Novel aluminum-based fuel: Facile preparation to improve thermal reactions
Fa-yang Guan,Hui Ren,Wan-jun Zhao,Xin-zhou Wu,Qing-jie Jiao
State Key Laboratory of Explosion Science and Technology,Beijing Institute of Technology,Beijing,100081,China
Keywords:Aluminum-based fuel Heterogeneous nucleation Molecular dynamics simulation Thermal reaction High energy laser-induced shockwave experiment
ABSTRACT To improve the thermal properties of aluminum (Al) in the energetic system,a coated structure with ammonium perchlorate (AP) was prepared by a facile approach.And N,N-Dimethylformamide (DMF)was chosen as an ideal solvent based on heterogeneous nucleation theory and molecular dynamics simulation.This coated structure could enlarge the contact area and improve the reaction environment to enhance the thermal properties.The addition of AP could accelerate oxidation temperature of Al with around 17.5 °C.And the heat release of 85@15 composition rises to 26.13 kJ/g and the reaction degree is 97.6% with higher peak pressure (254.6 kPa) and rise rate (1.397 MPa/s).An ideal ratio with 15 wt% AP was probed primarily.The high energy laser-induced shockwave experiment was utilized to simulate the reaction behavior in hot field.And the larger activated mixture of coated powder could release more energy to promote the growth of shockwave with higher speed up to 518.7 ± 55.9 m/s.In conclusion,85@15 composition is expected to be applied in energetic system as a novel metal fuel.
1.Introduction
Due to the high enthalpy up to 30.8 kJ/g or 83.3 kJ/cm[1,2],aluminum(Al)is extensively utilized as the fuel in thermites[3,4],explosives [5,6],propellants [7,8],and other energetic systems.However,Al cannot react completely and even acts as the inert substance during the second-oxidation process [9].Owing to the complexity of Al combustion in energetic surroundings,various factors would affect the heat release,including the particle size[10],agglomeration [11,12],and alumina shell [13,14].With the decrease of particle size,specific surface area rises gradually,enhancing the mass transformation and heat diffusion between fuels and oxidizer.The reaction degree and combustion rate would be improved accompanied with decreased ignition temperature.Although Al nanoparticles behave better in combustion than microscale particles[15],their active content,storage stability and particles agglomeration prohibit the application in engineering.Therefore,it is necessary to promote the reaction degree of ultrafine micron-sized Al particles.And many approaches have been attempted to improve the combustion performance.
From the perspective of oxidation reaction,it is necessary to weaken the obstacle effect of the alumina layer.Incorporating Al with other metals [16] and polymers is a feasible way to achieve this aim.The mass gain in main oxidation process of Al-Zn alloy prepared by centrifugal atomization process [17] increases greatly from 26.51%to 44.82%than pure Al.And fluorine-polymer could be applied to improve the combustion behavior,with is beneficial from the pre-ignition reaction [18,19].These additives react with the alumina shell to accelerate the oxidation of aluminum.However,as inert substance,compared with Al,added in the energetic system,high proportion of these additives(usually up to 10%~30%)would lower the ratio of fuel or oxygen balance in the formula,and the compatibility with high-energy materials remains to be solved.Another approach is to ameliorate reaction environment.From the investigations on the reaction mechanism of aluminum in combustion[20-24],the state of the particles in the flow field influence the reaction degree significantly.In solid propellant,agglomeration of Al would affect the heat diffusion and make the loss of two-phase flow up to 10%[25].Hence,an ideal Al-based fuel with high reaction degree ought to take these two aspects into account.
In this paper,a coated structure of Al with ammonium perchlorate(AP),commonly utilized in energetic formula with fine compatibility,has been prepared by heterogeneous recrystallization to realize the aims mentioned above.And molecular dynamics (MD) simulation was applied to select an ideal solvent.The morphology and structure have been characterized to determine the dispersion and contact state.The pressure changes with time and heat release have been measured and compared with physically mixed composition.Meanwhile,an ideal ratio of AP has been obtained preliminarily from combustion properties.High energy laser-induced shockwave experiment has been utilized to simulate the reaction condition in energetic system.And reaction mechanism has been proposed based on the combustion performance and ignition behavior characterization.
2.Theory and calculation
2.1.Heterogeneous nucleation theory
Solvent has an essential influence on the nucleation and growth of crystals.Classical nucleation theory[26-28]demonstrates that a stable crystal nucleus is formed from atom clusters with similar construction,named the critical crystal.And the rate of nucleation,,could be expressed in the form of Arrhenius reaction velocity.The size of critical crystal could be obtained through the maximum value of the free energy.The expression shows below:
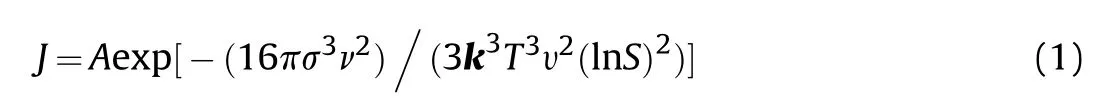
whereis the pre-exponential factor.σ,ν,,υ andindicate the interfacial energy,crystal volume,temperature,ionization number and degree of supersaturation.k is the Boltzmann constant.
In heterogeneous nucleation,due to the presence of solid particles in the liquid,crystals will adhere to the solid surface for nucleation.Although the radius of the critical nucleus is similar as that of the homogeneous system.Its volume will be reduced,lowering the nucleation work (Eq.(2) [28]) and increasing the heterogeneous nucleation rate (Eq.(3)).
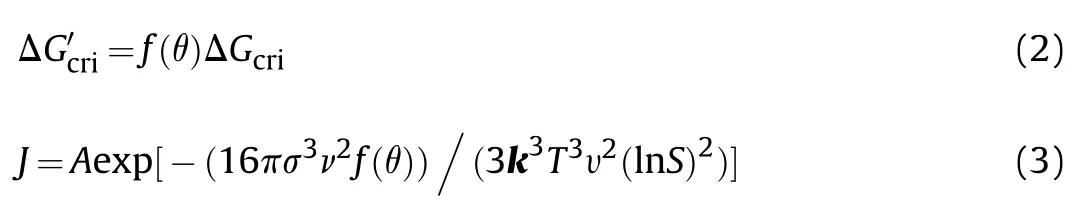
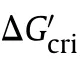
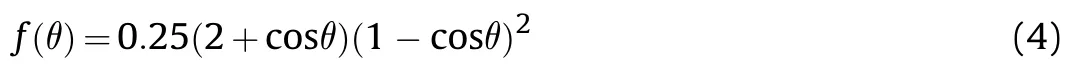
The contact angle,θ,is at the boundaries between two solids and one liquid,hard to be obtained from experiments directly.But it could be calculated from interfacial tension balance condition based on Eq.(5).And σ,σ,and σare interfacial energy respectively(see Fig.1).

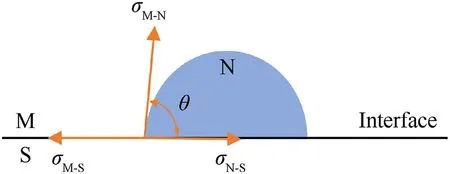
Fig.1.Interface diagram of heterogeneous nucleation.
To select suitable solvents of AP,some hypothesis has been taken: the size of nucleation is the same in various solvents.And the liquid is regarded as critical saturated solution.Thus,the interfacial energy between liquid and AP crystal,contact angle parameter and ionization number per mole would decide the nucleation rate.The solvent chosen parameter,ε,is defined as below and it has negative relationship with the rate of nucleation.

2.2.MD simulation
Interfacial condition and related properties between components could be obtained from MD simulation.Various solvents including water,methanol,ethylene glycol (EG),acetone,N,NDimethylformamide (DMF) and N,N-Dimethylacetamide (DMAC)was considered.The surface structure of γ-AlOis very similar to that of amorphous alumina [30],which is covered over the Al at room temperature with round 3-10 nm thickness.And investigation[31]indicates γ-AlO(110)surface are energetically the most stable.Meanwhile,from the morphology of AP (Fig.2(a) and Table S1),AP (101) surface with over 60% ratio in area has the greatest impact on the properties.Therefore,the interfacial energy between solutions and AP(101)surface,solutions and γ-AlO(110)surface as well as AP (101) surface and γ-AlO(110) surface was considered.Super cells of AP(101)with 5×9×1 and γ-AlO(110)with 9 × 5 × 1 was build to match the cell parameters and amorphous cell of solutions with 400 solvent molecules has a matched interface size(48×47 ?).To avoid the effect of periodic boundary,a vacuum layer with 15 ? was added.The constructed system,taking DMF solution as an example,is shown in Fig.2.
DREDING molecular force field can describe numbers of organic compounds,biological molecules and all inorganic molecules[32].Especially for energetic system [33],it presents wide applicability and good simulation results.γ-AlOand AP cell were calculated by DREDING force field.It is found that the errors of structure are within acceptable range.Therefore,the whole molecular dynamics work was carried out under DREDING force field.The simulations were implemented by Forcite module (Materials Studio from Accelary Inc.).Optimizations including geometry optimization and annealing process(heating from 300 K to 500 K and then cooling to 300 K with 5 cycles at ramps of 5 K) with fine calculation quality were implemented initially.Then A fixed time step of 1 fs was used in all cases and the whole runs of 1 ns duration were performed in the NVT system.The most stable configuration would be obtained from the last 100 ps,and equation of interfacial energy shows below.
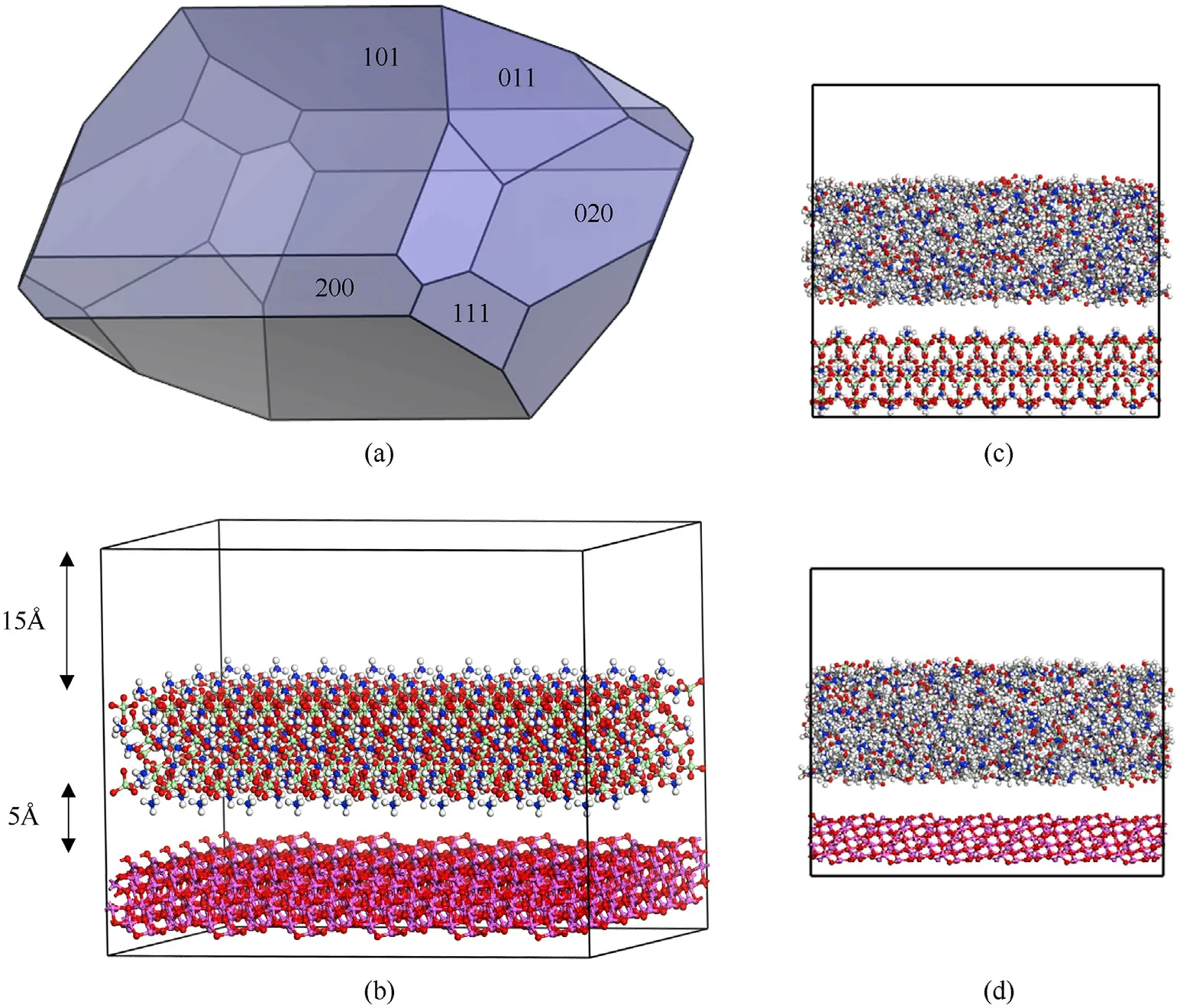
Fig.2.(a)Morphology of AP;(b)configurations AP(101)surface and γ-Al2O3(110)surface;(c)configurations AP(101) surface and DMF solution;(d)configurations γ-Al2O3(110)surface and DMF solution.

σis the interaction energy,σis the total energy of the compound configuration,σis the energy of removing B to get A in the compound configuration,σis the energy of removing A to get B in the compound configuration,andis the interface area.
3.Materials and methods
3.1.Sample preparation
The Al powders withof 1.3 μm (Purity 99%,Angang Group Aluminum Powder Co.,Ltd.,Anshan,China) and AP powders with the average size of 150 μm (AR,Aladdin Industrial Co.,Shanghai,China) were utilized in this work.After being ultrasonicated in ethanol(AR,Aladdin Industrial Co.,Shanghai,China)for 30 min to remove the impurities,Al powders were added into the AP solution(1.46 g/10 ml solvent) in N,N-Dimethylformamide (DMF,AR,Aladdin Industrial Co.,Shanghai,China) with various mass ratio of Al: AP (75:25,80:20,85:15,90:10 and 95:05).The dispersion was ultrasonicated for 2 h and then evaporated under stirring at 75C for 6 h.Finally,the composition was obtained after granulating and drying (60C,24 h).The preparation process is shown in Fig.3.
The contrast was prepared by physically mixing that Al and AP were added into the n-hexane(AR,Aladdin Industrial Co.,Shanghai,China)with certain mass ratio.The mixture was ultrasonicated for 2 h and then dried at 60C for 2 h.To characterize the physically mixed composition and coated composition,we define “@” as coated composition and “/” as mixed composition.The numbers before and after the symbol present the mass ratio of Al and AP,respectively (e.g.Al/AP presents the physical mixture;85@15 means the coated composition and the ratio of Al: AP is 85:15).
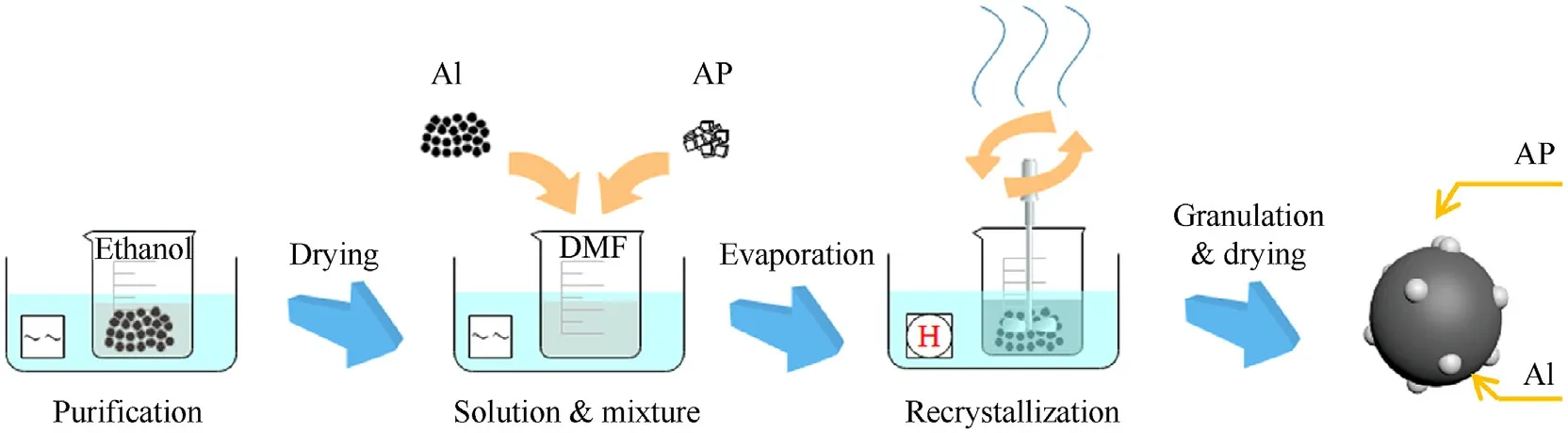
Fig.3.Preparation process of Al@AP composition.
3.2.Characterization
Scanning electron microscope,transmission electron microscopy and energy dispersive spectrometer(SEM,TEM&EDS)were performed by S-4800 (HITACHI,Japan) and Tecnai G2 F30 S-TWIN(FEI,USA)to characterize the morphology and element distribution of the samples.Infrared spectra were collected on Fourier transform infrared spectrometer (FTIR,VERTEX70,BRUKER,Germany)for characteristic peaks of AP.And the dispersion of specific groups on a single particle could be observed in nano-IR analysis (Ansys nanoIR2,BRUKER,Germany).In this instrument,a tapping-mode atomic force microscope (AFM) tip scanned the sample surface,and the IR signal of AP could be obtained simultaneously in nanometer scale.Even weak molecular vibrational resonances[34]could be probed.X-ray photoelectron spectroscope(XPS,PHI 5300,Al Kα,150 W,PerkinElmer Inc.USA)and X-ray diffraction(XRD,D8 Advance,Cu Kα,λ =1.5406 ?,BRUKER,Germany) were used to detect the reaction situation between Al and AP.The content of AP in the samples was converted from the mass percentage of nitrogen element by element analyzer (EA,vario El cube,Elementar,Germany).Only 85@15 sample was characterized by SEM,TEM,FTIR,nano-IR,XPS and XRD,and EA analyzed various ratios.
To investigate the thermal decomposition of pure aluminum,85@15 sample and 85/15 composition,differential scanning calorimetric (DSC) and thermogravimetry (TG) were carried out with NETZSCH STA 449(BRUKER,Germany)at heating rate of 20C/min from room temperature to 1200C under air condition.
3.3.Combustion and ignition experiments
Combustion of samples with different ratios were processed in an oxygen bomb calorimeter.Approximately 0.3 g of the sample was put into the calorimeter inflated with oxygen under 3 MPa,and the heat release was measured.The calculation method of reaction degree was listed in Supplementary Materials.The pressure changes with time()of samples were measured via pressure cell.0.3 g samples were weighed out and placed into the cell in a constant volume of 330 cm.The pressure change was recorded by sensor after the samples were ignited by electric wire.
The combustion behavior of Al,85@15 sample and 85/15 sample were observed in a combustion cell.Around 3.5 g powders were pressed into a 5 mm× 5 mm ×100 mm sample,and then ignited via a COlaser with power of 60 W irradiating for 200 ms.A highspeed camera (Optronis,Germany) was used to capture the photography images of combustion performance at 1000 fps.
The Laser-induced air shock on energetic materials experiment is a reliable measurement to simulate reaction condition of energetic materials [35-37].A high-energy laser pulse (700 mJ,1064 nm,SL1000,InnoLas,Germany) was focused the sample surface(diameter of 20 mm and height of around 2 mm)of the Al,85@15 and 85/15 powders respectively.The process of ignition and shockwave was captured by high-speed camera (SIMD8,Specialised Imaging Ltd.,England).
The structures of these equipments for combustion and ignition experiments are shown in Fig.S2~S4.
4.Results and discussion
4.1.Calculation analysis
The data of interaction energies and related parameters shows in Table 1.And the value of σis 0.733 kcal/mol?.Due to the value of(σ-σ)/σin aqueous system in-1.596,over the data range,heterogeneous crystallization might be hard to accomplish.The solvents with little solubility of AP behave badly in nucleation rate like methanol and acetone.And the σof EG is higher than σ,not conducive to the stable adsorption of AP on the γ-AlOsurface.Based on the calculation results,DMAC and DMF are expected to perform well as solvents in this system and DMF was chosen ultimately for its smaller value in ε.
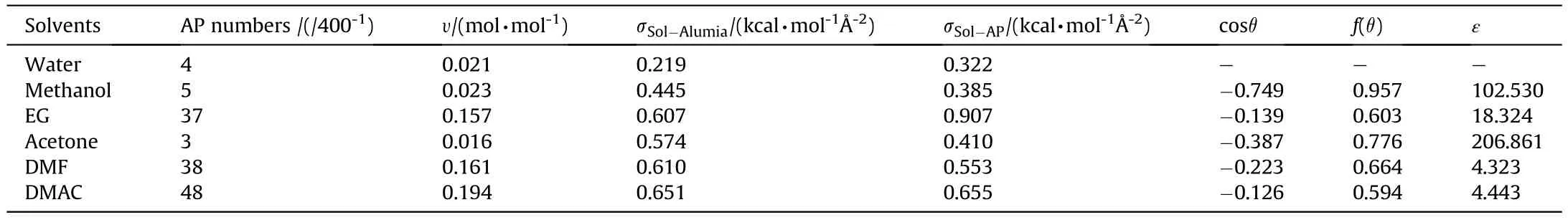
Table 1 Interaction energies and related parameters of various solvents.
4.2.Morphological and structure analysis
In SEM image from Fig.4(a)of single coated particle,tiny crystal could be found adhering on the surface of Al.And the morphology shows clearly from the TEM image in Fig.4(c) and (d).The distribution of oxygen element at the edge of the particle appears stratification obviously in Fig.4(b).Combining with the distribution of other elements,bright green band is the alumina layer with the thickness of around 4.2 nm.Outside the alumina,the irregular shape layer is the AP layer,and it distributes evenly around the particle from the EDS image of Cl element.
To further obtain the distribution of AP around the Al particle,nano-IR was used to characterize the intensity of specific wavelength throughout the mid-infrared fingerprint region at nanometer scales.The infrared single at a point of the particle shows the typical absorption bands of ammonium are 1634 cmand 1420 cm.The peaks of 1116 cmand 936 cmattributes to the stretching of perchlorate.Compared to the characteristic peaks of ammonium (1659 cmand 1415 cm) and perchlorate(1165 cmand 972 cm) from conventional FTIR curve,theresults vary slightly due to the shifts for vibrational resonances of the tip-sample coupling [38,39].Fig.5(a) shows the nano-IR scan taken on the top surface of the 85@15 microsphere at the frequency of 1116 cm,indicating strong intensity of AP in some areas of the surface.However,the distribution is uneven,which suggests the Al particle is not completely covered by AP.
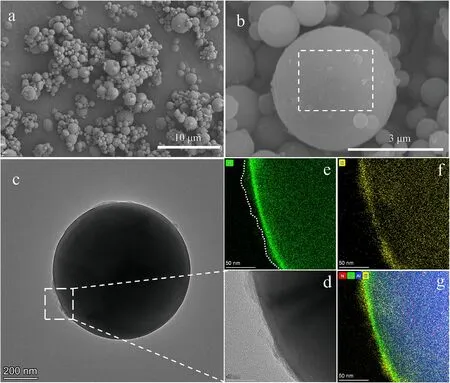
Fig.4.(a)SEM images of 85@15 particles;(b)SEM images of single 85@15 particle with high resolution;(c)TEM images of single 85@15 particle;(d)High resolution image of the edge;(e) EDS mapping of oxygen element;(f) EDS mapping of chlorine element;(g) EDS mapping of oxygen (green),nitrogen (red),aluminum (blue) and chlorine (yellow)elements.
The Al 2p XPS spectra of 85@15 sample is shown in Fig.5(d).Only two characteristic peaks of aluminum element assigned at 72.5 eV and 74.8 eV representing alumina and aluminum.From the XRD patterns of 85@15 sample in Fig.5(e),no new substance has generated in the compositions except Al and AP.Thus,AP is physical absorption on Al without any chemical reaction in the preparation.In other words,Al@AP composition might behave fine stability in the energetic system.From the data of EA in Table S3,the content of AP in the composition is similar with the feeding ratio of raw materials.
In summary,this facile preparation makes AP distribute evenly around the Al sphere as irregular crystal with superfine size.Simple physical absorption does not change the properties like stability,but it would increase the contact area greatly.
4.3.Thermal analysis
To compare the effects of structures on the thermal properties at slow heating rate,the thermal decomposition processes were considered.The whole decomposition of coated composition could be divided into 5 stages from Fig.6(a): the transformation of AP crystal.the decomposition of AP,the first exothermic reaction of Al,the melting of Al,and the second exothermic reaction of Al.The first stage is the transformation of AP crystal at around 245C.And then the AP begin to decompose with 14.7%mass loss.Stage III-Ⅴare the oxidation of Al powders.In the first oxidation of Al at 605.8C,the composition gains 3.3% mass growth and then the alumina shell hinders the reaction.When the temperature up to 663.6C,aluminum core begins to melt with a volume expansion [40].Meanwhile,the crystal form of alumina shell begins transforming from amorphous to γ-AlO,and the change of density induces the shell cracks [41-43].Then,the melting aluminum core releases more heat in the second exothermic stage with 52.1%weight gain.
Compared to the oxidation of 85/15 mixture and pure Al,the coated structure behaves better in the Stage II and Stage III.Due to the reduction of AP size,the first decomposition steps of AP became weak.Intermediate NHand HClOwould cover the surface of AP quickly and the dissociation and sublimation would begin directly[44-46].Large contact area accelerates the diffusion of oxygen decomposed from HClOto Al surface.Thus,the decomposition peak of 85@15 is 2.1C earlier than that of 85/15 mixture.In the first oxidation of Al powders,the heat transformation from the decomposition of AP accelerate heat accumulation of Al.And the reaction of 85/15 mixture is earlier than Al about 14.2C.Moreover,coated structure further advances the temperature benefiting from larger contact area.
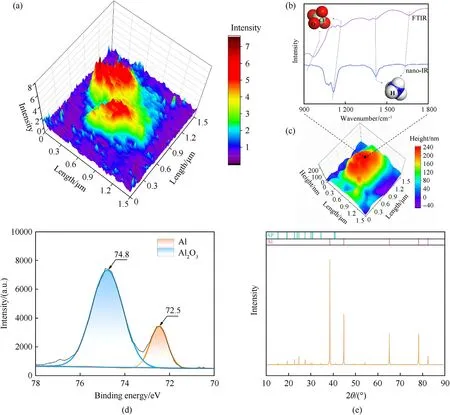
Fig.5.(a)Nano-IR intensity image of the microsphere at frequency of 1116 cm-1;(b)FTIR spectra and nano-IR absorption spectrum of 85@15 composition;(c)Topography of Al@AP particle.(d) Al 2p XPS spectra of 85@15 composition;(e) XRD spectra of 85@15 composition.
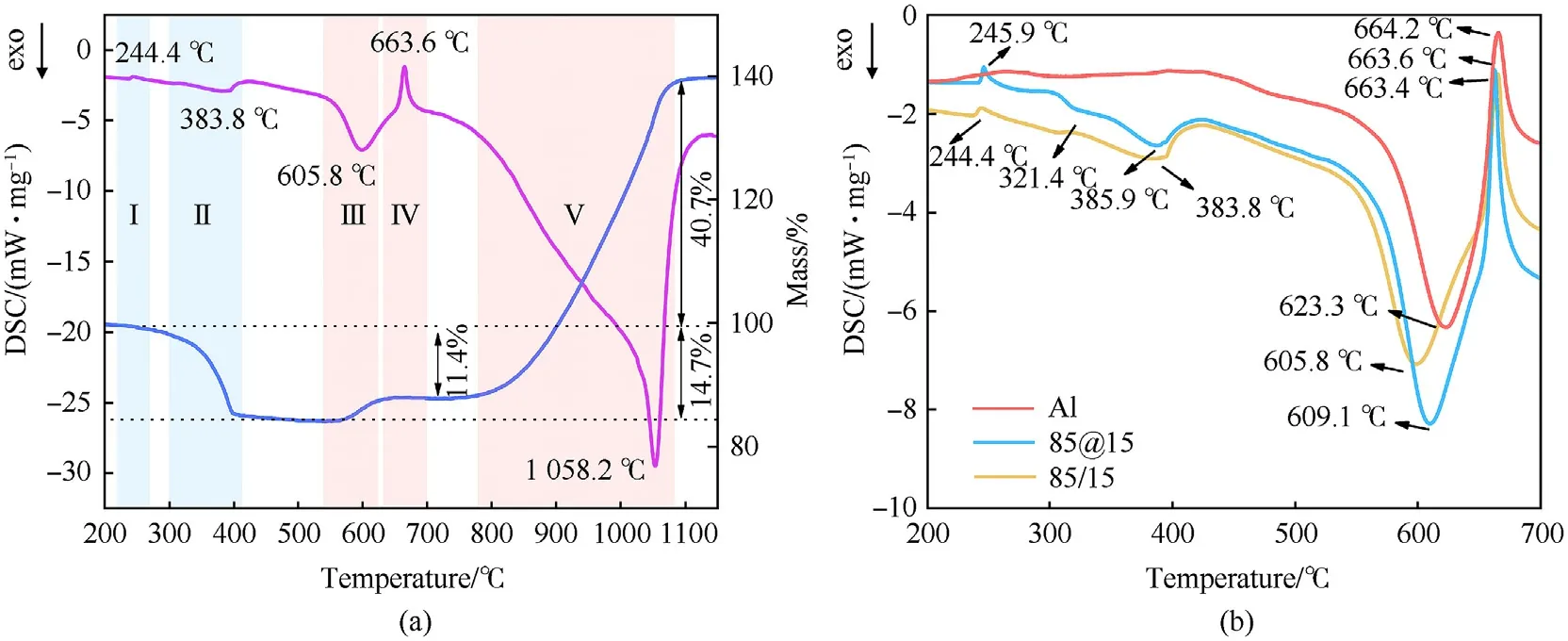
Fig.6.(a) TG-DSC curves of 85@15 powder;(b) DSC curves of Al,85/15 and 85@15 component.
In summary,due to the small size of AP crystal as well as the large contact area between AP and Al,the structure of Al@AP composition could decrease the decomposition temperature of AP and make the first exothermic process of Al in advance.
4.4.Combustion performance
The pressure change with time of samples could reflect the energy respond and work capacity under heat simulation.As shown in Table 2,higher peak pressure could be achieved with higher content of AP.And Al@AP shows higher pressure peak than that of Al/AP with the same ratio.Average boost speed behaves the same tendency.The compositions with 5% AP cannot be ignited under atmospheric air.In various ratios,the content of AP,decomposing to generate gases,attributes to the pressure growth dominantly.Besides,Al@AP composition would release more heat to expand the air compared to the Al/AP mixture with same AP ratio.Similar results could also be found from the data of heat release.
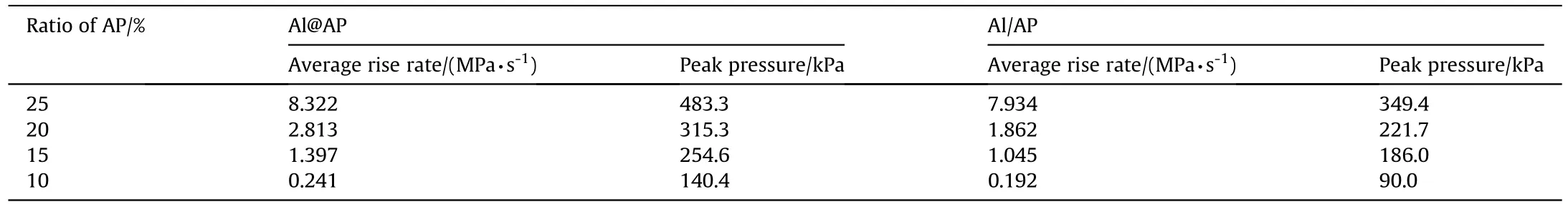
Table 2 Peak pressure and average boost speed of Al@AP and Al/AP compositions with various mass ratios.
As shown in Fig.7,with the increase of Al ratio growth,the heat release increases under oxygen saturation.And Al@AP composition release more heat than Al/AP composition with the same ratio.Moreover,the values of 85@15,90@10 and 95@05 composition are higher than the heat released from pure Al,suggesting Al@AP powder could increase the energy release and even behave better than traditional aluminum fuel.However,the increase is not obvious at the ratio of 75:25,and the exotherm is much lower than pure Al.It indicates AP only acts additives in the composition,existing an optimized ratio.We calculated the reaction degree of Al based on the heat release.The result shows that the addition of AP could increase the degree of oxidation,and the improvement relates to the content of AP.In agreement with the results of heat release and peak pressure,Al@AP composition performs fully oxidation reaction.Importantly,when the ratio of AP is up to 15%,the degree of oxidation stabilizes to the highest level,around 97.6%.
To probe the mechanism of Al@AP structure,combustion performance could be observed from Fig.8.Pure Al burns slowly with little spark.And 85@15 composition burns violently with visibly more intensive flame than 85/15 mixture at the same timing point of combustion process.However,the sparks from 85/15 composition are brighter with larger size.The dominate component of the sparks are melting aluminum,indication 85/15 composition burns insufficiently.The combustion speed of Al,85/15 and 85@15 are 0.047 m/s,0.132 m/s and 0.211 m/s respectively.The addition of AP could increase the burning rate and the coated structure performs better.The mechanism would be discussed below.
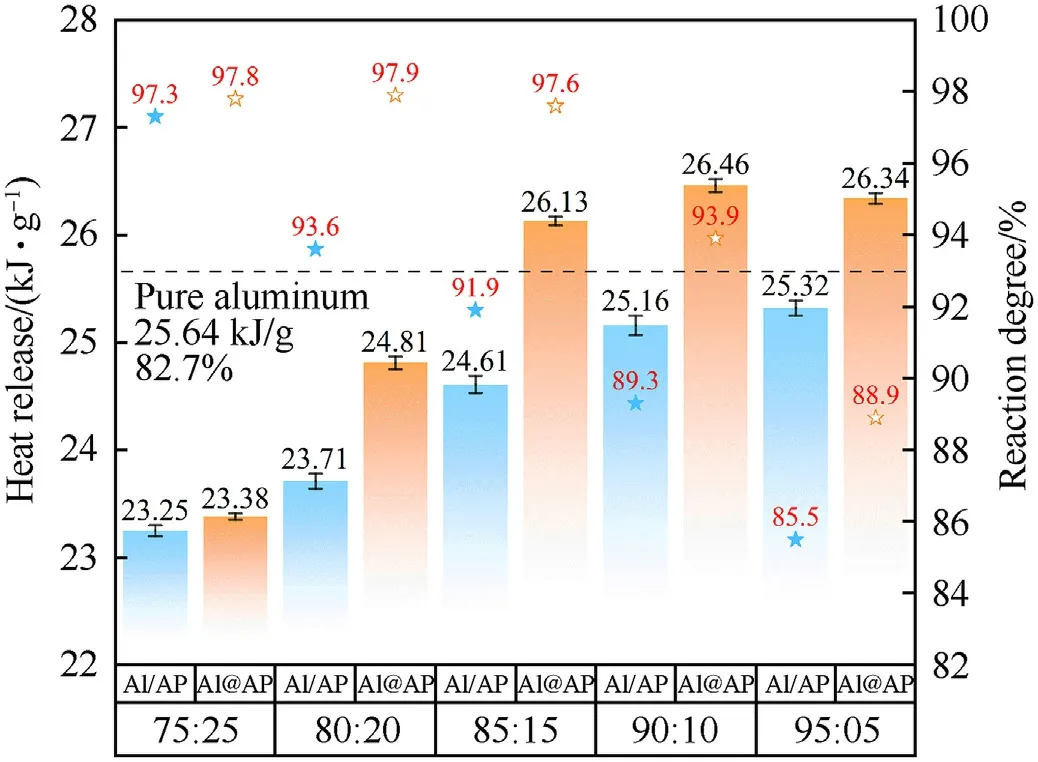
Fig.7.Heat release and reaction rate of Al@AP and Al/AP compositions with various ratios.
4.5.High energy laser induced shockwave
To simulate the reaction environment of energetic system with ultra-high temperature and activation within nanoseconds,high energy laser pulse was utilized to induce the shockwave.And the snapshots are shown in Fig.10.Based on the researches about the laser-induced shock wave on materials[47,48],the shock structure induced by plasma is target materials,ionized/shocked air,shocked air,and shocked front from inside to outside.When the compositions ignited by laser pulse,target materials were activated primarily.And then materials diffusion and shockwave begin to grow.As the reaction process undergoing,shockwave behaves faster and the front could be observed clearly after the time delay of 1.5 μs?In the delay of 2.55 μs,the shockwave separates from the activated materials because of the energy shortage from the reaction.Additionally,both substances wave and shockwave of 85@15 composition spread faster than that of 85/15.And pure Al has smallest active area and shockwave size.From the interval time and the growth of shock front,the average propagation velocity could be obtained that the value of Al is 477.9 ± 55.4 m/s,85/15 mixture is 490.4±52.5 m/s and 85@15 composition is 518.7±55.9 m/s.Rapid substances diffusion demonstrates that the products including the melting Al particle and decomposition gases of 85@15 composition disperses quickly and form a lager reaction area simulated by plasma.More energy released from the oxidation would promote the growth of shockwave with higher speed.Therefore,85@15 composition might have an expected behavior added in energetic system.
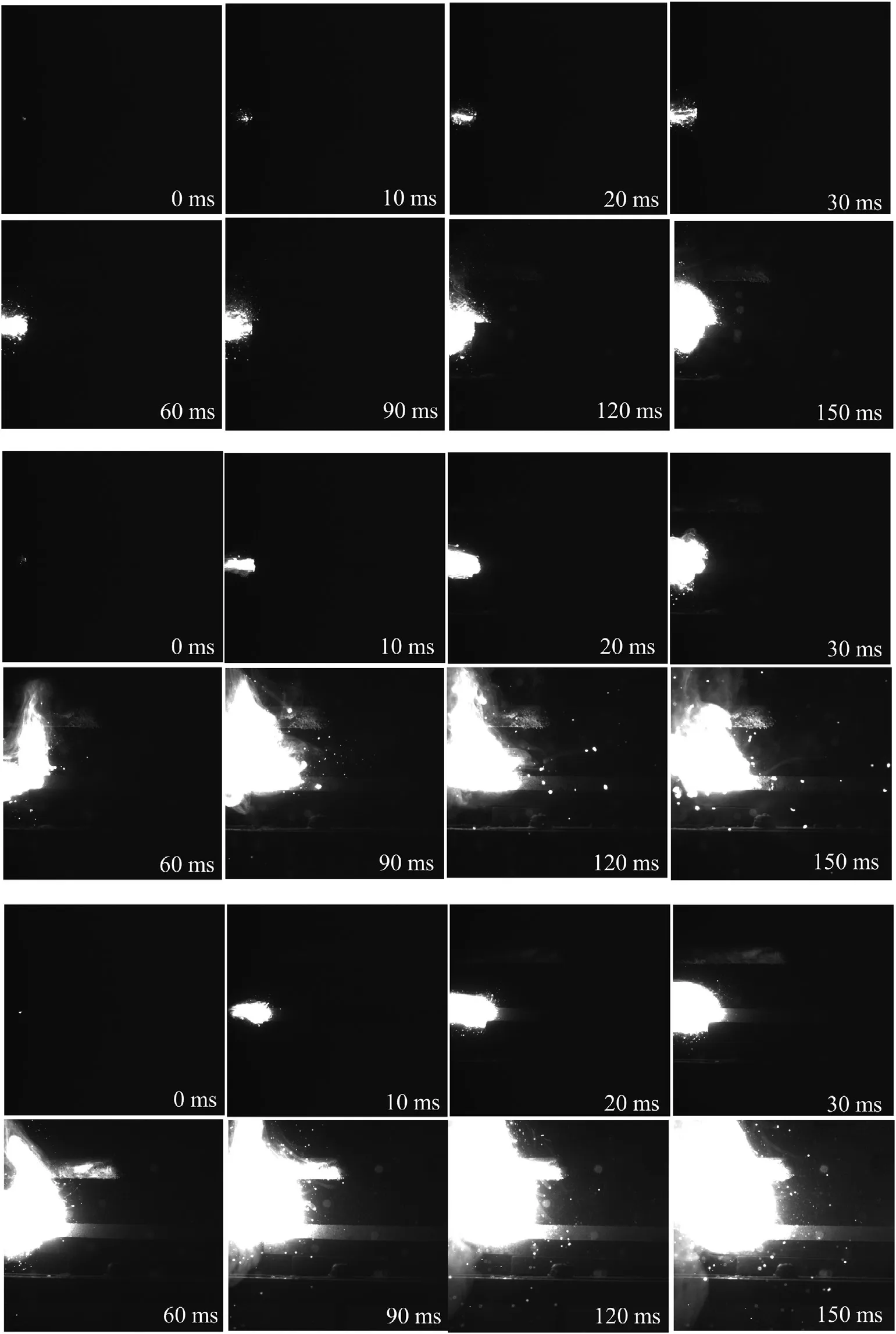
Fig.8.Combustion performance of Al (up),85/15 (middle) and 85@15 (down).
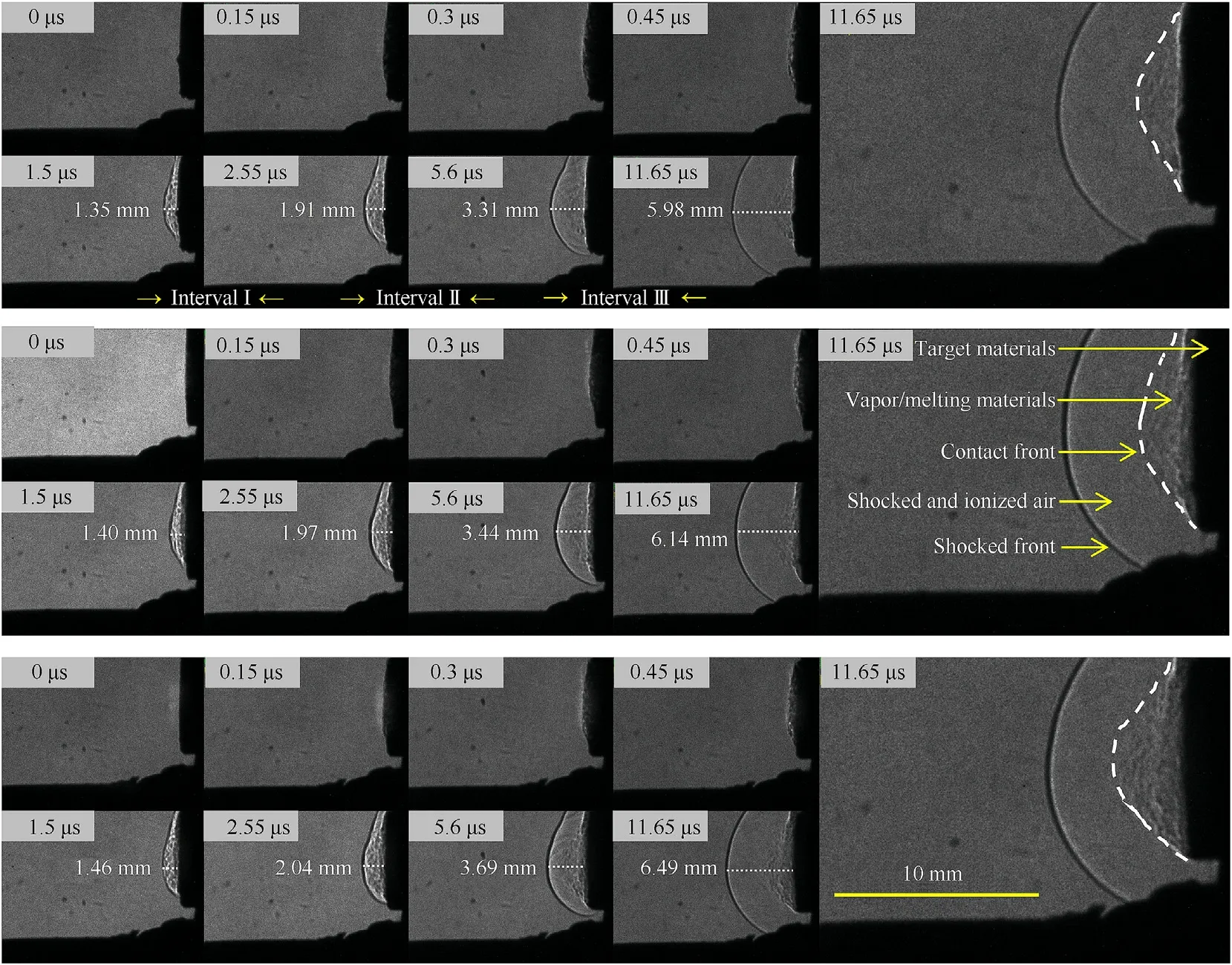
Fig.9.Ignition behaviors of Al (up),85/15 (middle) and 85@15 (down) composition under high energy laser pulse.
4.6.Reaction mechanism analysis
It is various reaction mechanism that lead to difference in combustion properties (see Fig.9).In pure Al system,the enlargement of reaction area is mainly owning to the thermal expansion of gases.Heat is conducted between particles with a long-time accumulation.Therefore,the combustion is modest relatively with low burning rate.
When AP is added into the system,the gases decomposed from AP expands the hot field greatly.And the heat from the decomposition of AP accelerate the heat accumulation of Al.Moreover,the acid gases production from AP could weaken the alumina shell[49]and oxygen from decomposition could improve the reactant concentration.Based on the effects above,Al/AP mixture possess more violent combustion performance,higher reaction degree and faster burning rate than Al.However,much gases from the decomposition of AP provides an instantaneous pushing function on the neighboring Al particles under a constrain space.In the reaction area,the particles with enough momentum in the opposite direction would extrude and fuse to form a cluster,difficult to react fully in the second oxidation stage.Moreover,much particles might be thrusted off hot area to form sparks,lowering energy level.
In contrast,this coated structure would perform a mutual impetus,which make the Al particles disperse more evenly in the environment with a lower momentum.This reaction mechanism could avoid generating sparks and further enlarge the reaction area.It is consistent with the researches of Galfetti [50] and DeLuca[51,52]that a positive relationship exists between the particle size of AP versus agglomeration in propellants because AP with smaller size will provide a gentler spraying effect.Meanwhile,this coated structure increases the contact area,promoting the heat diffusion and mass transformation.Hence,the Al@AP composition performs an extensive combustion behavior with high heat release,high reaction degree and fast burning rate.
5.Conclusion
In this work,Al@AP composition are fabricated by a facile preparation via heterogeneous nucleation.The AP crystals attach to the aluminum surface by physical absorption,enhancing the heat diffusion and mass transformation.This coated structure could make Al particles disperse evenly in the reaction,avoiding the formation of molten aluminum clusters with incomplete oxidation.In combustion performance,Al@AP composition behaves better with higher heat release and larger reaction field than Al/AP mixture.An ideal ratio of 15 wt%was concluded preliminarily from the data of exotherm and reaction degree.Meanwhile,85@15 possess higher activation,better diffusion of target materials,and quicker shockwave propagation when ignited by high-energy laser.Prospectively,85@15 composition is a promising aluminum-based fuel in consideration of its combustion performance in cold field and ignition behavior in hot field.
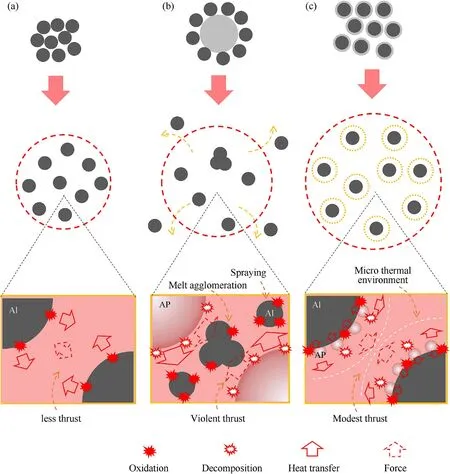
Fig.10.Mechanism diagram of Al (left),85/15 (middle) and 85@15 (right) composition.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work is supported by National Natural Science Foundation of China [No.21975024].
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2021.08.013.
- Defence Technology的其它文章
- Establishment,simulation and verification of firepower safety control model
- Burning characteristics of high density foamed GAP/CL-20 propellants
- Cell-type continuous electromagnetic radiation system generating millimeter waves for active denial system applications
- Sandwich structure for enhancing the interface reaction of hexanitrohexaazaisowurtzitane and nanoporous carbon scaffolds film to improve the thermal decomposition performance
- Ablation characteristics of insulator under high-temperature gas dualpulse erosion
- Influence of shaped charge structure parameters on the formation of linear explosively formed projectiles

