AA-stacked borophene–graphene bilayer as an anode material for alkali-metal ion batteries with a superhigh capacity
Yi-Bo Liang(梁藝博) Zhao Liu(劉釗) Jing Wang(王靜) and Ying Liu(劉英)
1Department of Physics and Hebei Advanced Thin Film Laboratory,Hebei Normal University,Shijiazhuang 050024,China
2National Key Laboratory for Materials Simulation and Design,Beijing 100083,China
As the lightest two-dimensional material,monolayer borophene exhibits great potential as electrode materials,but it suffers from stability issues in the free-standing form. Here,the striped-borophene and graphene bilayer(sB/Gr)is found to be a high-performance anode material for rechargeable alkali-metal ion batteries. The first-principles results show that all the three alkali-metal atoms,Li,Na,and K,can be strongly adsorbed on sB/Gr with ultra-low diffusion barriers than that on pristine borophene/graphene,indicating good charge-discharge rates. Remarkably,high storage capacities are proposed for LIBs(1880 mA·h/g),NIBs(1648 mA·h/g),and KIBs(470 mA·h/g)with relatively small lattice change rate(<2.9%)in the process of alkali-metal atoms intercalations. These intriguing features of sB/Gr make it an excellent choice for batteries.
Keywords: alkali-metal ion batteries,borophene–graphene bilayer,adsorption property,diffusion behavior
1. Introduction
The continuous increasing world’s energy demands as well as rising environmental problems have led to the improving of the energy conversion and more importantly its storage technology.[1]Nowadays, secondary batteries have been emerged as the most efficient solution.[2]Rechargeable lithium-ion batteries(LIBs)are successfully explored and widely used in people’s daily lives from mobile phones to laptops and electric cars.[3–6]Limited by the small abundance of Li in the Earth’s crust, the sodium-ion batteries (NIBs)and potassium-ion batteries(KIBs)are considered as ideal alternatives to LIBs due to their higher abundance, lower cost and similar chemical properties to Li.[7,8]For these rechargeable alkali-metal(Li,Na,and K)ion batteries(AMIBs),they show similar charging and discharging processes between the two electrodes, which play a vital role in the electrochemical performance.[2,9–12]In this respect,an abundant,eco-friendly,and stable anode material is highly desired for its low cost,high-energy density and long lifespan.
The two-dimensional (2D) materials have been explored as promising anode materials for AMIBs extremely fast due to their advantages in providing ample active sites and improving electrochemical reaction kinetics. In addition to graphene and its derivatives,[13–16]borophene,[17–20]as the lightest 2D material plus its unique polymorphism and metallicity,is becoming a great promising candidate for anode materials because of its large reversible capacity, high-rate capability and long cycle life.[21–25]Mortazaviet al.theoretically studied the interaction of alkali metals(Li and Na)with borophene and reported high storage capacity(1720 mA·h/g and 1380 mA·h/g)and low diffusion barriers of 25/317 meV for Li and 3/223 meV for Na in longitudinal/transverse directions of the surface forpmmnborophene.[21]Zhanget al.reportedβ12andχ3borophene as the electrode materials of LIBs and NIBs, and the computed storage capacities were 1984 mA·h/g inβ12phase and 1240 mA·h/g inχ3phase.[22]Jiangel al.reported the potential ofpmmnborophene as an anode of LIBs. They found that the fully lithiated borophene (Li0.75B) has a theoretical specific capacity of 1860 mA·h/g with a low energy barrier of 2.6 meV.[23]Raoet al.calculated the properties of a borophene anode material and ultrahigh energy storage and ultrafast ion diffusion were obtained for AMIBs (3306 mA·h/g and 3.95×10-7m2/s for LIBs).[24]However, the relatively large structural distortions of borophene in the process of charge and discharge,hinder its practical application as stable anode materials.[21,25]
Constructing 2D borophene-based heterostructures has been considered as an effective pathway to overcome these limitations, as well as keeping the merits of pristine borophene.[26]Borophene was firstly reported to form lateral and vertical heterostructures with graphene on the surface of Ag (111).[27]With the help of DFT, the structural, electronic, and elastic properties of single-layer striped borophene stacked on top of graphene (sB/Gr) were studied and good conductivity and excellent elastic properties were proposed.[28]Modified by a single metal atom, sB/Gr was reported to have a good catalytic effect in hydrogen evolution reaction.[29]When passivated by hydrogenation or fluorine, the band structure of sB/Gr was adjusted.[30]The DFT results also showed that transition metals doped borophene–graphene heterostructure can be used as robust polysulfide anchoring.[31]Houet al.demonstrated the potential application of a hydrogenated borophene/graphene (B/G)heterostructure for high-performance humidity sensor.[32]By using first-principles calculations, a van der Waalsαborophene/graphene heterostructure has been reported to be a promising anode material for LIBs with a theoretical capacity of 1469 mA·h/g.[33]Different from the van der Waals B/G,the sB/Gr bilayer presents an intralayer covalent bonding,which makes it thermally stable at high temperatures and able to stand at high mechanical stiffness. All these make sB/Gr promising for the design of next generation energy storage nanodevice.
Inspired by these discoveries,the first-principles calculations are employed in this work to explore the adsorption of alkali metals(Li,Na,and K)on the surface of sB/Gr for evaluating its performance as an anode material of AMIBs. The results show that sB/Gr has both high ion storage concentration and high theoretical specific capacity since boron and carbon belong to elements with low mass fraction. In the process of charge and discharge,it has very low diffusion barriers with relatively small lattice change rate, which ensures the cycle stability of the battery.
2. Computational details
The first-principles calculations are based on density functional theory (DFT) in conjunction with the projectedaugmented wave (PAW) method,[34]as executed in the Viennaab initiosimulation package(VASP).[35]The generalized gradient approximation (GGA)[36]with the Perdew–Burke–Enzerhof(PBE)functional[37]is used for electron exchange–correlation interactions. The Grimme’s semiclassical dispersion correction(DFT-D2)[38]is employed to take into account the long-range van der Waals(vdW)interactions between the adsorbed atoms and the layered systems.
In the process of geometric optimizations and total energy calculations,a sufficient vacuum space of approximately 25 ?A was constructed between layers to avoid the interaction between adjacent layers. The plane wave cut-off energy was chosen to be 500 eV and the convergence thresholds for energy and force were set to be 10-7eV and 0.02 eV/?A,respectively.All the configurations were fully relaxed with a 3×3×1kpoints in the Brillouin sampling. For static electronic calculations, the Heyd–Scuseria–Ernzerhof (HSE) method[39]was used to calculate the band structure and the Brillouin zone was sampled with ak-point grid of 20×20×1.The climbed image nudged elastic band(CI-NEB)method[40,41]was used to find the effective transition states and calculate the corresponding diffusion barriers. The phonon spectra was obtained from finite displacement method. Anab initiomolecular dynamics(AIMD) simulation of 5 ps with a time step of 1 fs at room temperature in the microcanonical ensemble (NVE) was employed to identify the stability of sB/Gr.
3. Results and discussion
3.1. Adsorption ability of alkali-metal atoms (Li, Na, and K)on sB/Gr
The sB/Gr bilayer was constructed by stacking a singlelayer striped borophene on the top of graphene with AA stacking mode and the optimized atomic configuration is shown in Figs.1(a)–1(c).Our calculations show that the equilibrium lattice constant was 2.702 ?A and the minimum distance between borophene and graphene was 1.713 ?A, which are within the range of the sum of atomic radii of boron and carbon atoms.This makes sB/Gr more stable than some other vdW structures. Figure 1(d)also shows the band structure of sB/Gr obtained at the level of HSE06,and the result indicates sB/Gr is metallic with a divergence of branches below the Fermi level at theKpoint in the first Brillouin zone.All these results are in consistence with the experimental and theoretical results.[27,28]
As shown in Fig.S3(a),the phonon band gap is not wide because the atomic mass in a single cell is very close (ΔE ≈5 THz).[28]sB/Gr has no negative frequency mode,therefore,it is dynamically stable at 0 K.In addition,Figs.S3(b)–S3(c)also show that sB/Gr can still maintain a stable periodic structure after AIMD simulation.

Fig.1. (a)Top, (b)bottom and(c)side views for sB/Gr. The possible adsorption sites and the unit cell are listed in(a)and(b)and the minimum distance between atoms is also illustrated in(c).(d)The electronic band structure of sB/Gr obtained at the level of HSE06.
To illustrate the adsorption abilities of alkali-metal atoms(Li, Na, and K) on sB/Gr, the first essential step is to identify the favorable adsorption sites of Li, Na, and K atoms.Here,a 4×4 supercell was chosen and eleven possible adsorption positions both on the surfaces of borophene and graphene were taken into account according to the symmetry of sB/Gr as marked on Figs.1(a)and 1(b). All the structures of Li/Na/Kadsorbed sB/Gr with different adsorption sites were fully relaxed. The adsorption energy(Ead)for alkali-metal atoms adsorbed on the surfaces of sB/Gr was calculated by using the following formula:[42,43]
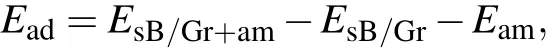
whereEsB/Gr+amis the total energy of the alkali-metaladsorbed sB/Gr,EsB/Gris the total energy of the pristine sB/Gr,andEamis the energy of an alkali-metal atom from its bulk crystal. According to the definition, a negative value ofEaminvolves an exothermic process and implies the adsorption of alkali-metal atom is energetically stable.
All the calculated results including the adsorption energy,the most stable adsorption site, and the height of the alkalimetal atom are listed in Table 1. Figures 2(a) and 2(e) show the optimized configurations with the most favorable adsorption positions on the sides of borophene and graphene,respectively. In general,all the three alkali-metal atoms(Li,Na,and K)prefer to the same adsorption sites neither on the surface of borophene or graphene. On borophene side,the Li/Na/K atom prefers to locate at site 1(S1),which is right above the boron atom in the grooves of borophene. On the graphene surface,the most stable adsorption site is site 9(S9),i.e.,the site right above the hollow of a six-membered carbon ring. The heights(H)of Li,Na,and K to the borophene/graphene surface were within the range of 1.594–2.528 ?A,which were slightly larger than the corresponding alkali-metal–B bond length. Both on the sides of borophene and graphene, they are in the law ofHLi<HNa<HK, which is consistent with the different atom radii of alkali-metal atoms and the same law also exists in the case of single-layer borophene.[24]The obtainedEadsare in the range from-1.388 eV to-1.805 eV,which indicates that the Li,Na,and K adsorptions on sB/Gr are all exothermic processes and energetically stable. It may not prevent the desorption of alkali-metal ions in the battery cycle. Thus,the sB/Gr is applicable for alkali-metal adsorptions with the relatively large adsorption energy and a proper adsorption height.

Table 1. The most stable adsorption sites,the adsorption energy(Ead),and the height(H)of adsorption atoms from the surfaces of borophene and graphene in sB/Gr.
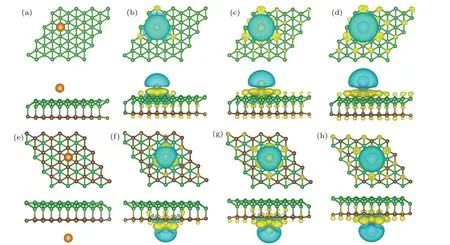
Fig.2. The most stable adsorption configurations and charge-density difference of Li,Na,and K adsorbed on sB/Gr. The isovalue is set to be 0.01 e/?A3. The blue and yellow regions represent the electron depletion and accumulation,respectively. (a)–(d)Correspond to the cases on the surface of borophene and(e)–(h)for the cases on graphene.
In order to gain deeper understanding of the alkali-metal adsorption behavior on the surface of sB/Gr, the charge redistributions were explored by plotting the charge density difference and carrying out the Bader charge analysis.[44]Figures 2(b)–2(d) and 2(f)–2(h) show the charge density differences for the most stable Li/Na/K adsorbed sB/Gr. Significant charge depletion was found around the alkali-metal atoms and near the borophene/graphene surface there was a large amount of charge accumulation. This further verified the high adsorption energies of alkali-metal atoms on sB/Gr, in which strong electrostatic interaction induced by the charge redistribution enhances the binding between alkali-metal atoms and borophene/graphene. Then we turn to the Bader charge analysis. It was found that during the adsorption process the alkalimetal atoms act as charge donors. When adsorbed on the surface of borophene,the Li,Na,and K atoms transferred 0.879e,0.876e,and 0.868e,respectively,and similar amount of charge transferred were found for the case of alkali-metal adsorbed on the surface of graphene,as listed in Table 1. All these results demonstrated that the Li,Na,and K atoms can be chemically adsorbed on the surface of borophene or graphene and they form chemical compounds, corresponding to the redox reaction in electrode materials.
Except for the surface of sB/Gr, we studied the adsorption of metal atoms in the cavities between borophene and graphene (Fig. S4). Firstly, when metal atoms are adsorbed in the cavity,the sB/Gr structure is greatly deformed,and even some B–B bonds are broken, which is not conducive to the cyclic stability of anode materials. In addition, the interlayer B–C bonds length of sB/Gr is only 1.713 ?A,and such a narrow space does not allow the diffusion of Li,Na,and K atoms.
In order to verify whether the most stable adsorption sites of multiple metal atoms will change when they are adsorbed on the sB/Gr surface at the same time, we studied the simultaneous adsorption of two and four metal atoms. When two metal atoms are adsorbed on the two nearest S9 sites at the same time,after optimization,the two Li atoms can be stably adsorbed on the S9 sites(Fig.S5(a)), while Na and K have a certain deviation, and the deviation of K is greater than that of Na (Figs. S5(b) and S5(c)). This is only due to the large atomic radius of Na and K, because from the charge-density difference(Figs.S5(d)–S5(i)),there is a lack of electrons between metal atoms, and the electrons gather between metal atoms and sB/Gr. After that,we studied four metal atoms uniformly adsorbed on the non-adjacent S9 sites on the sB/Gr surface.The optimized results are shown in Figs.S6(a)–S6(c).It was found that all the metal atoms could be stably adsorbed on the S9 sites,and the charge-density difference(Figs.S6(d)–S6(f))showed that there was still no interaction between metal atoms. In other words,when adsorbing multiple metal atoms,the most stable adsorption site of Na and K do have a certain deviation, but this is only due to the large atomic radius of metal atoms. When the number of adsorbed atoms on the upper and lower surfaces of sB/Gr can reach one layer,the metal atoms can still be stably adsorbed on S9 after optimization(Fig.S7).
In order to ensure that the metal atoms can be stably adsorbed at the S9 sites at high concentration (to avoid the deviation caused by the large atomic radius of Na and K),when there are many adsorbed metal atoms, the distance between the metal atoms is increased. The metal atoms are evenly adsorbed on the nonadjacent S9 sites,which improves the cycle stability of the battery to a certain extent.But at the same time,
this is also one of the reasons why the maximum concentration of Na and K stored by sB/Gr is smaller than that of Li.
3.2. Diffusion of alkali-metal atoms on sB/Gr
It is well known that the diffusion barrier of ions on the surface of electrode materials is the key factor affecting the charge and discharge rate. To investigate the diffusion properties of alkali-metal atoms(Li,Na,and K)on sB/Gr,we examined the optimal diffusion paths and computed the corresponding diffusion barriers based on NEB method.For sB/Gr,a total of four possible diffusion paths were considered by taking account of the most stable adsorption positions of alkali-metal atoms. The top views of the migration paths on the surfaces of borophene (denoted by P1, P2) and graphene (P3, P4) are shown schematically in Figs. 3(a) and 4(a). For P1/P3, the alkali metals directly diffuse between the two nearest moststable positions(S1/S9). The P2/P4 corresponds to the diffusion between the two nearest most-stable positions by passing a sub stable location.

Fig.3.(a)The top view of possible diffusion pathways for Li,Na and K on the surface of borophene and the calculated diffusion energy profiles along the two favorable paths for(b)Li,(c)Na,and(d)K on the surface of borophene in sB/Gr.
After calculations, the obtained energy barriers for Li,Na, and K both on the surfaces of borophene and graphene are summarized in Figs.3(b)–3(d)and 4(b)–4(d). Obviously,the optimal diffusion paths of Li, Na and K on the surface of borophene were P2, and the lowest diffusion barriers were calculated to be 0.39 eV, 0.19 eV and 0.09 eV, respectively. In the process of diffusion, metal atoms need to overcome the electrostatic potential on the surface of the electrode material.[45,46]The atomic radius gradually increases from Li to K,and their distance from the surface of the electrode material gradually increases. Therefore, the influence of sB/Gr surface electrostatic potential on K is the smallest,making the diffusion barrier of K the lowest. So the diffusion barriers decrease with the adsorbed metal change from Li to K atoms.

Fig. 4. The top view of possible diffusion pathways for Li, Na and K on the surface of graphene and the calculated diffusion energy profiles along the two favorable paths for(b)Li,(c)Na,and(d)K on the surface of graphene in sB/Gr.
Compared to the diffusion barriers of Li and Na on pristinepmmnborophene (10.53 meV and 2.55 meV),[24]the diffusion barriers slightly increased due to the formation of B–C bonds in sB/Gr, in which the borophene layer is reconstructed and the surface of borophene is not flat enough.However,it is still comparable toα-borophene/graphene heterostructure (0.353 eV for Li diffuse in the interlayer ofαborophene/graphene with 4.5 ?A interlayer distance)and much lower than many other 2D materials (0.46 eV for Li on the o-Mg2BC surface).[33,47]It was found that P4 has the lowest diffusion barrier on the surface of graphene, with the energy values of 0.18 eV for Li, 0.15 eV for Na, and 0.09 eV for K. These are equivalent to the diffusion barriers on pristine graphene monolayer(0.34 eV for Li,0.13 eV for Na,0.09 eV for K).[48]Next,we presented further discussions on the diffusion properties by roughly estimating the diffusion coefficient(D)according to the Arrhenius equation[49]
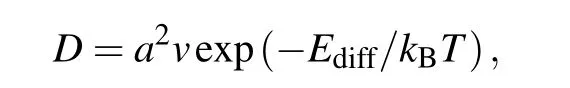
whereais the lattice constant of sB/Gr(diffusion distance of ions),v=1013Hz is the vibration frequency,Ediffis the diffusion barrier, andkBTis 0.026 eV atT=300 K. The obtained diffusion coefficients, as well as the diffusion barriers,are summarized in Table 2. On the whole, Li has the lowest diffusion coefficient among the three alkali-metal atoms,then is Na, and K has the largest one. The maximum absolute diffusion coefficient was~2.3×10-4cm2/s for K on both sides of sB/Gr, which is~107times higher than that of Li on graphene (~2×10-11cm2/s). Even for the lowest diffusion coefficient,~3×10-10cm2/s for Li on the borophene side, it was still higher than that of Li on the surface of graphite (~10-11cm2/s),[50]and considerable with other previous studies.[47]In addition to relatively low diffusion barriers,the ultrahigh ion mobilities of Li,Na,and K will provide more evidence for sB/Gr as potential anode materials for AMIBs.
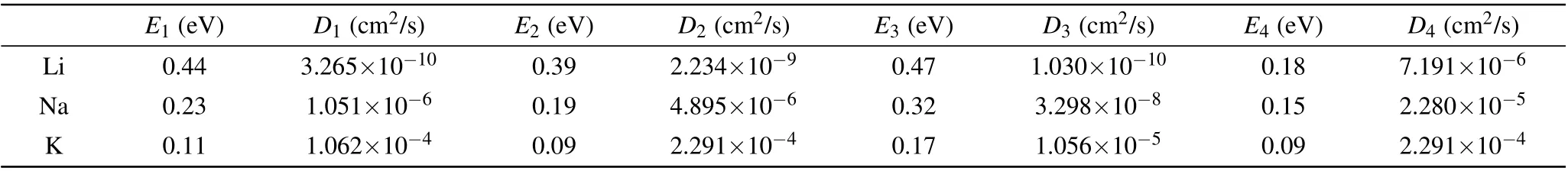
Table 2. The diffusion energy barrier(Ex)and diffusion coefficient(Dx)of Li,Na,and K along the four possible paths Px (x=1,2,3,4)on the surfaces of sB/Gr.
3.3. Storage capacity and open circuit voltage
Storage capacity is another key parameter to estimate the performance for electrode materials in AMIBs. Here,we employed the 4×4 supercell to evaluate the adsorption capacity of sB/Gr as the number of the adsorbed Li/Na/K atom increases. Generally, the average adsorption energies of alkalimetal atoms decreased with the increasing of adsorption concentration, as shown in Fig. 5. When the second layer of alkali-metal atoms adsorbed, the average adsorption energies remain negative. Further increasing the concentration,the adsorption energy will become positive for all possible adsorptions. It implies that additional adsorptions more than two layers are energetically unfavorable. Therefore, at most two layers of Li,Na,and K atoms can be adsorbed on each side of a 4×4 sB/Gr supercell,with the corresponding chemical formulas of Li2CB1.5,Na1.56CB1.5,and K0.5CB1.5,respectively.
Combined with the above results, the maximum theoretical specific capacity(C)was calculated according to the following equation:[45]
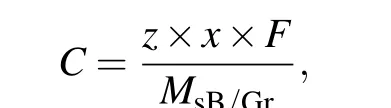
wherezrepresents the valence electron number of the metal atom (z= 1 for Li, Na, and K),xrepresents the maximum adsorption concentration,Fis the Faraday constant (26801 mA·h/mol), andMsB/Gris the relative atomic mass of sB/Gr in g/mol. Accordingly, the calculated specific capacities for Li-, Na- and K-adsorbed sB/Gr systems were 1880.7 mA·h/g, 1468.9 mA·h/g, and 470.2 mA·h/g,respectively. It is higher than the Li storage capacity ofα-borophene/graphene heterostructure.[33]And these results are much higher than many other 2D heterostructures,such as arsenene/graphene heterostructure(409.9 mA·h/g),[45]ZrS2/graphene heterostructure(380 mA·h/g).[51]

Fig. 5. The average adsorption energy as a function of the number of adatom for alkali-metal atoms adsorbed on sB/Gr. The inserted are some typical adsorption configurations.
The most important aspect to understand the performance of electrode materials is to obtain the voltage distribution.The open circuit voltage (OCV) was calculated at different concentrations[52]
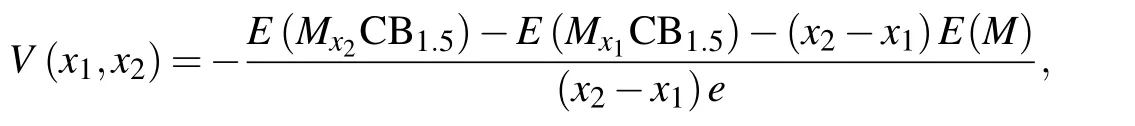
whereE(Mx2CB1.5) andE(Mx1CB1.5) represent the total energies of the systems with the adsorption concentrations ofx2andx1,respectively.E(M)is the cohesive energy per atom of the alkali-metal atoms in their bulk phase. Figure 6 displays the voltage profiles as a function of the concentration (x) for Li,Na and K-intercalated sB/Gr. With the increase of the concentration,the OCV showed a decreasing trend. The obtained average OCVs of sB/Gr were 0.81 V, 0.62 V and 0.67 V for Li, Na, and K, respectively. All these results are within the acceptable voltage range of 0.0–1.0 V for commercial anode materials of AMIBs.[43]The positive values of OCVs indicated that alkali metals tend to maintain ions rather than form clusters on sB/Gr surface, which ensures that metal ions can participate in electrode reactions.[53]Usually,the safety of the battery significantly depends on the stability of the electrode materials. Thus,the volume expansion upon the adsorption of a large number of metal atoms would play an important role in the actual use of the electrode materials. Here, the lattice change rates at the maximum adsorption concentration were calculated by(l-l0)/l0and the results were only 2.2%,2.9%,and 0.5%for Li, Na, and K-adsorbed sB/Gr systems, respectively. They were far less than the lattice change rate of some previously reported graphene based heterostructures, such as arsenene/graphene as MIBs (15.4%).[45]This shows that the sB/Gr has good stability as anode materials of AMIBs.
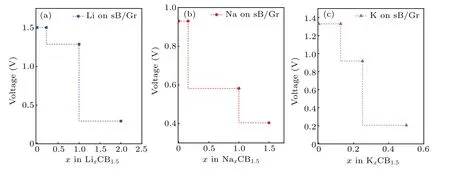
Fig.6. The voltage profiles for Li,Na,and K adsorption on sB/Gr with energetically stable concentrations.
4. Conclusion and perspectives
In conclusion,we systematically studied the properties of sB/Gr as anode materials for alkali-metal ion batteries by using the first principles calculations. The results showed that all the three alkali-metal atoms, Li, Na, and K, can strongly adsorb on both surfaces of sB/Gr and the adsorptions were all exothermic processes. At the same time,the three alkali-metal atoms had relatively low diffusion barriers and ultrahigh ion mobilities providing more evidence for sB/Gr as potential anode materials for AMIBs. The maximum storage capacities of sB/Gr in LIBs and NIBs were extremely high with the theoretical values of 1880 mA·h/g and 1648 mA·h/g,which are several times higher than some other typical 2D-heterostructure based electrode materials. In addition, the lattice change rate of sB/Gr was very small (<2.9%) in the process of alkalimetal atoms adsorptions. All these results suggested that the sB/Gr can meet all the necessary requirements for a good electrode material. Thus,the sB/Gr was a highly promising anode material for the rational design of highly performance AMIBs.
Acknowledgements
Project supported by the National Natural Science Foundation of China (Grant No. 12174084), the Scientific and Technological Research Foundation of Hebei Province,China(Grant No. ZD2021065), and the Key Program of Natural Science Foundation of Hebei Province, China (Grant No.A2021205024).
- Chinese Physics B的其它文章
- Microwave absorption properties regulation and bandwidth formula of oriented Y2Fe17N3-δ@SiO2/PU composite synthesized by reduction–diffusion method
- Amplitude modulation excitation for cancellous bone evaluation using a portable ultrasonic backscatter instrumentation
- Laser-modified luminescence for optical data storage
- Electron delocalization enhances the thermoelectric performance of misfit layer compound(Sn1-xBixS)1.2(TiS2)2
- TiO2/SnO2 electron transport double layers with ultrathin SnO2 for efficient planar perovskite solar cells
- Sputtered SnO2 as an interlayer for efficient semitransparent perovskite solar cells

