caudalanteriorcingulate) and the parietal lobe(posteriorcingulate).
The prefrontal cortex plays an important role in pain regulation through cognitive control mechanisms.Cognitive pain processing includes attention,anticipation,and appraisal of pain,all of which are influenced by previous pain experience and pain memory[41].The results showed that MO and MA patients have increased functional connectivity in the prefrontal cortex under somatosensory stimulation,suggesting abnormal cognitive processing of pain and increased pain anticipation in migraine patients.
The temporal lobe is a multisensory association area involved in the integration of multisensory stimulation,including pain,auditory,olfactory,and visual stimulation[42],and is associated with emotional responses to pain[43].We found increased functional connectivity in several brain regions of the temporal lobe in MO and MA patients compared to controls,suggesting that migraine patients overreact to somatosensory stimulation.
The cingulate gyrus is involved in emotional responses to pain and subjective sensations,as well as pain-related attention and memory[44].Enhanced functional connectivity in the isthmus cingulate in migraine patients indicates increased sensitivity to nociception.And reduced functional connectivity in the caudalanteriorcingulate and posteriorcingulate indicates reduced emotional regulation of pain in migraine patients.
The occipital lobe is primarily responsible for visual perception and processing.Previous studies[45]have shown abnormalities in the structure and function of the occipital lobe in MO and MA patients.The lingual gyrus is part of the occipital cortex with a significant role in vision and memory.In migraine patients,this region may be involved in the generation and propagation of migraine aura[46].Enhanced functional connectivity of lingual gyrus in MO and MA patients in our study may reflect increased visual processing of the cortex.
In conclusion,our results suggest that functional connectivity in MO and MA patients during the interictal period is atypical compared to controls under median nerve somatosensory stimulation,and that the abnormal functional connectivity mainly involves areas of sensory discrimination,pain modulation,emotional cognition,and visual processing.
3.2 Comparison of EEG functional connectivity between MO and MA
MO and MA are the two main clinical subtypes of migraine[47].A previous study of steady-state visual evoked potentials[48]showed differences between MO and MA patients,suggesting hyperexcitability of the visual cortex in MA.Another meta-analysis of interictal pattern-reversal visual evoked potentials in migraine patients showed no statistical differences between MO and MA patients[49].Few electrophysiological studies of interictal MO and MA have been reported,and the evidence in the literature is contradictory and controversial.
Our study demonstrated that there is no significant difference in functional connectivity during median nerve somatosensory stimulation between MO and MA patients.Therefore,the cerebral cortex in MO and MA patients may possess a common way of responding to somatosensory stimulation.A previous study[50]found increased information flow in the beta band in MA patients during intermittent visual stimulation,compared to MO patients.Another study[51]analyzed EEG signals during pattern-reversal visual stimulation and showed that MA patients had increased occipital cortical activation and dissociated connections in posterior regions compared to MO patients and controls.
No significant differences were found in the EEG functional connectivity between MO and MA patients under somatosensory stimulation in this study,possibly due to the use of a whole-brain data-driven analysis with overly stringent statistical thresholds.Differential functional connectivity might be identified in our study if a seed-point-based approach were used,or if parameters for statistical significance were eased.In addition,unmeasured confounding variables could have influenced the study results.Finally,migraine is a heterogeneous disorder with different disease duration,attack frequency,headache intensity,and associated symptoms,which might have caused variations in results between studies.Notably,Denuelleet al.[52]found posterior cortical hypoperfusion in MO patients using positron emission tomography (PET),with changes similar to those previously found in MA[53],and speculated a common pathogenesis of migraine with and without aura.The correlation between the pathological and physiological mechanisms of these two migraine subtypes may partially explain our results.Our study is an exploration of the functional connectivity of EEG under somatosensory stimulation in migraine patients,and future studies with larger samples are needed for further elucidation.
3.3 Correlation between EEG functional connectivity and clinical features in migraine patients
Correlation analysis revealed significant correlations between coherence in the theta band and clinical features in migraine patients.Anxiety score on the HADS was positively correlated with the strength of functional connectivity in the caudalanteriorcingulate and posteriorcingulate,and negatively correlated with the strength of functional connectivity in the isthmuscingulate and lingual.The cingulate gyrus is involved in cognitive and emotional responses to pain,and the lingual gyrus is an important area for visual processing.This suggests that as anxiety levels increase,migraine patients have increased pain anticipation,as well as impaired pain regulation and visual processing.Migraine duration was positively correlated with the strength of functional connectivity in several regions,including the inferiortemporal,isthmuscingulate,lingual,parsorbitalis,and parstriangularis,while negatively correlated with the strength of functional connectivity in the caudalanteriorcingulate and posteriorcingulate.This suggests that the degree of dysfunctional connectivity increases with years of migraine duration,mainly involves areas of pain modulation and visual processing.In conclusion,the functional connectivity abnormalities in migraine patients have correlations with clinical features and may partly reflect the severity of migraine.
3.4 Limitations
This study has several limitations.Firstly,we did not compare the difference in functional connectivity between resting state and somatosensory stimulation state,and could not completely exclude the effect of somatosensory evoked potentials induced by median nerve electrical stimulation on the functional connectivity analysis.However,considering that the present study is a preliminary exploration of EEG functional connectivity under somatosensory stimulation in migraine patients,resting-state EEG analysis should be included in future experiments.Secondly,this was a cross-sectional study,and we could not prove the role of functional connectivity abnormalities in migraine attacks and disease progression.Thirdly,the sample size of subjects in our study was relatively small.Finally,the signals recorded by EEG mainly reflect the electrical activity of the cerebral cortex,and when combined with functional magnetic resonance imaging,may be more useful in elucidating the pathophysiological mechanisms in migraine patients.
4 Conclusion
In this study,we have investigated cortical responses to somatosensory stimulation in migraine patients during the interictal period.Our data provide the first evidence of EEG functional connectivity in migraine patients under somatosensory stimulation.The results demonstrated that functional connectivity in MO and MA patients is atypical compared to controls under median nerve somatosensory stimulation,mainly involving areas of sensory discrimination,pain modulation,emotional cognition,and visual processing.The cerebral cortex in MO and MA patients may possess a common way of responding to somatosensory stimulation.These findings suggest that the dysfunction in the brain network may be involved in the pathological process of migraine.
SupplementaryAvailable online (http://www.pibb.ac.cn or http://www.cnki.net):
PIBB_20230166_Table_S1.pdf
PIBB_20230166_Table_S2.pdf
PIBB_20230166_Table_S3.pdf

