Ag Nanoparticle?Doped Activated Microporous Carbon Spheres:An Efficient Adsorption/Catalysis Bifunctional Material for the Removal of Congo Red
XU Peng DAI WeiWANG Sha?Sha WU Zhen?Guo CHEN Nan?Nan WANG Yang LIU Jian
(Co?innovation Center for Efficient Processing and Utilization of Forest Products,Nanjing Forestry University,Nanjing 210037,China)
Abstract:Herein,we fabricated silver nanoparticle?doped activated microporous carbon spheres(Ag/AMCSs)through a combining method of spray drying,carbonization,and KOH activation by using chitosan as a precursor.Based on a series of characterization and performance studies,Ag/AMCSs are confirmed to be able to function as both an adsorbent and a catalyst for the reduction of Congo red(CR)in the presence of NaBH4due to the high poros?ity and high dispersion of silver nanoparticles.Adsorption studies were performed by varying pH,contact time,and initial concentration,and it was found that the adsorption process could be well estimated by the pseudo?second?order kinetic plot and Langmuir models,and the maximum adsorption capacity of Ag/AMCSs was estimated as 445 mg·g?1.For the reduction of CR,the rate constant k was calculated up to 0.311 min?1,and the conversion efficiency of CR remained at 95% after 5 successive cycles.
Keywords:carbon;silver;activation;catalyst;adsorbent
0 Introduction
Currently,synthetic dyes have been used widely in several manufacturing fields,including the textile,paper,and printing industries[1?4].However,most of the dye contaminants in water bodies are toxic and can not be degraded naturally,which seriously endangers human health and environmental security[1,5?6].There?fore,it is important to find a quick and efficient method for removing dye contaminants.
To date,various techniques,such as adsorption[7],catalytic degradation[8?9],photocatalytic degradation[10],membrane filtration[11],and electrochemical methods[12]have been exploited to remove dye pollutants.Among these reported strategies,adsorption has been regarded as a remarkable prospective technique for the removal of dyes owing to its low cost,easy operation,and environmental compatibility[13?16],and many convention?al absorbents such as clay,zeolites,natural polymers,and porous SiO2have been reported to remove dye?contaminated water[17?29],but major of them are suffering from low adsorption capacity with remarkable low sen?sitivity and selectively.In addition,the adsorption pro?cess does not break down dye molecules,and addition?al post?treatment is indispensable after adsorption alone.It is reported that catalytic reduction/oxidation of dyes by noble metal nanoparticles can efficiently degrade the dyes in the aqueous solution[30?31].However,due to the high surface energy and high reactivity,noble nanoparticles are apt to aggregate into large sized agglomerates,leading to the decreasing of the properties of catalysts.Taking the advantages of the above methods into consideration,combining the merits of the adsorption and catalysis is an intelligent strategy to treat organic pollutants[5,32?33].Hence,it is critical to seek ideal bifunctional materials for the synergistic removal of dyes via adsorption and catalytic degrada?tion.
Recently,porous carbon sphere,as a functional carbon material,has been received continuous interest in adsorption,catalysis,hydrogen storage,and drug release due to its excellent chemical stability,large specific surface area,and excellent biocompatibility.In wastewater treatment,porous carbon sphere adsor?bents have been used to adsorb organic pollutants,heavy metal ions,and dyes in aqueous solutions[34].In addition,the porous architectures of the carbon spheres provide numerous anchoring sites for the steady immobilization of active species,which can be highly dispersed in the porous matrix and preserve their activity[35].For example,Yang et al.fabricated mesoporous carbon spheres immobilized ultra?small gold nanoparticles as an excellent catalyst for the reduction of 4?nitrophenol[35].Shao et al.successfully fabricated tiny gold nanoparticles loaded magnetic mes?oporous carbon spheres and investigated their catalytic activity towards reduction of 4 ?nitrophenol[36].There?fore,the porous carbon sphere is expected to become an ideal bifunctional material with adsorption and cata?lytic degradation of dye molecules,however,practical application is still a challenge mainly due to their com?plex synthetic procedures,high costs,and low yields.Therefore,the present work aims to develop a facile and mass?producible process for preparing a porous carbon sphere?based bifunctional material with excel?lent catalytic and adsorption functions.
Herein,we demonstrate a simple and high?yield method to construct a bifunctional material with silver nanoparticle?doped activated microporous carbon spheres(Ag/AMCSs)for adsorption and catalytic appli?cations.Intermediate Ag nanoparticles embedded chi?tosan microspheres(denoted Ag/chitosan)were first fabricated by a spray drying method,in which chitosan was designed as a carbon precursor and silver stabiliz?er.After carbonization and KOH activation of Ag/chito?san,the resulting carbon composite Ag/AMCSs exhibit?ed abundant micropores and a high specific surface area.Finally,Congo red(CR)was opted as a model dye to explore the adsorption and catalytic properties of Ag/AMCSs.
1 Experimental
1.1 Chemical and reagents
Acetic acid(CH3COOH),potassium hydroxide(KOH),and silver nitrate(AgNO3)were obtained from Nanjing Chemical Reagent Co.,Ltd.China.Chitosan and sodium borohydride(NaBH4)were purchased from Sinopharm Chemical Reagent Co.,Ltd.China.CR orig?inated from Tianjin Chemical Reagent Co.,Ltd.China.There was no further purification of chemicals,and deionized water was used in all experiments.
1.2 Material preparation
1.2.1 Precursor preparation
A solution of chitosan in 2% acetic acid solution(100 mL,10 mg·mL?1)was first prepared,and 1 mL of 0.6 mol·L?1AgNO3was added to the solution.Then,1 mL of ice?cold 1.3 mol·L?1NaBH4was added drop?wise,and the solution was continued for another 3 h.
1.2.2 Preparation of silver nanoparticles embedded chitosan spheres via the spray drying technique
Mini spray dryer B ?290(BüCHI Labortechnik AG,Flawil,Switzerland)was used for spray drying.In the spray drying process,the dry airflow rate,liquid feeder pump flow,aspiration,and inlet drying air tem?perature of the mini spray dryer were set to 600 L·h?1,30%,100%,and 180℃,respectively.The obtained sil?ver nanoparticles embedded in chitosan microspheres were denoted as Ag/chitosan.
1.2.3 Carbonization and activation process
Both carbonization and activation were carried out under nitrogen protection.For the carbonization pro?cess,Ag/chitosan was carbonized at 700℃for 2 h,and the heating procedure was separated into two stages.In the initial stage,the temperature was increased from room temperature to 400℃,and in the next stage,the temperature was increased to 700℃.The heating rates were 6 and 3 ℃ ·min?1,respectively.The obtained materials were marked as silver nanoparticle?doped carbon spheres(Ag/CSs).To activate Ag/CSs,the mate?rials and base were uniformly mixed with the mass ratio of Ag/CSs to KOH was 1∶4.The chemical activa?tion process was executed by heating the samples with a ramp rate of 5 ℃·min?1,and the samples were activat?ed at 750℃for 1.5 h[37].After the activation process,the product was completely washed several times with deionized water until the alkali was completely washed away.The prepared activated samples were denoted as silver nanoparticle?doped activated microporous car?bon spheres(Ag/AMCSs).
1.3 Adsorption properties of Ag/AMCSs
The adsorption capacity of Ag/AMCSs was mea?sured by selecting CR as a model dye.In a typical adsorption experiment,5 mg of Ag/AMCSs was dis?persed in 25 mL of CR solution and maintained for 12 h,and the absorbance of the solution after the experi?ment was recorded by UV?Vis spectroscopy.To investi?gate the influence of pH on adsorption properties,CR solutions with different initial pH values(4 to 11)were prepared by adding 0.1 mol·L?1HCl or NaOH solution.Different initial CR concentration at neutral(from 20 to 1 000 mg·L?1)was used to investigate the effect of ini?tial concentration on adsorption performance,and the contact time was set as 12 h.For adsorption kinetics studies,5 mg of Ag/AMCSs was dispersed in CR solu?tion(100 mg·L?1)and the absorbance of the solution was recorded at intervals.
The equilibrium adsorption capacity(qe,mg·g?1),adsorption capacity(qt,mg·g?1)at given timet,and re?moval rate(%)were calculated according to the follow?ing equations[38]:
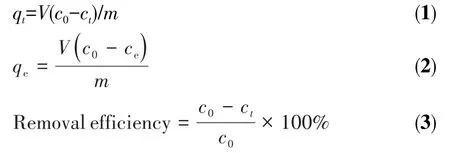
wherec0(mg·L?1),ct(mg·L?1),andce(mg·L?1)repre?sent concentrations of CR solution at initial,timet,and equilibrium status,respectively.V(L)denotes the solu?tion volume,andm(g)is the mass of Ag/AMCSs.
1.4 Catalytic performance
CR was also used to evaluate the catalytic perfor?mance of Ag/AMCSs.6 mL of CR(60 mg·L?1)solution was mixed with 2 mL of 0.5 mol·L?1NaBH4in a tube,resulting in a light red solution,and different masses(0.5 or 1 mg)of Ag/AMCSs was added to act as a cata?lyst.UV?Vis spectroscopy was used to monitor the reac?tion.The reaction rate constant(k)can be obtained with the following equation:

wherec0andctare the concentrations of solution at the initial time and timet(min),respectively.
1.5 Recycling experiment
A cycling test was used to investigate the stability and reusability of Ag/AMCSs.For adsorption experi?ments,CR solution(25 mL,100 mg·L?1)was used in the tests.After the first cycle,the suspension of CR and Ag/AMCSs was centrifuged to separate the frac?tions,and a large amount of alcohol?containing lye was used to desorb dyes.Deionized water was used to wash out the residual alkali and ethanol before the next test.In determining catalytic performance,after a complete catalytic experiment,Ag/AMCSs were recovered and washed several times to thoroughly remove the impuri?ties before the next run.
1.6 Characterization
The morphologies of Ag NPs in chitosan solution and Ag/AMCSs were observed by transmission electron microscopy(TEM,JEM?1400,Japan)and the accelera?tion voltage was 300 kV.The surface morphologies of Ag/chitosan,Ag/CSs,and Ag/AMCSs were observed by scanning electron microscopy(JSM?7600F,Rigaku,Japan)with energy dispersive spectrometry (EDS)employing an operating voltage of 5 or 25 kV.The ther?mal properties of the materials were analyzed by a ther?mal analyzer(Q5000IR,TA,USA)in N2atmosphere with a heating rate of 10 ℃·min?1and nitrogen gas flow rate of 10 mL·min?1,and the temperature was pro?grammed to rise from 20 to 750℃.N2adsorption iso?therm wasmeasuredbyanASAP 2020system(Micromeritics,USA)at?196℃,the samples were degassed at 30℃for 10 h before the measurement.The specific surface areas,pore size distributions,and total pore volumes were determined by nitrogen adsorp?tion measurements(ASAP 2000 system,Micromeritics,USA).The Brunauer?Emmett?Teller(BET)and Barrett?Joyner?Halenda(BJH)methods were used to analyze detailed data.The crystal structure of the obtained materials was characterized by X?ray diffraction(XRD,Rigaku,Japan),operating at 40 kV and 100 mA,scan?ning rate of 5(°)·min?1from 2θ=10°to 90°,using CuKαas a radiation source andλwas 0.150 6 nm.The surface chemical composition and binding energy were characterized on an AXIS UltraDLD X?ray photo?electron spectroscopy(XPS,AXIS UltraDLD,UK).The surface functional groups of the sample were measured using a Fourier transform infrared spectrometer(FT?IR Spectrum Two,Perkin Elmer)(KBr pellets,scanning range 4 000?1 000 cm?1).A UV?visible spectrophotome?ter(Shimadzu UV?2450,Japan)was employed to record the absorbance of the target solution.
2 Results and discussion
2.1 Characterizations of Ag/chitosan,Ag/CSs,and Ag/AMCSs
Chitosan is an attractive metal chelating ligand due to its abundance of reactive amino and hydroxyl groups,and after the addition of NaBH4,the color of the AgNO3/chitosan solution rapidly changed from light blue to purplish?red(inset in Fig.1),indicating that the chelated Ag ions were reduced to Ag NPs[39].More evidently,the UV?Vis spectra(Fig.1)recorded for the purplish?red solution showed a characteristic surface plasmon resonance(SPR)band at approximately 420 nm[40],while the light blue solution exhibited no typical absorption peak,indicating that Ag NPs were success?fully synthesized by the reduction of NaBH4.The TEM image of Ag NPs in chitosan solution is shown in Fig.2a,revealing that spherical Ag NPs with diameters of approximately 3 nm(Fig.2b)were successfully pre?pared.Besides,the selected area electron diffraction analysis shows diffraction rings of face?centered cubic silver(inset in Fig.2a),proving that these nanoparticles are polycrystalline[41].
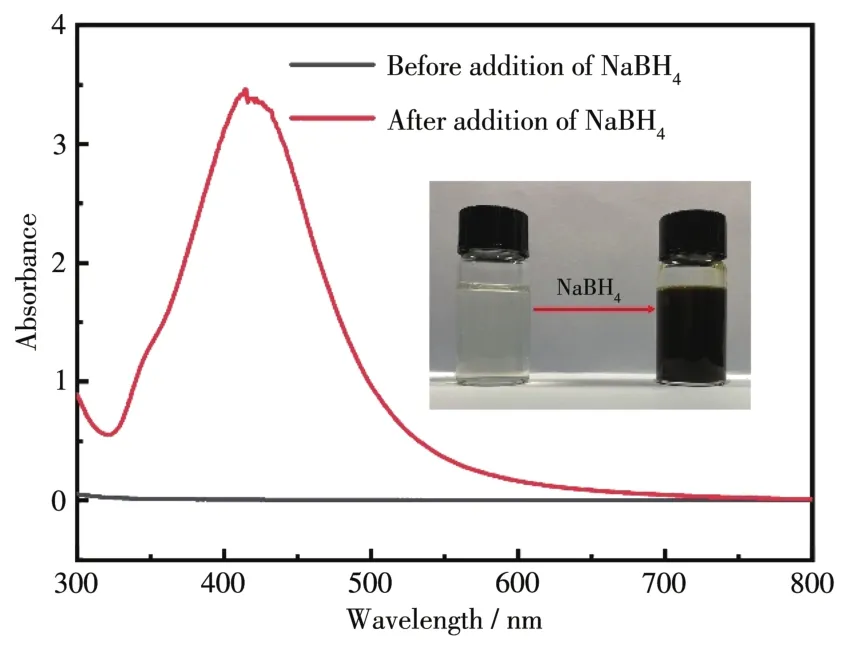
Fig.1 UV?Vis spectra of Ag NPs stabilized with chitosan before and after the addition of NaBH4

Fig.2 TEM image(a)and size distribution(b)of Ag NPs in chitosan solution
SEM was used to reveal the distributions and structures of Ag/chitosan,Ag/CSs,and Ag/AMCSs.As shown in Fig.3a and 3d,Ag/chitosan obtained from the spray drying process has a spherical creased morpholo?gy,which may be due to the inhomogeneous shrinkage caused by the momentary spray drying process.After pyrolysis,the spherical structures of the composites were retained,but the sizes of the spheres decreased significantly owing to the shrinkage of the chitosan framework during carbonization(Fig.3b and 3e).In addition,as revealed in Fig.3c and 3f,considerable amounts of Ag NPs were exposed after KOH activation.Fig.4 displays a representative SEM image(Inset in Fig.4a)and the corresponding EDS mappings of C(36.69%,mass fraction),N(15.33%,mass fraction),O(43.03%,mass fraction),and Ag(4.95%,mass fraction)(Fig.4b?4e)elements in Ag/AMCSs,further indicating that Ag/AMCSs are formed with relatively homoge?neous distributions of these four elements.

Fig.3 SEM images of Ag/chitosan(a,d),Ag/CSs(b,e),and Ag/AMCSs(c,f)
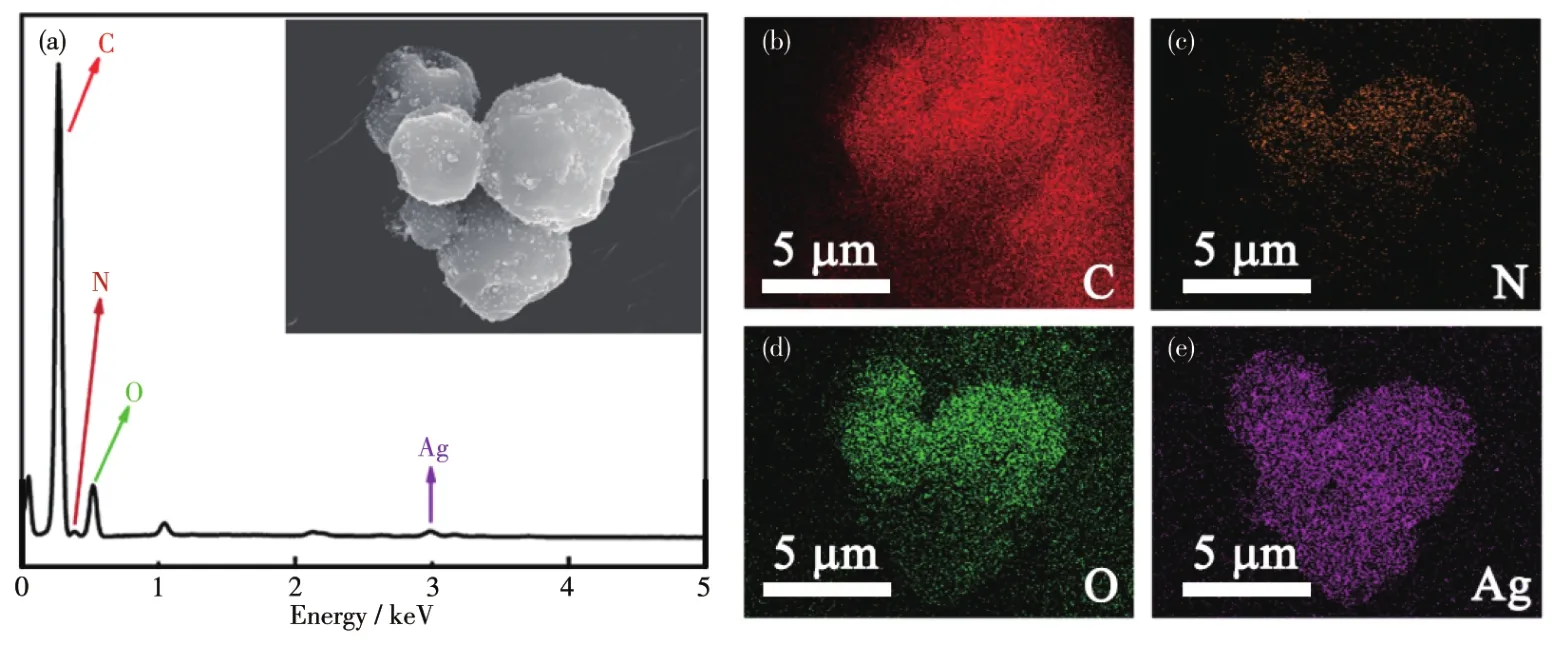
Fig.4 EDS spectrum(a)and corresponding mappings of C,N,O,and Ag elements(b?e)in Ag/AMCSs
Thermogravimetric analysis(TGA)was performed to study mass loss during the carbonization process and the thermal stability of carbonized and activated materials.TG curves of Ag/chitosan,Ag/CSs,and Ag/AMCSs are shown in Fig.5a.For Ag/chitosan,the ini?tial mass loss was due to the release of absorbed water[41].After the dehydration process,10% of the mass was lost in the temperature range of 100?200 ℃owing to the removal of surface hydroxyl groups,and there was a mass loss of 37% in the temperature range of 200?400 ℃ due to the decomposition of chitosan[42].The final weight was lost(400?800 ℃)as a result of the decomposition of residual organic compounds in chito?san spheres[43?44].In contrast,Ag/CSs and Ag/AMCSs displayed good thermal stability at high temperatures.
The XRD patterns for Ag/chitosan,Ag/CSs,and Ag/AMCSs are shown in Fig.5b.Peaks located at 2θ=38.29°,44.58°,64.64°,77.35°,and 81.63°correspond to the(111),(200),(220),(311),and(222)planes of Ag crystals[45?46],respectively,proving the existence of Ag crystals with face?centered cubic structures in all three composites.In addition,the XRD pattern of Ag/chito?san not only shows the diffraction peaks for Ag crystals but also shows peaks for Ag2O at 32.58°(110),38.29°(111),and 54.9°(200)due to the relatively long spray drying time and oxidation in the air[47].
Fig.5 TG curves(a)and XRD patterns(b)for Ag/chitosan,Ag/CSs,and Ag/AMCSs
Spherical Ag NPs are uniformly dispersed in the grated Ag/AMCs(Fig.6a).The representative TEM image(Inset in Fig.6a)shows that Ag NPs had good morphology,and the average particle size was about 13.7 nm,which is consistent with the histogram(13±5)nm in Fig.6b.Compared with Ag NPs in chitosan solution before pyrolysis,Ag NPs are agglomerated,and the particle size increased about 10 nm,which is mainly due to the fusion during the process of high?temperature pyrolysis.
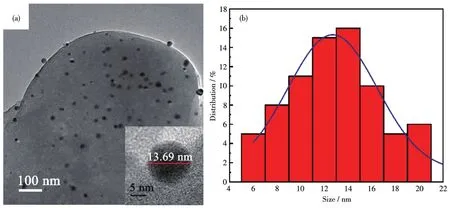
Fig.6 TEM images(a)and size distribution(b)of Ag NPs in Ag/AMCSs
N2sorption experiments were carried out to ana?lyze the specific surface areas and pore size distribu?tions of Ag/chitosan,Ag/CSs,and Ag/AMCSs samples.Fig.7a and 7b show that Ag/chitosan and Ag/CSs exhib?it type Ⅳ isotherms,and there are H3 hysteresis loops in their isotherms.The types of isotherms and hystere?sis loops indicate that both Ag/chitosan and Ag/CSs have mesoporous structures[48].The isotherm of Ag/AMCSs(Fig.6c)shows a typeⅠisotherm typical of microporous materials,indicating that a large propor?tion of dominant micropores were created in the activa?tion process[49].The specific surface areas of the sam?ples calculated by BET,and total pore volumes and pore sizes calculated by BJH are listed in Table 1.Ag/chitosan and Ag/CSs both exhibited small amounts of mesopores with pore sizes distributions centered at approximately 4.52 and 2.60 nm,while Ag/AMCSs had abundant micropores with sizes centered at 1.83 nm,which is more clearly shown in Fig.7d.In addition,the surface area of Ag/AMCSs was 1 276 m2·g?1,which is far beyond those of materials that are not carbonized(10 m2·g?1)and activated(11 m2·g?1).
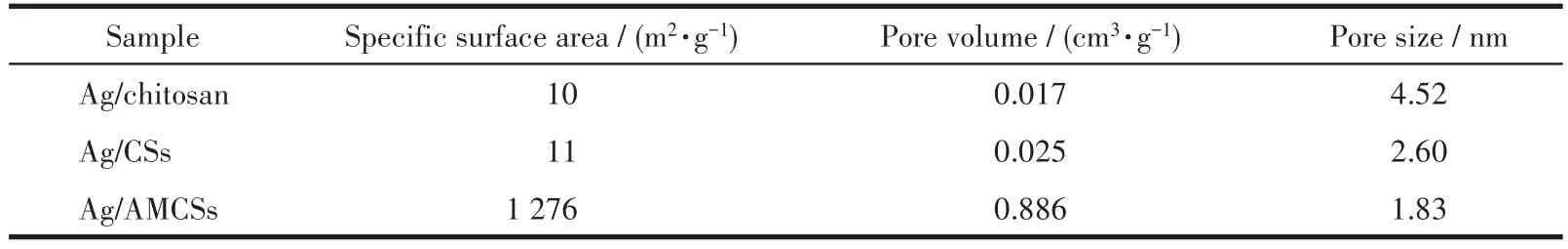
Table 1 N2sorption data for Ag/chitosan,Ag/CSs,and Ag/AMCSs
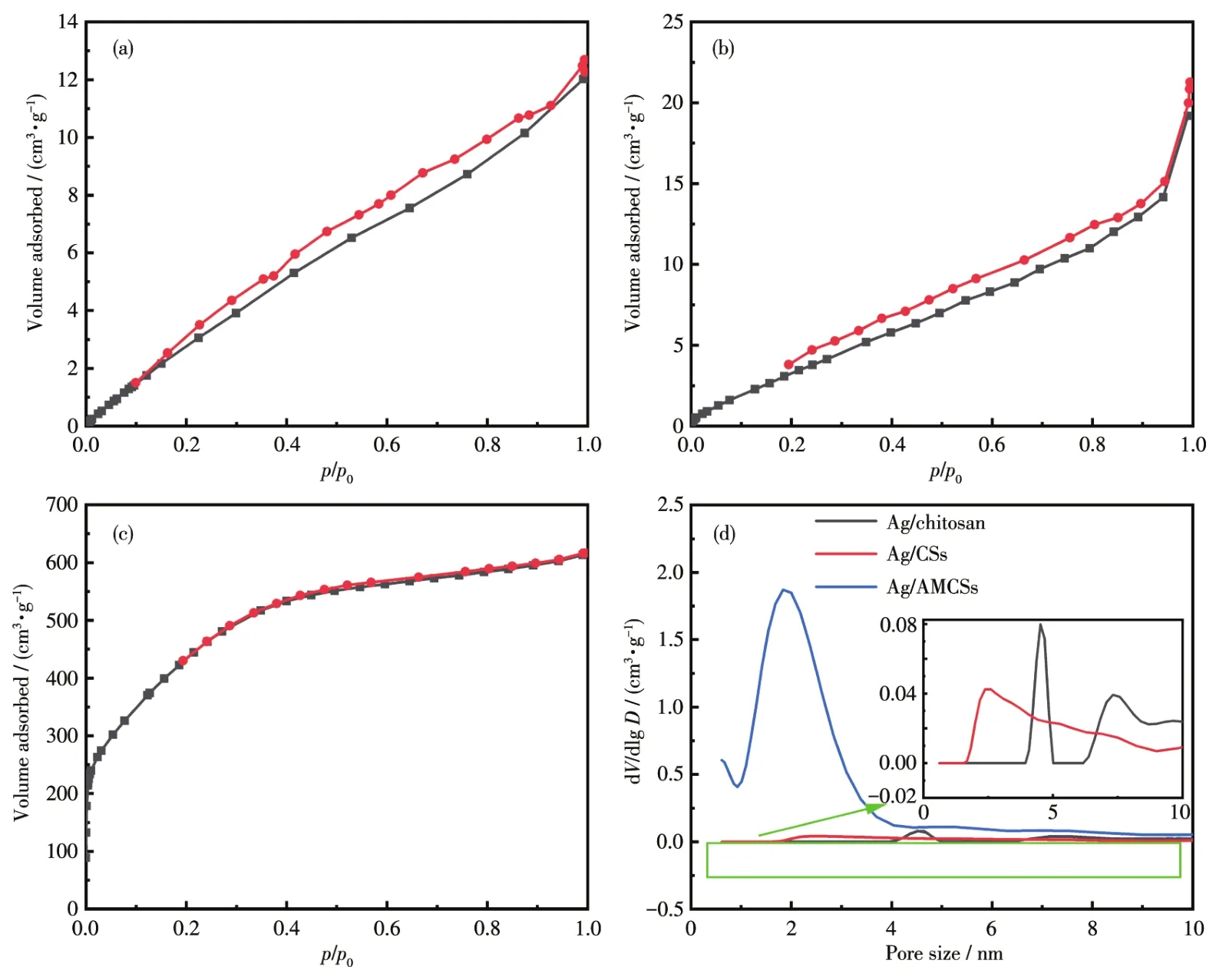
Fig.7 N2adsorption?desorption isotherms of Ag/chitosan(a),Ag/CSs(b),and Ag/AMCSs(c)and pore size distributions determined after different synthetic steps(d)
The binding energies and electronic states of Ag/AMCSs were analyzed by XPS,and the results are dis?played in Fig.8.The typical peaks for C1s,N1s,O1s,and Ag3dionizations were observed in the XPS full survey(Fig.8a).The inset in Fig.8a shows the mass fractions of C,N,O,and Ag in Ag/AMCSs were 70.69%,0.45%,26.76%,and 2.09%,respectively,indicating that Ag/AMCSs has a high carbon content and abundant oxygen?containing groups after KOH activation.In Fig.8b,the peak was split into three peaks occurring at 284.99,286.76,and 289.52 eV,cor?responding to C—C/C=C,C—O,and C=O groups,respectively[50].N1speak,illustrated in Fig.8c,is split into two characteristic peaks at approximately 398.94 and 400.71 eV,and the two peaks can be assigned to pyridinic N and graphitic N,respectively[51].Additional?ly,to evaluate the electronic state of dropped Ag,XPS analysis was performed with the results presented in Fig.8d.XPS spectrum of Ag3dshows the Ag3d5/2and Ag3d3/2located in the range of 367 to 371 eV and 373 to 376 eV,respectively.The binding energy of deconvo?luted peaks after correction for this particular sample is 368.96 eV for Ag3d5/2and 374.98 eV for Ag3d3/2.The peaks at 368.96 and 374.98 eV represent the for?mation of metallic Ag in Ag/AMCS samples.
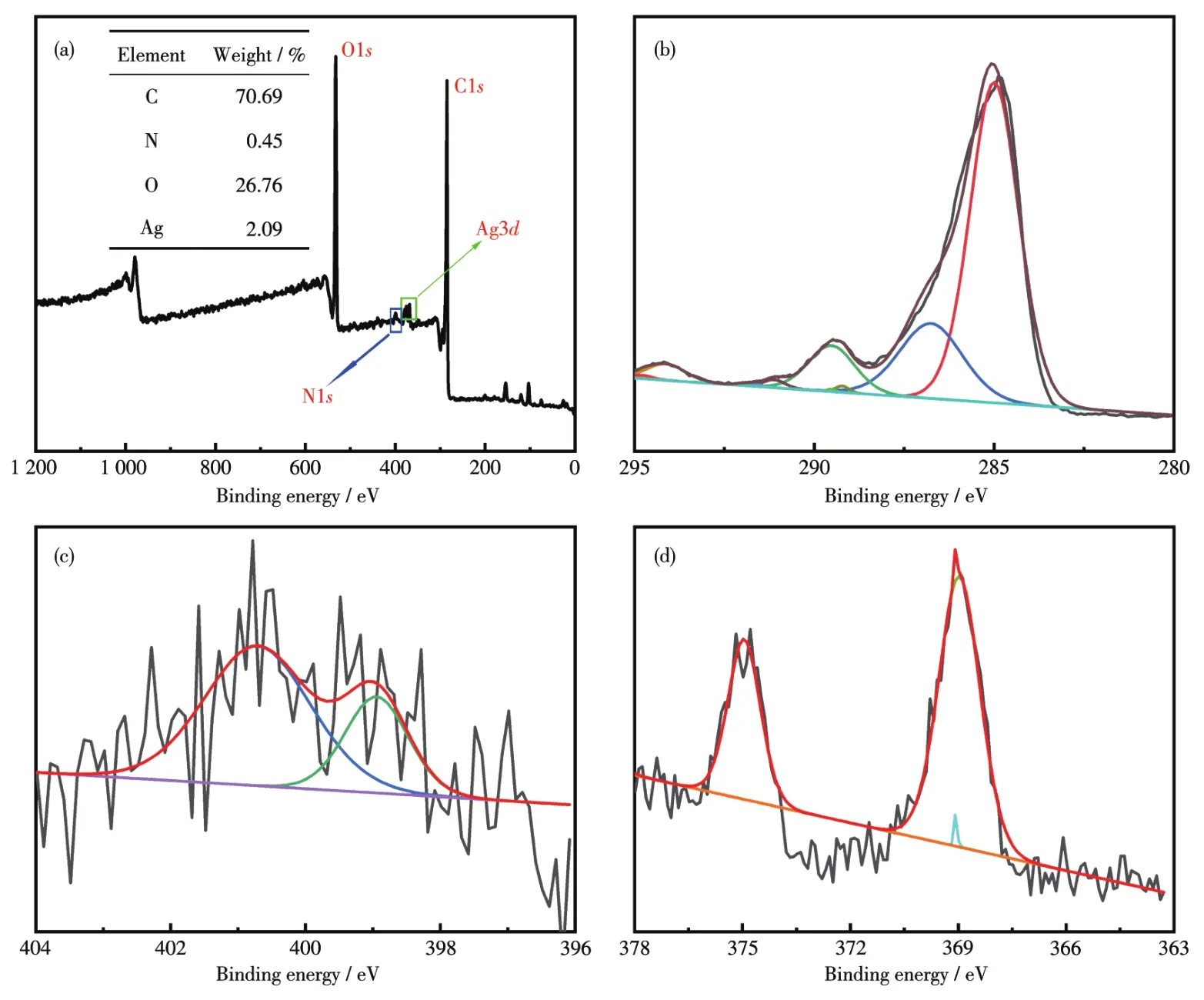
Fig.8 Survey(a)and high?resolution XPS spectra of C1s(b),N1s(c),and Ag3d(d)of Ag/AMCSs
2.2 Adsorption performance
The removal efficiency for the CR solution at dif?ferent initial pH values was investigated,and the adsorption efficiency(Fig.9a)for the CR in solution apparently decreased as the solution pH increased from 4 to 11.However,since the color of the CR solu?tion changed under acidic conditions,a pH of 7 was selected for later studies.In addition,the effect of reac?tion time for the evaluation of equilibrium CR dye adsorption(Fig.S1,Supporting information)and the effect of initial concentrations of CR dye for the evalua?tion of maximum CR removal(Fig.S2)was investigated and discussed in additional discussion of Fig.S1.In the study of adsorption isotherms,Freundlich and Langmuir isotherms were selected to investigate the mechanism of the adsorption reaction[52?55].The corresponding equa?tions and detailed descriptions for the model fitting are given in experimental of Fig.S1,and the plots of Freun?dlich and Langmuir adsorption isotherm are shown in Fig.9b.With the increase of initial CR concentration,the capacities increased until the maximum capacity was achieved.As shown in Table 2,the correlation coefficient(R2)of the Langmuir and Freundlich isotherms were 0.96 and 0.72,respectively.Moreover,the MSE(mean square error)obtained from Langmuir isotherm was smaller than which obtained from Freundlich isotherm.Thus,the adsorption data are consistent with the Langmuir model,and the maximum adsorption capacity(qm)was 445 mg·g?1.
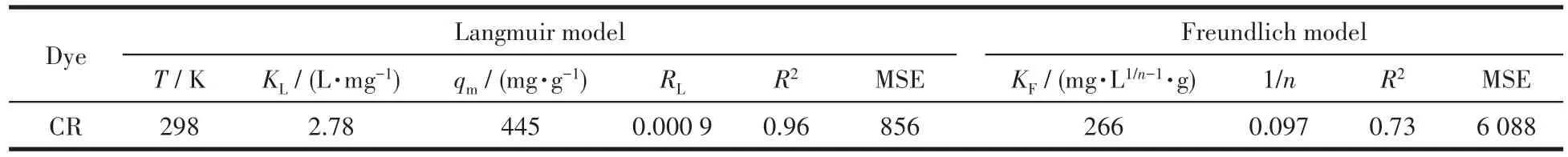
Table 2 Langmuir and Freundlich isotherm parameters
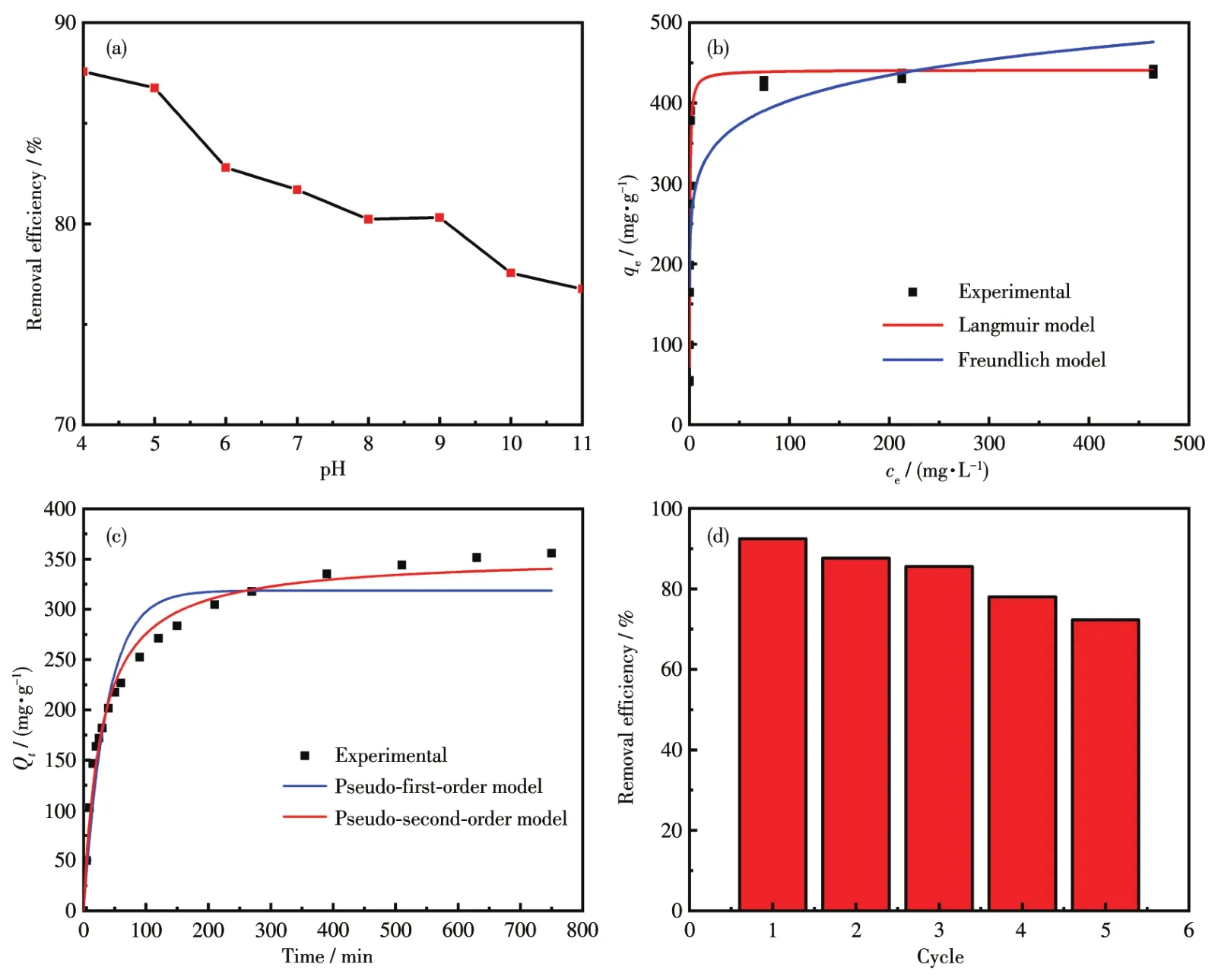
Fig.9 (a)Effect of pH values on the removal of CR solution(absorbent:5 mg,CR:25 mL,T:298 K);(b)Adsorption isotherms of CR on Ag/AMCSs;(c)Kinetic curves for adsorption of CR on Ag/AMCSs;(d)Recyclability of Ag/AMCSs
To further explore the adsorption process of CR on Ag/AMCSs.The adsorption kinetics were measured and the experimental data were fitted and analyzed by pseudo?first?order kinetic model and pseudo?second?order kinetic model[56].
The pseudo?first?order kinetic equation can be described as:

The pseudo?second?order kinetic equation can be described as:

Whereqe(mg·g?1)andqt(mg·g?1)are the adsorption capacities of quinoline on adsorbents at equilibrium state and different contact timet,respectively.k1(min?1)andk2(g·mg?1·min?1)stand for the adsorption rate constants of pseudo?first?order and pseudo?second?order,respectively.
Fig.9c and Table 3 show the pseudo?first?order and pseudo?second?order fitting curves and related parameters of CR adsorption by Ag/AMCSs.TheR2determined with the pseudo?second?order model was higher than the pseudo?first?order model,and the cal?culated adsorption capacity was in good agreement with the experimental adsorption capacity.Thus,CR adsorption closely follows the pseudo?second?order mechanism,showing that the adsorption is of chemical adsorption nature.As shown in Fig.9d,after five cycles of adsorption and desorption, recyclability of Ag/AMCSs remained over 70% demonstrating as?prepared material can be used repeatedly.The compar?ison of adsorption capacities of Ag/AMCSs with other adsorbents for removal of CR is given in Table S1,it is found that the prepared Ag/AMCSs exhibit relatively larger adsorption capacities than most reported adsor?bents.

Table 3 Kinetic parameters for adsorption of CR on Ag/AMCSs
2.3 Catalytic performance
To evaluate the catalytic activity of Ag/AMCSs,degradation of CR was opted as a model reaction.In our previous study[57],it has been proved that NaBH4alone cannot degrade CR,and the adsorption of CR is a relatively slow process;thus,catalytic degradation would have great application potential.For the mixture of CR and NaBH4with the addition of a small amount of Ag/AMCSs,the red color gradually decreased as the reaction proceeded.Fig.10a shows that CR was degrad?ed completely within 17 min by using 0.5 mg of Ag/AMCSs as a catalyst.With the increase of Ag/AMCSs,the degradation time decreased rapidly(Fig.10b).The concentration of NaBH4was much higher than that of CR,and the linear relationship of ln(ct/c0)versus time could be considered a pseudo?first?order reaction.As displayed in Fig.10c,the rate constants,calculated by Equation 4,were 0.086 6 and 0.311 min?1,respectively.As shown in Fig.10d,the degradation efficiencies of CR remained at a very high level,i.e.,more than 95%,and the morphology of Ag/AMCSs remained well(Fig.S3)after 5 treatment periods,so that the prepared cata?lyst of Ag/AMCSs can be used for several cycles.
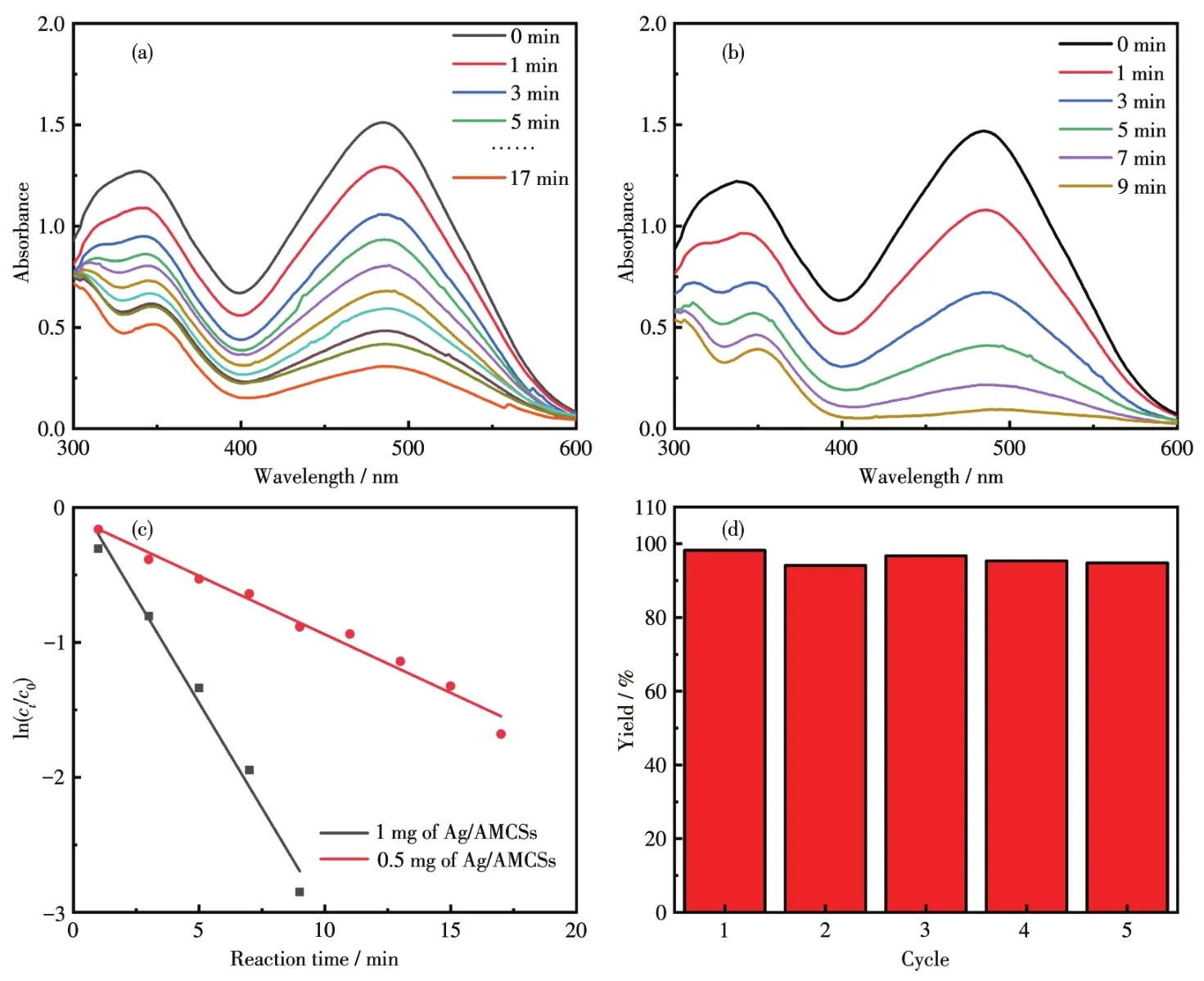
Fig.10 UV?Vis spectra for the reduction of CR in the presence of NaBH4and Ag/AMCSs catalyst(a:0.5 mg;b:1 mg)at room temperature;ln(ct/c0)versus time plots for reactions with different amounts of additives(c);Degradation of CR catalyzed by Ag/AMCSs over five successive cycles(d)
3 Conclusions
In summary,a novel Ag/AMCSs adsorption/cataly?sis bifunctional material was successfully fabricated by the method of spray drying,carbonization,and KOH activation,in which Ag NPs with an average size of 10 nm were evenly dispersed in porous carbon micro?spheres.Ag/AMCSs exhibited an excellent adsorption capacity for CR with the maximum adsorption capacity of 445 mg·g?1,the pseudo?second?order and Langmuir models fit well in the adsorption process.Ag/AMCSs also showed superior catalytic performance for reduc?tion of CR,with thekof 0.311 min?1.Thus,the bifunc?tional Ag/AMCSs hold great potential in practical wastewater treatment.
Acknowledgments:This investigation was supported by the Jiangsu Province Natural Science Foundation(Grant No.BK20130969),the National Natural Science Foundation of China(Grant No.81971675,21603106),and the Jiangsu Key Lab of Biomass?Based Green Fuels and Chemicals.
Supporting information is available at http://www.wjhxxb.cn
- 無機化學(xué)學(xué)報的其它文章
- 《無機化學(xué)學(xué)報》投稿須知(NOTICE TO AUTHORS)
- N4O2?Donor Macrocyclic Schiff Base Ni(Ⅱ) Complex:Synthesis,Crystal Structure,DFT Study and Urease Inhibition Study
- Photocurrent Response Properties of 2,6?Bis(3′?pyridyl)?tetrathiafulvalene Based Zn/Co Coordination Polymer
- A Highly Sensitive and Multi?responsive Zn?MOF Fluorescent Sensor for Detection of Fe3+,2,4,6?Trinitrophenol,and Ornidazole
- Two 3D Microporous Zn?MOF for Fluorescence Sensing of Fe3+,Cr2O7 2-,and Acetone in Aqueous Solution
- Structures,Photoluminescent and Magnetic Properties of Three 2D Lanthanide Complexes

