Physiological and Molecular Analyses of Low-Salinity Stress Response in the Cuttlefish (Sepia pharaonis) Juveniles
XIN Hongwei, WU Kunlan, YUAN Yimeng, ZHAO Yun,SONG Weiwei, 2), *,WANG Chunlin, 2), *, MU Changkao, 2), and LI Ronghua, 2)
Physiological and Molecular Analyses of Low-Salinity Stress Response in the Cuttlefish () Juveniles
XIN Hongwei1), WU Kunlan1), YUAN Yimeng1), ZHAO Yun1),SONG Weiwei1), 2), *,WANG Chunlin1), 2), *, MU Changkao1), 2), and LI Ronghua1), 2)
1),,,315832,2),,315832,
As a stenohaline species, the survival ofcan be affected bysalinity significantly. This study aimed to explore the function of decreasing salinity on the survival of, which can provide an advanced production guide on the culture ofin the rainy season. Salinity was gradually decreased from 29 to 22 within 48h to acclimateto a low-salinity environment. After ten days of breeding under low-salinity of 22, the death rate was high. In this process, changes in tissue and cell structures in the larval liver, biochemical indicators, and osmoregulation-related gene expression were examined. In- terestingly, hepatocytes in the low-salinity group were irregular, had dissolved tissue inclusions, and contained vacuolized cells. There- fore, low salinity caused severe damages at a cellular level that can elevate the mortality rate. A gradual decline in salinity limited the full adaptation of. Biochemical indicators and osmoregulation-related gene expression changed similarly. For instance, the trend of malondialdehyde (MAD) as a product of lipid peroxidation reflected the degree of damage to the body by free radicals. The antioxidant system ofcould cope with oxidative stress caused by the change in salinity to a certain extent. Osmo- regulation-related genes’ expression also showed an optimistic result, that is,responded positively to the change in sali- nity by adjusting the expression of osmoregulation-related genes. Conversely, the increase in mortality at day 10 also proved the weakadaptation capability of. This study indicated thatcan adapt to a low-salinity environment with a li- mited extent.
; low salinity; physiological responses; biochemical indicators; osmoregulation-related genes
1 Introduction
Salinity is among the primary factors that affect the growth, physiological performance, and survival rate of aquatic animals (Wang., 2019). In the aquaculture in- dustry, the mechanism underlying the response to salinity stress should be comprehensively explored. The effects of salinity on the embryonic and larval development of Chi- nese mitten crab () have been extensive- ly explored; furthermore, studies have provided vital infor- mation on the protection of their spawning ground(Wang., 2019)Another study on white shrimp () has revealed that low salinity potentially de- creases its immunity, which is a key factor in breeding (Jaf- fer., 2019). Moreover, numerous studies have focusedon the effect of salinity on euryhaline aquatic animals, such as pacific white shrimp (Li., 2017; Xu., 2017), flounder (Zou., 2019), and stickleback (DeFaveri Me-ril?, 2014; Heckwolf., 2018). However, the research on stenohaline sea species is limited till present.
Various cephalopods adapt differently to common en- vironmental factors. For instance,, a euryhaline cephalopod mollusk, potentially tolerates sali- nity from 17.5 to 36 (Hendrix Jr.., 1981)., another cephalopod species, is sensitive to salinity fluctuation. Peng. (2016) studied the effect of salinityon the embryonic development ofby quanti-fying the hatching rate, incubation period, hatching period, wet weight of hatchling cuttlefish, and yolk utilization ef- ficiency ratio. Although salinity ranges from 27 to 33, the established optimal salinity foregg hatching is 30. No eggs were hatched at a low-salinity range of 18– 21. Yin. (2018) observed the survival rate oflarvae significantly reduced at 21. Generally, these findings confirmed that low salinity impedesadaptability. However, in our previous studies, as the water salinity of the open-air pool decreased because of rain- fall, the mortality did not increase even the salinity once reached 14. Hence,can adapt to an environ- ment with low salinity that decreases gradually. This phe- nomenon indicated that a previous research on the rela- tionship betweenand salinity is not compre- hensive. Therefore, mechanisms underlying the responses ofto salinity should be further studied.
A low-salinity environment can cause oxidative stress to stenohaline aquatic species. Some studies onhave examined its superoxide dismutase (SOD) and glu- tathione (GSH) activities and MDA contents which have antioxidative stress functions (Yin., 2018). Therefore, the effects and responsive mechanisms ofon gradual changes in salinity were determined by assessing the variations in the tissue and cell structures of the larval liver and its biochemical indicators. The expression levels of two osmoregulation-related genes,and, werealso examined to study the effects of low salinity onat a molecular level. A zinc-finger protein genewith a significantly different expression inunder different salinity conditions was selected for the study (PRJNA430775; Ren., 2020). This study wasperformed to obtain in-depth knowledge on the nature of mortality due to a decrease in salinity and provide an ad- vanced production guide on the survival rate ofin the rainy season. Consequently, this study wouldre- veal in-depth knowledge on the responses of stenohaline species to low-salinity environments.
2 Materials and Methods
2.1 Experimental Animals
Cuttlefish ()juveniles were obtained from a breedingnursery based in Ningbo City, China (29?35?N, 121?59?E). Larvae (mantle length of 4.17cm±0.46cm and body weight of 13.85g±3.42g) with uniform size and good vitality were selected and maintained in different cementponds for temporary cultivation. Thereafter, the larvae were temporarily maintained in 12 blue plastic barrels (40 in-dividuals/barrel; barrel dimensions: diameter 1.05m; height, 0.6m) for 3 days before the experiment. The maintained lar- vae were fed with small shrimps, which were washed andthen dried, twice per day. The feces and food remains were drained after 30min. The experimental conditions were as follows: water temperature of 24.2℃±1.5℃, salinity of 29.1±0.2, and water oxygen of 4.6mgL?1. Up to 80% of the water was changed daily.
2.2 Experimental Protocol
2.2.1 Salinity stress in cuttlefish juveniles
Seawater salinity was gradually decreased to attain three schemes to determine the tolerance of cuttlefish to sali- nity. In plan A, salinity was lowered by 4; in plan B, sali- nity was decreased by 3; in plan C, salinity was reduced by 2; and in the control group, salinity was maintained at 29. Salinity was decreased in the three experimental groupswithin 24h. The salinity was decreased with mixing fresh- water. The survival rate was analyzed at 24, 48, 72, and 96h. The identified dead juveniles were immediately re- moved. Ten individuals were included in each group. The procedure was repeated three times.
2.2.2 Long-term exposure to low salinity
The larvae were temporarily maintained in 12 blue plas-tic barrels (40 individuals per barrel; barrel dimensions: diameter 1.0m; height, 0.6m). The larvae were randomly categorized into four groups (control group 1, control group 2, low-salinity group 1, and low-salinity group 2) and ac- climatized to two different salinity stresses (22 and 29). Control group 1 and low-salinity group 1 were used to ana- lyze the change of survival rate in a long-term low-sali- nity farming process. Subsequently, five cuttlefish were ran- domly sampled from control group 2 and low-salinity group 2 at six time points (0, 0.5, 1, 2, 7, and 14d) before they were narcotized with 5% alcohol (diluted with seawater). The cuttlefish samples were dissected, and their removed livers were immediately stored in liquid nitrogen. The ex- periments were conducted in compliance with the guide- lines and approved by the Animal Care and Use Commit- tee of Ningbo University.
2.3 Hematoxylin and Eosin (H-E) Staining
The isolated gills and livers were preserved in 10% for- malin solution and embedded in paraffin. The tissues were sliced into 4μm sections, dewaxed, and rehydrated. They were stained with H-E and examined under a standard light microscope.
2.4 Transmission Electron Microscopy Analysis
Tissues were immediately isolated and cut into 1mm3blocks that were fixed in a fixative solution overnight at 4℃. The tissue blocks were washed several times in pho- sphate-buffered saline, postfixed in 1% (w/v) osmium te- troxide, and dehydrated with a series of washing at diffe- rent ethanol concentrations as follows: 50% (v/v) ethanol for 15?min, 75% (v/v) ethanol for 15?min, 95% (v/v) etha- nol for 15?min (twice), and absolute ethanol for 30?min (twice). The tissue blocks were dehydrated with absolute acetone for 10?min twice, embedded with Spurr’s resin, and cured in an oven at 60℃ for 48?h. Ultrathin sections (90?nm thickness) of the tissue blocks were cut using a Leica EMUC7 ultrathin slicer and collected on a 150-mesh cop- per grid. The sections on the copper grid were double stain- ed with uranyl acetate and lead citrate, then viewed under an H-7650 transmission electron microscope (Hitachi-Sci- ence & Technology, Japan).
2.5 Determination of Biochemical Indicators
The liver was immediately removed and washed with normal saline and 10% homogenate prepared in 1.15% (w/v) of potassium chloride. The homogenate was centrifuged at 7000×for 10min at 4℃, and the supernatant was used to determine oxidative stress through antioxidant enzyme estimations. Superoxide dismutase, glutathione peroxidase (GSH-Px), catalase (CAT), and malondialdehyde (MDA) concentrations were determined using their respective anti- oxidant enzyme analysis kits in accordance with the manu- facturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.6 Expression Profiles of Osmoregulation-Related Genes
The gill transcriptome ofwas mapped with an Illumina Hiseq2500 tool. Genes related to salinity stress were identified (PRJNA430775; Ren., 2020). The re- lative expression levels of osmoregulation-related genes Na+/K+-ATPase α subunit (), water-channel pro- tein (), and AN1 type zinc-finger protein 4 () were detected. Total RNA was isolated from frozen liver tissues by using TRIZOL reagent (Invitrogen Corp., Carls- bad, CA) in accordance with the manufacturer’s instructions. The total RNA concentration was measured at 260nm with Nanodrop 2000, an ultra-trace nucleic acid ana- lyzer. Reverse transcription was performed using M-MLV Reverse Transcriptase (Promega Co., Beijing, China) in ac-cordance with the manufacturer’s instructions. RT-PCR was conducted in a 20μL reaction mixture system containing 10μL of SYBR reagent, 5μL of template cDNA, 3μL of water and 2μL of each primer. The reaction procedure was as follows: Initial denaturation at 95℃ for 5min and 45 cycles at 95℃ for 10s; 60℃ for 20s; and 72℃ for 30s. The melting reaction procedure was as follows: 95℃ for 5s; 65℃ for 1min; and 97℃ for 15s.was used as an internal reference gene (Table 1).

Table 1 Primer sequences
2.7 Statistical Analysis
Statistical data were analyzed with Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) version 19.0. The groups were compared at different time points by using Student’s-tests, and results were expressed in mean±SD. For comparisons,<0.05 was considered sta- tistically significant, whereas<0.01 was considered high- ly significant. Charts were developed using Prism 5 (ver- sion 5.01, GraphPad Software Inc.).
3 Results
3.1 Effects of Saline Gradient Schemes on the Survival Rate of Cuttlefish Larvae
An abrupt change in salinity caused the death of. A 4 decrease in salinity at an interval of 24h led to the death of all cuttlefish within 48h, while a reduction of 3 salinity every 24h led to 50% of the survival rate at 48h. Furthermore, the 96h cumulative survival rate was 10%. After the salinity was decreased by 2 every 24h, the cumulative survival rate in 96h was 75%. The results re- vealed that plan C was an effective scheme for a gradual reduction in salinity. Eventually, salinity was maintained at 22 for long-term culture. (Fig.1a).
3.2 Long-Term Exposure to Low Salinity on the Survival Rate of Cuttlefish Larvae
In the first ten days of breeding, the survival rate of the control group was not significantly different from that of the experimental group (Fig.1b). After ten days of breeding, the survival rates of the low-salinity group and the control group gradually decreased by 40% and about 80% on day 14, respectively.
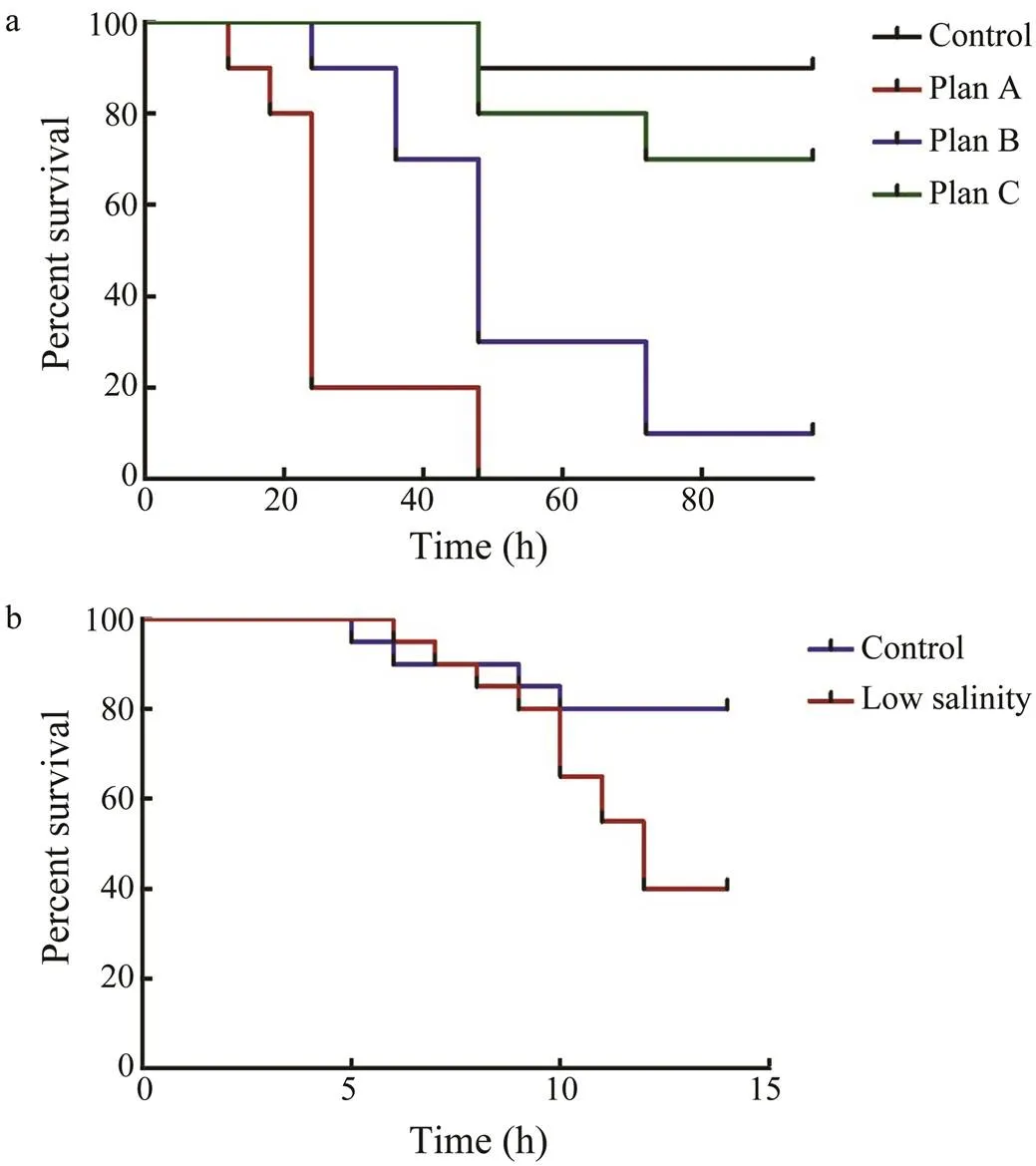
Fig.1 Survival rate of cuttlefish larvae cultured with low sa-linity. a, survival rate of different saline gradient schemes; b, survival rate of long-term exposure to low salinity.
3.3 Histological and Electron Microscopic Evaluations of Livers
The liver tissue sections in the control group are shown in Fig.2a. In the control group, the hepatocytes were close- ly arranged, had a clear outline, had clear and interlaced cell cords, were complete in a nuclear structure, and had rich contents. However, in the low-salinity group, the he- patocytes were irregular, had an incomplete cell cord struc- ture, and an incomplete nuclear structure that deviated from the cell center. The hepatocyte inclusions were dissolved and had vacuolized cells (Fig.2b). Electron microscopy re- vealed that the organelles around the hepatocytes of the control group were complete and abundant (rough endo- plasmic reticulum, mitochondria; Fig.3a). The cytoplasm of the control group was well developed and had a flat-tened cystic shape (Fig.3c). Microscopy results (Fig.3b) confirmed the description of hepatocytes and determined the increased mitochondria in the low-salinity group, which had long bars (Fig.3d).
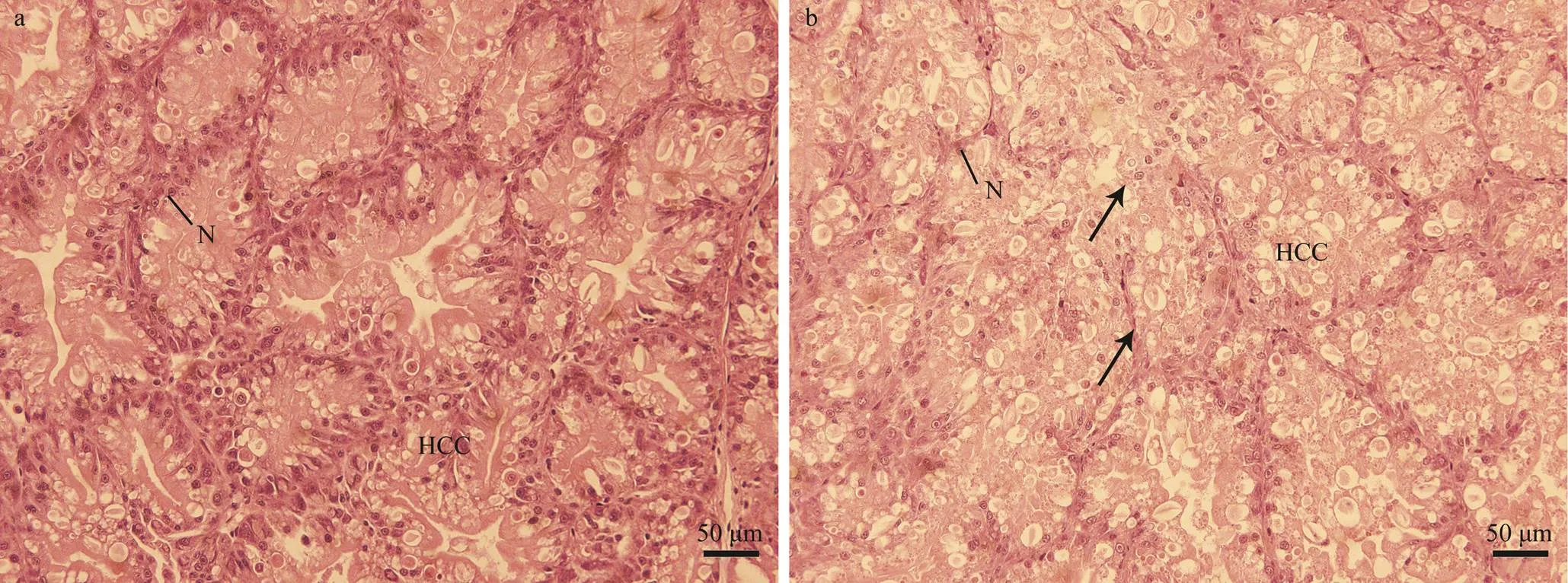
Fig.2 Images of S. pharaonis liver under a light microscope. a, liver in the control group, ×200; b, liver in the low-salinity group, ×200. Arrow showed that the nucleus was dissolved, and the cytoplasm was hollow. N, nucleus; HCC, cell cord.

Fig.3 Transmission electron microscopy images of the liver of S. pharaonis larvae. a, multiple liver cells in the control group, ×8000; b, multiple hepatocytes in the low-salinity group, ×8000; c, single hepatocyte in the control group, ×12000; d, single hepatocyte of the low-salinity group, ×12000. M, mitochondria; N, nucleus; RER, rough endoplasmic reticulum.
3.4 Key Antioxidant Enzymes Levels in the Liver
After the SOD activity increased with time, the maxi- mum value was attained at day 2. However, the SOD ac- tivity was significantly lower than that of the control group at the beginning and day 0.5 (<0.05). Interestingly, the SOD activity was significantly higher than that of the con- trol group at day 7 (<0.05). The general trend of SODwas similar to that of GSH. Nevertheless, the GSH con- tent in the control group fluctuated at around 37μmol(gprot)?1, but it was significantly higher in the low-salinity group than in the control group at 0.5d (<0.05). TheSOD activity and GSH content increased rapidly from day 0.5 to day 1 with a fast response of GSH and a slow re- sponse of SOD enzyme (Figs.4a, c).
The CAT enzyme activity level in the liver of the cut- tlefish larvae in the low-salinity group gradually decreased with time. In the low-salinity group it was lower than that in the control group at day 0.5. Although the CAT level in the control group decreased with time, it subsequently flat-tened compared with that in the low-salinity group (Fig.4b).
The MDA content in the low-salinity group initially in- creased and then decreased with time to reach a maximum value at day 1 (Fig.4d). The MDA content in the low-sa- linity group was significantly higher than that in the con- trol group in the experiment at all time points (<0.05).
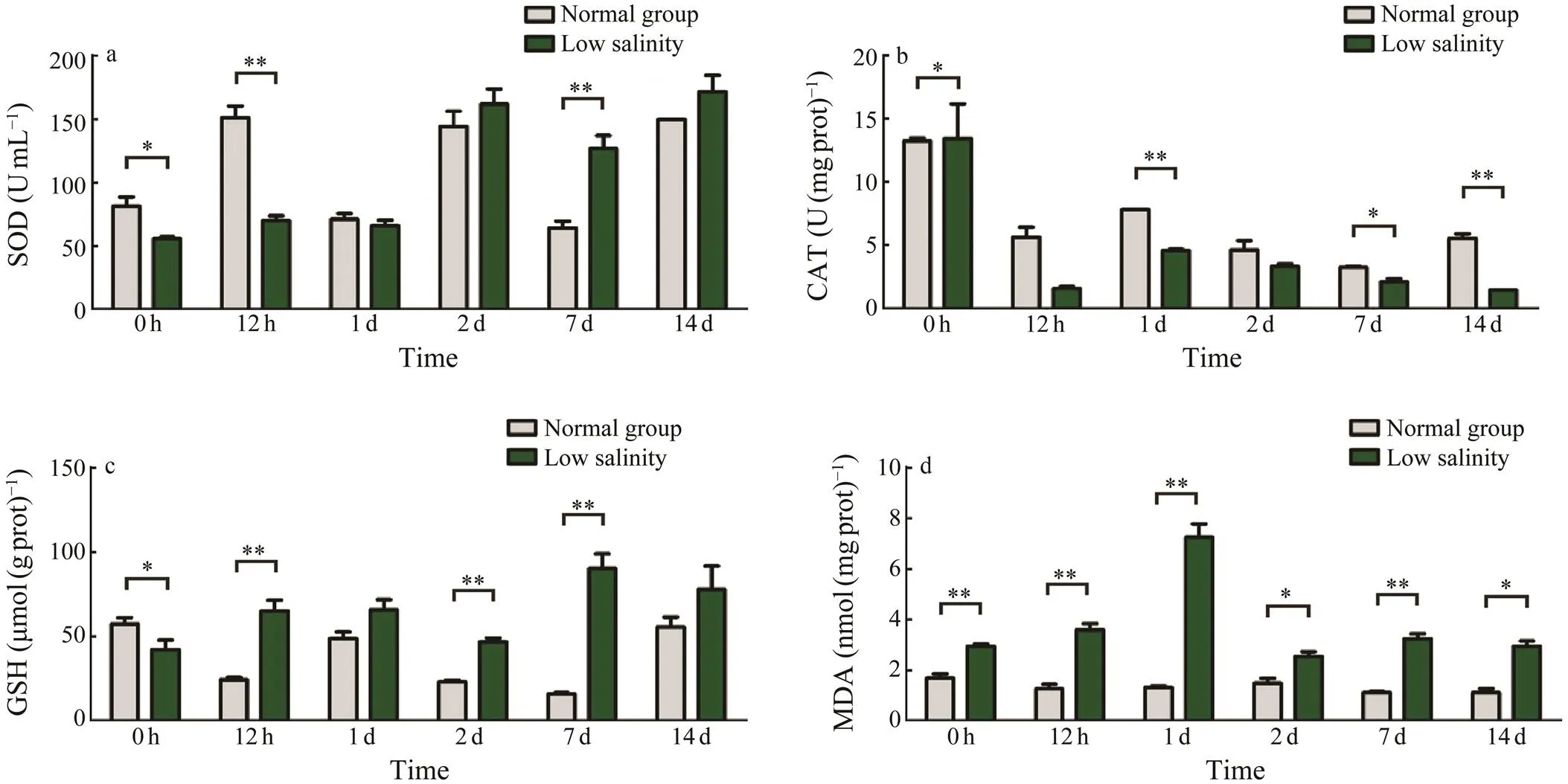
Fig.4 Effect of low salinity on biochemical indicators in the antioxidation system. a, SOD activity changes; b, CAT acti- vity changes; c, GSH activity changes; d, MDA content. Values are shown as the mean±SD (n=5). Statistical significance is set at P<0.05.
3.5 Changes in Osmotic Pressure Regulation-Related Gene Activity Under Different Salinity Levels
The expression of thegene sharply fluctuated with time (Fig.5a). At 0 and 10d, the expression levelswere significantly lower than those of the control group (<0.05). Thegene expression at 0d was the lowest. Conversely, it was higher than that of the control group at day 0.5 and day 2. Moreover, the maximum va- lue was reached at day 0.5.
The expression of thegene showed volatility (Fig.5b). It reached the maximum value at day 0.5, which was significantly higher than the control group (<0.05). The relative expression of genes in the control group re- mained stable.
As a whole, the expression of thegene initially decreased and then increased with time (Fig.5c). The ex- pression of thegene at day 0 was significantly high- er than that in the control group but was significantly low- er at day 2 (<0.05). Briefly, the expressions of thegene at other time points showed no significant difference between the low-salinity and control groups.
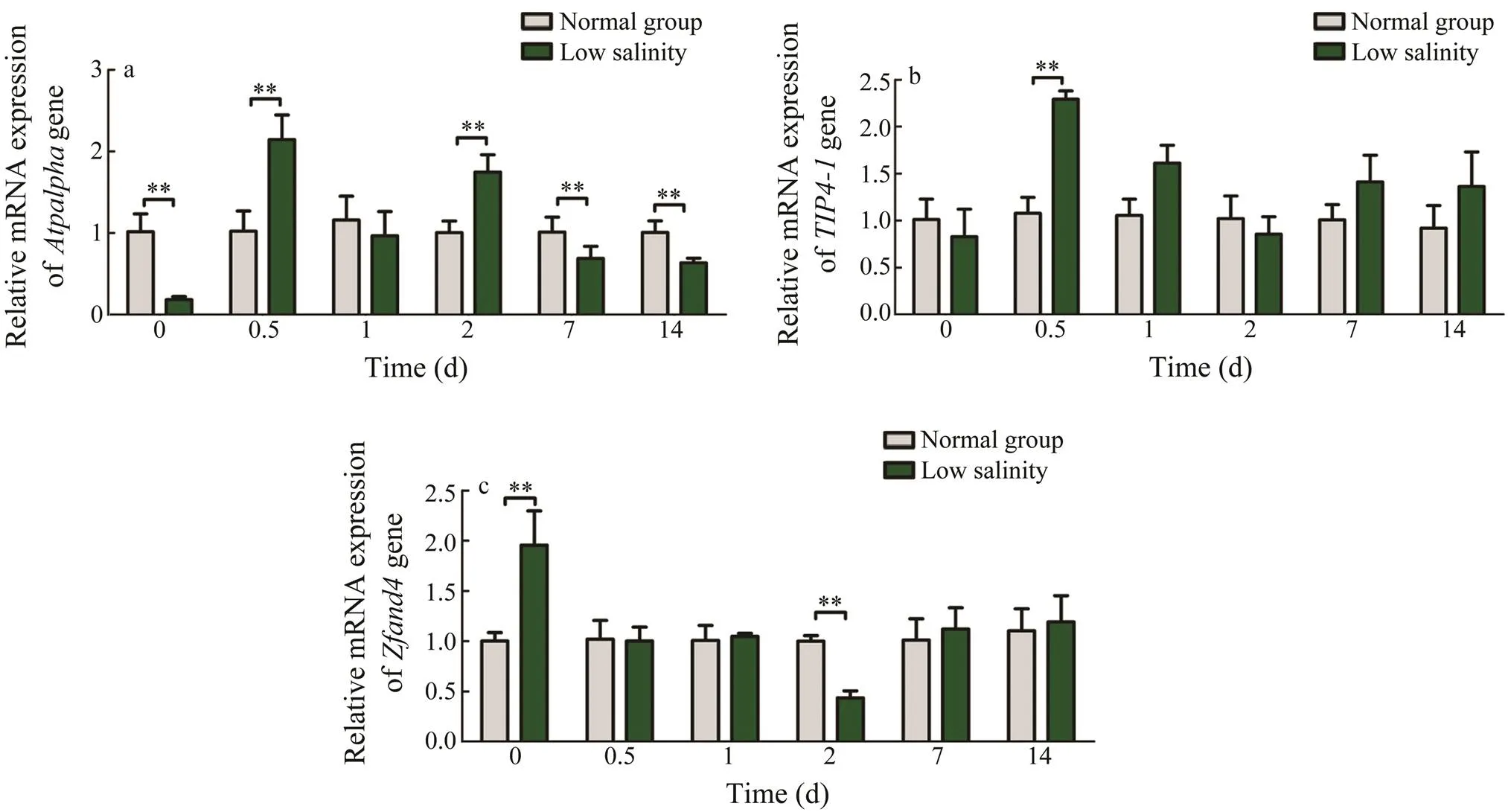
Fig.5 Expression of osmotic pressure regulatory genes in the livers of S. pharaonis larvae with different culture time. a, Atpalpha gene; b, TIP4-1 gene; c, Zfand4 gene. Values are shown as the mean±SD (n=5). Statistical significance is set at P<0.05.
4 Discussion and Summary
is a stenohaline species that shows mini- mal fluctuation and compatibility in salinity. The optimal salinity for the cultivation oflarvae ranged from 24 to 30. Our findings showed that when the water salinity of the open-air pool decreased due to rainfall (sa- linity once reached 14), mortality oflarvae did not increase. Therefore,can adapt to a gra-dual decrease of salinity. Moreover, when salinity gradu-ally decreased from 29 to 22 in 48h,can ac- climate to the low salinity. After the species were bred un- der low-salinity conditions for 10d, substantive deaths oc- curred and hysteresis was evident, which had not been re- ported before.
The hepatocytes in the low-salinity group were irregu- lar with dissolved inclusions and vacuolized cells. A simi- lar histological observation was in large croaker () and. The possibi- lity of cell vacuolization in the hepatocytes ofwas similar to that of. Body energy is used to regulate osmotic pressure and adapt to a low-salinity environment. Sugar metabolism diverts toward the direc- tion of glycolysis by regulating hormones. As the result, glycogen is stored in the liver to provide energy for os- motic pressure regulation in the body. Consequently, thestored glycogen in the liver develops numerous vacuoles in hepatocytes. Therefore, the histological findings in the liver suggested that a low-salinity environment damaged the structures and functions of normal tissues. However,some results still indicated thatcould adapt to low salinity.
SOD and CAT are two antioxidant enzymes that can re- move reactive oxygen species from the body. GSH plays an important role in the nonenzymatic antioxidant system. Our findings indicated that the SOD activity in the low- salinity group gradually increased with time, while super- oxide radical increased with time. In contrast to SOD, the CAT activity decreased with time. CAT is the first enzyme in theliver to resist the damage from exces- sive free radicals. When the extent of damage was beyond the tolerance of the body, the CAT activity was inhibited. The trends of SOD and CAT inwere differ- ent from those in, whose SOD and CAT ac- tivities are similar (Sun., 2010). Various species have different antioxidant strategies, which are hypothesized to yield different results. The content of GSH increased gra- dually from lower than the control group to higher than the control group. By contrast, Yin. (2018) obtained different results. In their results, the survival rate, the his- tological structure, antioxidative stress parameters, and GSH level in the liver ofafter 48h of breeding in different salinities significantly decreased compared with that of the control group. Differences in salinity changes(gradual or direct) can contribute to various outcomes. GSH potentially protects the tissues from oxidative damage in- flicted by reactive oxygen species (Leeuwenburgh., 1997). The different outcomes of the gradual decrease in salinity could providethe potential ability to resist oxidative damage from the change in salinity. The content of MDA, as a product of lipid peroxidation, re- flects the degree of damage to the body by free radicals. The MDA content trend showed that numerous free radi- cals were produced in theliver before the an- tioxidant system was activated. Thereafter, the SOD acti- vity and GSH level significantly increased, scavenging a large number of free radicals and reducing the peroxida- tion degree. As a result, the MDA content decreased. There- fore,can deal with low-salinity stress by acti- vating the antioxidant system.
In this study, the expression levels of three osmoregula- tion-related genes were assessed, including Na+/K+-ATPase α subunit gene, aquaporingene, andgene. The α subunit of Na+/K+-ATPase contains nucleotide, cation- binding sites, catalytic sites, chemical modification regu- latory sites, and ligand binding sites that can activate and inhibit enzyme activities as the core function of Na+/K+- ATPase (James., 1999; He., 2001). Studies have shown that the expression of Na+/K+-ATPase α subunit gene is related to salinity stress in rainbow trout(Richards., 2003), eel(Cutler., 1995),and(Deane., 2005), tilapia(Hwang., 1998), and crabs, such as(Han., 2015) and Chinese mitten crab(Sun., 2012). the Na+/ K+-ATPase activity trend of juvenileanddecreases (Bystriansky., 2011; Sun., 2012). However, in our study, the expression of Na+/K+- ATP α subunit in the liver ofinthe low-sa- linity group sharply increases with longer culture period.larvae can produce a large amount of Na+/K+- ATPase in response to the change in the salinity of the external environment. The gradual adaptation to the new environment simultaneously led to the recovery of expres- sion levels to the normal state.
Aquaporins are a family of water-channel proteins. Theyplay an important role in the osmotic regulation of fish, such as golden sea bream () (Deane., 2006), zebrafish () (Tingaud-Sequeira., 2010), Atlantic salmon () (Tipsmark., 2010), and European eel () (MacIver., 2009). Our results revealed that the expression of aqua- porinlarvae changed dramatically after low-salinity stress.main- tained homeostasis in their cells by balancing the osmotic pressure in the body and regulating the expression of.
A20/AN1 zinc-finger proteins were demonstrated to re- present the common elements of stress response in plants and animals (Vij., 2008). In the present study, the ex- pression level of thegene in the liver oflarvae decreased with longer culture time, and the highest expression was found at 0d (significantly higher than the control group,<0.01). These results suggested thatadapted to environmental changes gradually.
In summary, biochemical parameters, such as the trend of antioxidant enzymes, confirmed thatcould potentially adapt to a low-salinity environment. The his- tological results showed hepatocytes were critically da- maged at a cellular level by low salinity, which might be the reason that low salinity induced a dramatic increase in mortality. This result indicated thatcould fully adapt to the gradual decrease of salinity that was changed from 29 to 22 in 48h. Therefore, a gradual decrease in sa- linity could facilitate the efficient acclimation of. The high mortality at 10d indicated the weak adap- tation capability of.
Acknowledgements
This study was supported by the Ningbo Agricultural Major Projects (No. 201401C1111001) and the Foundation of Zhejiang Educational Committee (No. Y201940957), and all the authors were sponsored by K. C. Wong Magna Fund in Ningbo University.
Bystriansky, J. S., and Schulte, P. M., 2011. Changes in gill H+-ATPase and Na+/K+-ATPase expression and activity during fresh- water acclimation of Atlantic salmon ()., 214 (14): 2435, https://doi.org/10.1242/jeb.050633.
Cutler, C. P., Sanders, I. L., Hazon, N., and Cramb, G., 1995. Pri-mary sequence, tissue specificity and expression of the Na+, K+-ATPase α 1 subunit in the European eel ().,111 (4): 567-573, https://doi.org/10.1016/0305-0491(95)00037-9.
Deane, E. E., and Woo, N. Y., 2005. Cloning and characteriza- tion of sea bream Na+-K+-ATPase α and β subunit genes:effects of hormones on transcriptional and translational expression.,331 (4): 1229-1238, https://doi.org/10.1016/j.bbrc.2005.04.038.
Deane, E. E., and Woo, N. Y., 2006. Tissue distribution, effects of salinity acclimation, and ontogeny of aquaporin 3 in the ma- rine teleost, silver sea bream ().,8 (6): 663-671, https://doi.org/10.1007/s10126-006-6001-0.
DeFaveri, J., and Meril?, J., 2014. Local adaptation to salinity in the three-spined stickleback?,27 (2): 290-302, https://doi.org/10.1111/jeb.12289.
Han, X., Liu, P., Gao, B., Wang, H., Duan, Y., Xu, W.,., 2015. Na+/K+-ATPase α-subunit in swimming crab: Molecular cloning, characterization, and expres- sion under low salinity stress.,33 (004): 828-837, https://doi.org/10.1007/s00343-015-4018-9.
He, S., Shelly, D. A., Moseley, A. E., James, P. F., James, J. H., Paul, R. J.,., 2001. The α1- and α2-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility.,281 (3): R917-R925, https://doi.org/ 10.1152/ajpregu.2001.281.3.R917.
Heckwolf, M. J., Meyer, B. S., D?ring, T., Eizaguirre, C., and Reusch, T. B., 2018. Transgenerational plasticity and selection shape the adaptive potential of sticklebacks to salinity change.,11 (10): 1873-1885, https://doi.org/10.1111/eva.12688.
Hendrix Jr., J., Hulet, W., and Greenberg, M., 1981. Salinity to- lerance and the responses to hypoosmotic stress of the bay squid, a euryhaline cephalopod mollusc.,69 (4): 641-648, https://doi.org/10.1016/0300-9629(81)90150-X.
Hwang, P., Fang, M., Tsai, J.C., Huang, C., and Chen, S., 1998. Expression of mRNA and protein of Na+-K+-ATPase α subunit in gills of tilapia ().,18 (4): 363-373, https://doi.org/10.1023/A:1007711606064.
Jaffer, Y. D., Saraswathy, R., Ishfaq, M., Antony, J., and Sharma, P. C., 2019. Effect of low salinity on the growth and survival of juvenile Pacific white shrimp,: A revival.,515: 734561, https://doi.org/10.1016/j.aquaculture.2019.734561.
James, P. F., Grupp, I. L., Grupp, G., Woo, A. L., Askew, G. R., Croyle, M. L.,., 1999. Identification of a specific role for the Na, K-ATPase α2 isoform as a regulator of calcium in the heart.,3 (5): 555-563, https://doi.org/10.1016/S1097-2765(00)80349-4.
Leeuwenburgh, C., Hollander, J., Leichtweis, S., Griffiths, M., Gore, M., and Ji, L. L., 1997. Adaptations of glutathione an- tioxidant system to endurance training are tissue and muscle fiber specific.–, 272 (1): R363-R369, https://doi.org/10.1152/ajpregu.1997.272.1.R363.
Li, E., Wang, X., Chen, K., Xu, C., Qin, J. G., and Chen, L., 2017. Physiological change and nutritional requirement of Pacific white shrimpat low salinity.,9 (1): 57-75, https://doi.org/10.1111/raq.12104.
MacIver, B., Cutler, C. P., Yin, J., Hill, M. G., Zeidel, M. L., and Hill, W. G., 2009. Expression and functional characterization of four aquaporin water channels from the European eel ().,212 (17): 2856-2863, https://doi.org/10.1242/jeb.025882.
Peng, R. B., Wang, P. S., Jiang, M. W., Peng, R., Han, Q. X., and Jiang, X. M., 2016. Effect of salinity on embryonic develop- ment of the cuttlefish., 48 (4): 666-675, https://doi.org/10.1111/jwas.12321.
Richards, J. G., Semple, J. W., Bystriansky, J. S., and Schulte, P. M., 2003. Na+/K+-ATPase α-isoform switching in gills of rainbow trout () during salinity transfer.,206 (24): 4475-4486, https://doi.org/10.1242/jeb.00701.
Sun, M., Jiang, K., Zhang, F., Zhang, D., Shen, A., Jiang, M.,.,2012. Effects of various salinities on Na+-K+-ATPase, Hsp70 and Hsp90 expression profiles in juvenile mitten crabs,.,11 (2): 978-986, https://doi.org/10.4238/2012.April.19.3.
Sun, P., Peng, S. M., Yin, F., and Shi, Z. H., 2010. Effects of sa- linity on activity of Na+/-K+-ATPase in juvenile.,34 (8): 1204-1209.
Tingaud-Sequeira, A., Calusinska, M., Finn, R. N., Chauvigné, F., Lozano, J., and Cerdà, J., 2010. The zebrafish genome en- codes the largest vertebrate repertoire of functional aquapo- rins with dual paralogy and substrate specificities similar to mammals., 10 (1): 38, https://doi.org/10.1186/1471-2148-10-38.
Tipsmark, C. K., S?rensen, K. J., and Madsen, S., 2010., Aquapo-rin expression dynamics in osmoregulatory tissues of Atlantic salmon during smoltification and seawater acclimation., 213 (3): 368-379, https://doi.org/10.1242/jeb.034785.
Vij, S., and Tyagi, A. K., 2008. A20/AN1 zinc-finger domain-containing proteins in plants and animals represent common elements in stress response.,8 (3): 301-307, https://doi.org/10.1007/s10142-008-0078-7.
Wang, R., Huang, X., Wang, H., Lu, J., Shi, X., Feng, G.,.,2019. Effects of salinity on embryonic and larval developmentof Chinese mitten crab(Decapoda: Bra-chyura) and salinity-induced physiological changes.,37 (5): 1777-1788, https://doi.org/10.1007/s00343-019-8190-1.
Xu, C., Li, E., Liu, Y., Wang, X., Qin, J. G., and Chen, L., 2017. Comparative proteome analysis of the hepatopancreas from the Pacific white shrimpunder long-term low salinity stress.,162: 1-10, https://doi.org/10.1016/j.jprot.2017.04.013.
Yin, S.J., Zhang, L., Zhang, L., Wan, J., Song, W., Jiang, X.,.,2018. Metabolic responses and arginine kinase expression of juvenile cuttlefish () under salinity stress.,113: 881-888, https://doi.org/10.1016/j.ijbiomac.2018.03.036.
Zou, H., He, F., Lan, Z., Tang, L., and Lu, W., 2019., The person- ality of Japanese flounder () and gene expression related with osmoregulatory capacity in the gills., 500: 221-227, https://doi.org/10.1016/j.aquaculture.2018.10.013.
December 15, 2020;
February 16, 2021;
August 3, 2021
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
. E-mail: songweiwei@nbu.edu.cn
E-mail: wangchunlin@nbu.edu.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2022年4期
Journal of Ocean University of China2022年4期
- Journal of Ocean University of China的其它文章
- Complete Mitochondrial Genome of Myra affinis (Decapoda:Brachyura: Leucosiidae) and Its Phylogenetic Implications for Brachyura
- Multisource Target Classification Based on Underwater Channel Cepstral Features
- Joint Model of Wind Speed and Corresponding Direction Based on Wind Rose for Wind Energy Exploitation
- Elastic-Wave Reverse Time Migration Random Boundary-Noise Suppression Based on CycleGAN
- Identification, Phylogeny and Expressional Profiles of Peptidoglycan Recognition Protein (PGRP) Gene Family in Sinonovacula constricta
- Molecular Characterization,Expression Pattern and Transcriptional Regulation of Figla During Gonad Development in Japanese Founder(Paralichthys olivaceus)
