α-Synuclein as a Diagnostic Marker and Therapeutic Target for Parkinson Disease*
LUAN Ming?Yue,WANG Zhao?Xia,DENG Jian?Wen
(1)Department of Neurology,Peking University First Hospital,Beijing 100034,China;2)Beijing Key Laboratory of Neurovascular Disease Discovery,Beijing 100034,China)
Abstract Parkinson disease (PD) is an insidious and progressive neurodegenerative disease.It is the second most common neurodegenerative disease affecting 1%-3% of the population over the age of 65.While the etiology of PD is complex,α?synuclein(α?syn)misfolding and aggregation is identified as the hallmark of PD.Due to lack of the reliable biomarkers,early diagnosis of this disease is difficult.In this review,we discuss recent research progresses in the development of PD biomarkers based on the detection of α?synuclein from different samples,including body fluids (cerebrospinal fluid,blood,saliva) and peripheral tissues (skin,olfactory mucosa,salivary gland,gut mucosa).Furthermore,we summarize the recent advances on the therapeutic approaches targeting α?synuclein in treating PD.
Key words Parkinson disease,α?synuclein,biomarkers,therapy
Parkinson disease (PD) is one of the most common neurodegenerative diseases.Clinical features of PD include both motor and non?motor symptoms.Rest tremor,bradykinesia,rigidity,gait dysfunction and postural instability are typical motor symptoms.Non?motor symptoms manifest as gastrointestinal and autonomic nervous system abnormalities,as well as neuropsychiatric and cognitive dysfunction[1?3].The global prevalence of PD is estimated around 0.3% of the general population.It affects 1%of people over 60 years of age,and increases sharply with age to 3% at the age of 80 years or older[4?5].However,the diagnostic criteria for PD are still based on the identification of only motor symptoms,which occur years after the neurodegenerative process has begun[6].Compared with clinical diagnosis,body fluid biomarkers have many advantages in confirming the diagnosis.Of note,they are objective indicators,so they are less likely to be affected by the bias which may lead to a misdiagnosis rate of PD up to 20%[7].Studies have used enzyme?linked immunosorbent assay (ELISA),Western blot,mass spectrometry and other techniques to detect total α?synuclein (t?α?syn)and its oligomeric and phosphorylated isomers inbody fluids,such as cerebrospinal fluid(CSF),plasma and saliva[8].
α?Syn is the main component of Lewy bodies[9],which has the propensity to misfold,become undissolved and form β?sheet?rich amyloid aggregates that accumulate and constitute intracellular inclusions[10].Currently,the clinical diagnostic accuracy of PD remains suboptimal and there is no cure or preventive therapy for PD.In view of the role of α?syn in the pathogenesis of PD,it is considered as a promising target for diagnosis and treatment.In this review,we summarize the recent advances in the development of biomarkers and treating approaches related to α?syn.
1 Biological characteristics of α-synuclein
In 1988,a presynaptic protein in the electric organ ofTorpedo californicawas isolated.It is localized to a region of the nucleus as well as the presynaptic,hence the name“synuclein”[11].The three forms of synuclein α?,β?,γ?synuclein are modest size soluble proteins (140,134,and 127 amino acids,respectively)that contain an acidic stretch towards the C?terminus and an absence of cysteines and tryptophans in the full length[12].α?Syn contains a characteristic 11?residue sequence,which is repeated seven times and forms an amphipathic α?helix similar to apolipoproteins upon lipid binding[13].
Human α?syn is mainly expressed by neurons and erythrocytes[14?15]and consists of three different regions[16].The N?terminal domain (amino acids 1-60) of the protein binds lipids and contains all known disease?linked mutations.The central domain is known as non?amyloid component (NAC) (amino acids 61-95) that is relatively hydrophobic and aggregation?prone.The acidic and glutamate?rich C?terminal sequence (amino acids 96-140) that contains most of the phosphorylation sites,especially serine 129,accounts for most interactions with other proteins and small molecules[17?18].α?Syn aggregation and transmission,especially a link to mitochondria and lysosome[19],are actively associated with PD pathogenesis (Figure 1).α?Syn has a natural propensity to aggregate in amyloid structures in a nucleation dependent process[20]that acquires neurotoxic properties during a pathogenetic process in which soluble monomers form oligomers to small protofibrils and eventually large,insoluble α?syn fibrils (that is,Lewy bodies)[21?22].It is in a state of equilibrium between the soluble and the membrane?bound state,and its secondary structure depends on its state[19].So far,the exact physiological function of α?syn remains unclear but its misfolding and aggregation is currently regarded as the main cause of synaptic dysfunction.α?Syn plays a role in regulating neurotransmitter release,synaptic function,and plasticity[23?24].
2 Biomarkers for Parkinson disease
Clinical diagnosis of PD is based on the typical motor symptoms,but clinical overlap among parkinsonian disorders causes difficulties for differential diagnosis,especially at early disease stages[25?26].Delayed diagnosis and misdiagnosis can reduce the therapeutic effect.Hence,there is an urgent need to discover and identify reliable and accurate biomarkers for PD.In addition to α?syn,other studies have also reported PD?related biomarkers,such as neurofilament light chain (NfL),total tau protein (t?tau),phosphorylated tau protein (p?tau),and amyloid?beta 42 (Aβ42),urate,urine kynurenine (KYN),deglycase(DJ?1),small molecule RNAs(microRNAs,miRNAs) and so on,although the evidence of these biomarkers for the diagnosis of PD is still insufficient.Compared with healthy controls (HCs),the diagnostic accuracy of NfL in the CSF and serum of PD patients is poor,and the NfL levels of patients with other cognitive or neurodegenerative diseases are generally higher than those of PD[27].Although the ratio of t?tau/α?syn in Alzheimer’s disease (AD) was significantly higher than that in HCs,which was strongly positively correlated with the rate of tau phosphorylation[28],another study revealed that the levels of t?tau,p?tau,and Aβ42 were not significant changes in PD[29].A large two?sample Mendelian randomization (MR)design evaluated the causal relationship between plasma urate and PD risk,and found no evidence that urate has a linear causal protective effect on PD risk[30].Urine KYN levels were significantly associated with PD severity and mild cognitive impairment[31],but this study is a very preliminary exploratory study with a small sample.DJ?1 exerts its neuronal cell protection through a multi?modal mechanism in response to different cellular stress to prevent oxidative damage[32].Therefore,the potential of DJ?1 in CSF as a PD biomarker has been analyzed,but the results are unclear and conflicting[33?34].A valuable finding in A53T α?syn mice is that there is an interaction between DJ?1 and α?syn,which increases the expression level of A53T α?syn and negatively correlates with the expression level of DJ?1[35].As DJ?1 and α?syn are closely related,DJ?1 may be a potential biomarker for PD.A study performed meta?analyses of miRNAs evaluated in brain,blood,and CSF?derived samples and identified 13 miRNAs that were consistently differentially expressed in the brain or blood of PD patients and controls,but this analysis cannot rule out selective reporting exaggerations some meta?analysis results[36].Future studies need larger sample size to verify the sensitivity and specificity of the above biomarkers.
Since phosphorylated α?syn (p?α?syn) is the hallmark pathological change of PD,many closely related biomarkers have been studied.A growing chorus of studies have used it as a non?invasive diagnostic biomarker.Based on the characteristics of α?syn that is less affected by other factors and easy to detect,this review will focus on the research of α?syn as a PD diagnostic marker.The major isoforms of α?syn have been detected in various biofluids and peripheral tissues,as biomarkers for PD[37].
2.1 Body fluids
2.1.1 α?Syn in the CSF as a biomarker for PD
Different laboratories have found consistent results that t?α?syn levels in CSF significantly decreased in PD patients compared to HCs and other non?PD neurological disease controls(NDCs)[38?42].To improve diagnostic specificity,other α?syn species including oligomeric α?synuclein (o?α?syn) and p?α?syn have also been evaluated as potential biomarkers for PD[43?44].
New methods for the detection of protein aggregates in biological fluids have emerged,such as real?time quaking induced conversion (RT?QuIC) and protein misfolding cyclic amplification (PMCA).Studies have revealed that misfolded p?α?syn possesses prion like aggregation seeding activity[45].RT?QuIC is a novel assay,which is originally developed as a specific and quantitative diagnostic test for prion diseases based on the“prion replication principle”.Misfolded proteins are seeds that serve as template for imparting their conformation to normal isoform[46].Fairfoul and colleagues[47]initially employed RT?QuIC to detect abnormal CSF α?syn in dementia with Lewy bodies (DLB) and PD with a sensitivity of 92%-95%,respectively,with 100%specificity.Another study employed PMCA to detect attomole quantities of o?α?syn and identify 76 PD patients and 97 individuals with other neurologic disorders,which yielded 88.5% sensitivity and 96.9%specificity.Moreover,they observed good correlation between the α?syn?PMCA results and the disease progression[48].The cost effectiveness and practicality of the α?syn RT?QuIC analyses of CSF was enhanced by shortening time to only 1-2 d[49].A recent study has demonstrated α?syn?PMCA can discriminate PD and multiple system atrophy (MSA) by CSF and has found structural differences between α?syn aggregates of them[50].Research also applied RT?QuIC to a large cohort of 439 CSF samples from clinically well?characterized,or post?mortem verified patients with parkinsonism or dementia.They also studied patients with isolated rapid?eye?movement (REM) sleep behavior disorder (IRBD) and pure autonomic failure(PAF) with 95.3% sensitivity and 98% specifcity[51].A longitudinal observational study observed 52 patients with IRBD and 40 HCs that concluded CSF α?synuclein RT?QuIC assay was positive in 47 (90%)patients with IRBD and in 4 (10%) controls,resulting in 90.4% sensitivity and 90.0% specifcity[52].Altogether,studies demonstrate that CSF may be a promising potential biomarker for diagnosis of PD.
2.1.2 α?Syn in the blood as a biomarker for PD
Blood is more accessible and requires a comparatively less invasive procedure to obtain compared to CSF,it would be preferable clinically as a biomarker containing α?syn.Studies have explored α?syn levels in the blood,including serum,plasma and red blood cells(RBCs).
Abundant studies have assessed t?α?syn levels in serum and plasma as a promising biomarker for PD.However,results from different studies are inconsistent and the α?syn levels were either higher,lower,or not significantly different in patients with PD compared with controls[53?60].The easy contamination of RBCs that are a major source of α?syn (>99%) in blood could be a key contributor to the conflicting results[61].Therefore,studies have shifted focus to RBCs and specific forms of α?syn including oligomeric,p?α?syn.A study found in RBCs t? α ?syn showed an increase in PD patients using Western blot[62].Yet in a larger cohort,a more sensitive detection method proved that a reduction in RBCs t?α?syn of PD compared to controls[63].A recent study revealed that total and aggregated α?syn in the membrane fraction of erythrocytes significantly increased and p?α?syn at Ser129 (pS129?α?syn) in cytosolic fraction of erythrocytes was remarkably elevated in PD subjects in comparison to that in HCs[64].Earlier research has shown significantly elevated o?α?syn in the blood plasma of PD patients compared to NDCs.Subsequent studies reported o?α?syn to be increased in plasma[65],serum[66],and RBCs[67],whereas other studies reported no significant differences[68].In a similar study,Fouldset al.[69]measured concentrations of t?,o?,or pS129?α?syn in blood plasma of PD patients compared to HCs.They reported elevated pS129?α?syn levels in PD patients,but no difference in levels of t? or o?α?syn in both groups.Miranda and colleagues[70]also found an increase in glycation,Y39 nitration and Y125 phosphorylation of α?syn.
2.1.3 α?Syn in the saliva as a biomarker for PD
Due to the non?invasive features and easy accessibility of saliva,it has gradually become a hot spot in current research.Many studies reported a lower salivary level of t?α?syn in PD patients than in HCs[71?72]as well as reduced levels of salivary t?α?syn in older patients.However,there was no significant correlation between the t?α?syn concentration and disease severity,suggesting that t?α?syn cannot be qualified as a single biomarker in saliva.Other study confirmed salivary t?α?syn levels decreased in PD patients compared to HCs,whereas salivary o?α?syn levels were higher in PD patients.Consequently,the o?α?syn/t?α?syn ratio was significantly higher in PD patients than in HCs[73].A larger study group drew a same conclusion[74].A cohort study of 25 patients with PD and 15 HCs showed that the total and oligomeric forms of salivary α?syn could be quantified and correlated with disease severity.They also found an increase of the o?α?syn/t?α?syn ratio in PD patients compared to HCs,and a decrease of t?α?syn in salivary samples[75].
A study innovatively detected t?α?syn and o?α?syn in extracellular vesicles obtained from the saliva of 74 PD patients and 60 HCs.They reported an increase in the o?α?syn levels in the extracellular vesicles of PD patients compared to HC subjects and the o?α?syn/t?α?syn ratio in the extracellular vesicles also increased in PD patients[76].Another study quantified the levels of t?α?syn,o?α?syn and the o?α?syn/t?α?syn ratio in the saliva samples of 100 PD patients,80 HCs,and 20 progressive supranuclear palsy (PSP) patients.They displayed a decrease in t?α?syn in the saliva of PD patients compared to HCs and a significant increase of the salivary o?α?syn in PD patients.Consequently,the o?α?syn/t?α?syn ratio increased in PD patients compared to HCs[77].These studies indicate that salivary o?α?syn/t?α?syn ratio,could be a potential biomarker for diagnosing PD and monitoring disease progression.
2.2 Peripheral tissue
2.2.1 α?Syn in the skin as a biomarker for PD
As the largest organ of human body,skin is closely related to pathological α?syn deposition.Stacks of research studies have used skin biopsy as an available diagnostic tool for synucleinopathies that can identify misfolded α?syn in cutaneous nerves.
Since 2014,Donadioet al.[78]investigated whether the skin biopsies could be used to detect p?α?syn deposits in skin nerve fibers,which could represent a useful biomarker for idiopathic Parkinson disease (IPD),and explain the underlying pathogenesis of peripheral neuropathy associated withIPD.P?α?synuclein was found in all patients with IPD in the cervical skin site,while not in control subjects.In their subsequent studies,p?α?syn was detected both in proximal and distal sites of small nerve fibers from DLB patients[79].As for α?syn gene (SNCA) mutation(E46K?SNCA) carriers,researcher found they had p?α?syn aggregates in intraepidermal nerve fibers,and the severity of the skin abnormalities was correlated with sudomotor dysfunction in hands[80].The distribution difference of p?α?syn in cutaneous nerves was proved to distinguish MSA parkinsonism type from PD with orthostatic hypotension[81].Using a proximity ligation assay,a recent study detected o?α?syn in autonomic nerve terminals of skin biopsy samples.They showed that a significantly higher deposition of o?α?syn was observed in 38 PD patients compared to 29 HCs[82].Rodríguez?Leyva and colleagues[83]studied the immunopositivity pattern of p?tau and α?syn in skin of PD,PSP and HCs,indicating that PD group showed significantly higher α?syn and AT8 immunopositivity,while PSP group only expressed higher AT8 immunopositivity than HCs.Other study agreed with the previous experiments and pointed out that immunohistochemical analysis of skin biopsies[84]showed increased t?α?syn staining in PD patients compared to controls.Meanwhile,Kuzkinaet al.[85]reported that there was no significant difference between α?syn deposits in dermal cutaneous nerve fibers and midbrains of PD patients.
RT?QuIC/PMCA,as a novel technology,has been used to detect α?syn in skin.It found that skin α?syn had aggregation seeding activity which was significantly higher in subjects of PD and other synucleinopathies,compared with those with tauopathies and NDCs[86].A blinded study determined the seeding kinetics of α?syn present in the skin sections from autopsied PD and controls indicated that the feasibility of using skin tissues for clinical diagnosis of PD by detecting pathological α?syn[87].
2.2.2 α?Syn in the olfactory mucosa as a biomarker for PD
Olfactory dysfunction in PD is common and takes place prior to the manifestation of the motor symptoms,suggesting α?syn deposition in olfactory nuclei,mucosa or epithelia could relate to impaired sense of smell in PD.In 2009,a study used an antibody against pS129?α?syn to evaluate immunohistochemical analysis of olfactory bulb samples from post?mortem PD patients,with 95%sensitivity and 91% specificity compared to age?matched postmortem non?PD controls,revealing that pS129?α?syn in the olfactory bulb can be a biomarker to diagnose PD[88].However,an ante?mortem study reported similar levels of α?syn expression and distribution across the different groups[89].Remarkably,a recent study found pS129?α?syn staining was in the olfactory epithelium of 75% PD patients but not in HCs[90].RT?QuIC assay was used to assess the ability of olfactory mucosa from clinically confirmed PD and MSA to induce α?syn aggregation,and both of them showed a significant percentage of α?syn aggregation with high efficiency while the final RT?QuIC aggregates of MSA and PD samples owned unique morphological features[91].Recently,Stefani and colleagues[92]further explored RT?QuIC detection of α?syn aggregates in olfactory mucosa among three groups with IRBD compared to PD patients and control subjects.While the sensitivity for isolated RBD plus PD versus controls was 45.2%,specificity was high(89.8%).
2.2.3 α?Syn in the salivary gland as a biomarker for PD
One of the characteristics of PD is sialorrhoea,thus a study conducted salivary gland biopsies of PD patients compared to healthy age?matched controls.It found that elevated t?α?syn in PD rather than in controls[93].Experiments with larger sample sizes came to the same conclusion[94].But another study demonstrated that in the submandibular gland t?α?syn in PD cases was no difference to that in controls while the level of o?α?syn was higher in PD[95].Similarly,pS129?α?syn in all PD patients showed positive staining compared to age?matched controls.Adleret al.[96]and Iranzoet al.[97]detected submandibular gland and labial salivary glands biopsy respectively.They both believed that the deposition of p?α?syn in PD patients was also probably for clinical application.Other studies analyzed pS129?α?syn immunoreactivity in nerve fibres obtained from salivary gland biopsy of PD patients and HCs,which claimed this could be reliably and safely to obtain sufficient glandular parenchyma and neural structures to evaluate the α?syn pathology[98?99].These results suggest that the detection of α?syn especially special species in submandibular gland biopsies may be a useful means of PD diagnosis.Furthermore,biopsy samples of submandibular glands were reported to have higher sensitivity and specificity than minor salivary glands[100].The RT?QuIC technique was also used to quantify pathological α?syn level in submandibular glands of PD patients and HCs with 100%sensitivity and 94%specificity[101].
2.2.4 α?Syn in the gut mucosa as a biomarker for PD
Since Braaket al.[102]proposed that a toxic agent originating in the gut and/or olfactory system accessed the central nervous system (CNS)viathe olfactory and vagus nerves,researchers had put a lot of effort into understanding the role of enteric nervous system in PD through colonic biopsies.A study performed immunohistochemical analysis of PD patients’ ascending and descending colon compared to age?matched HCs,using antibody against pS129?α?syn.It showed that 72% of the PD patients revealed positive staining for pS129?α?syn and LB pathology,while controls were negative[103].Likewise,another study examined pS129?α?syn staining in the descending colon biopsy of PD patients and age?matched HCs displayed that 4 out of 9 of PD patients showing positive staining in the colonic submucosa,while 1/3 patients showed positive staining in the colonic mucosa[104].Depending on the location of the biopsy,ascending and descending colon biopsies were assessed respectively,Lewy neurites were detected in 23%-65% of PD patients but not in controls[105].Researchers also found that a high level of o?α?syn in PD patients as compared to HCs[106].Yet the detection of pathological α?syn conformational variants is considered inadequate for the diagnosis or prediction of PD,in spite of using different methods for ideal specificity and sensitivity[107].Using an antibody against α?syn,a pilot study performed immunohistochemical analysis of the colon biopsy samples obtained from PD patients 2-5 years prior to PD onset,as well as HCs[108].They found 100% PD patients showed positive staining for α?syn,while controls were not observed.However,recent studies have got contradictory results[109].They found that there was no difference in α?syn staining of right and left colon biopsies of idiopathic PD patients and HCs.Similarly,a study observed no significantly difference in α?syn immunoreactivity in gastric and colonic mucosal tissues of PD patients and HCs[110].
3 Comparisons of different biomarkers
The levels of t?α?syn in the CSF of PD patients are relatively consistent and the operation of clinical CSF extraction is very mature.However,the sensitivity and specificity of CSF as a biomarker of PD need to be improved.The challenge of using blood as a biomarker for PD is enormous as RBCs influence the detection of t?α?syn.Differences in the processing and testing of blood in different laboratories also lead to inconsistencies in the detection of specific forms of protein.Saliva is non?invasive and easily accessible compared to CSF and blood,but t?α?syn in it seems to be frivolous.Using o?α?syn in saliva as a PD biomarker may be feasible.As for peripheral tissue,the immunostaining results of skin,olfactory mucosa,salivary gland and gut mucosa fluctuate greatly.pS129?α?syn staining is more specific than t?α?syn staining,but t?α?syn is more sensitive.Since the immunostaining results of these peripheral tissues are not sufficient to diagnose or predict PD,RT?QuIC assay is being developed to increase the sensitivity and specificity of PD diagnosis.RT?QuIC assay on skin and salivary gland shows great specificity and sensitivity.Due to the varying experimental conditions explored by different laboratories,further studies of more stable experimental conditions and more samples are required.The comparison of different biomarkers is as follows(Figure 2).
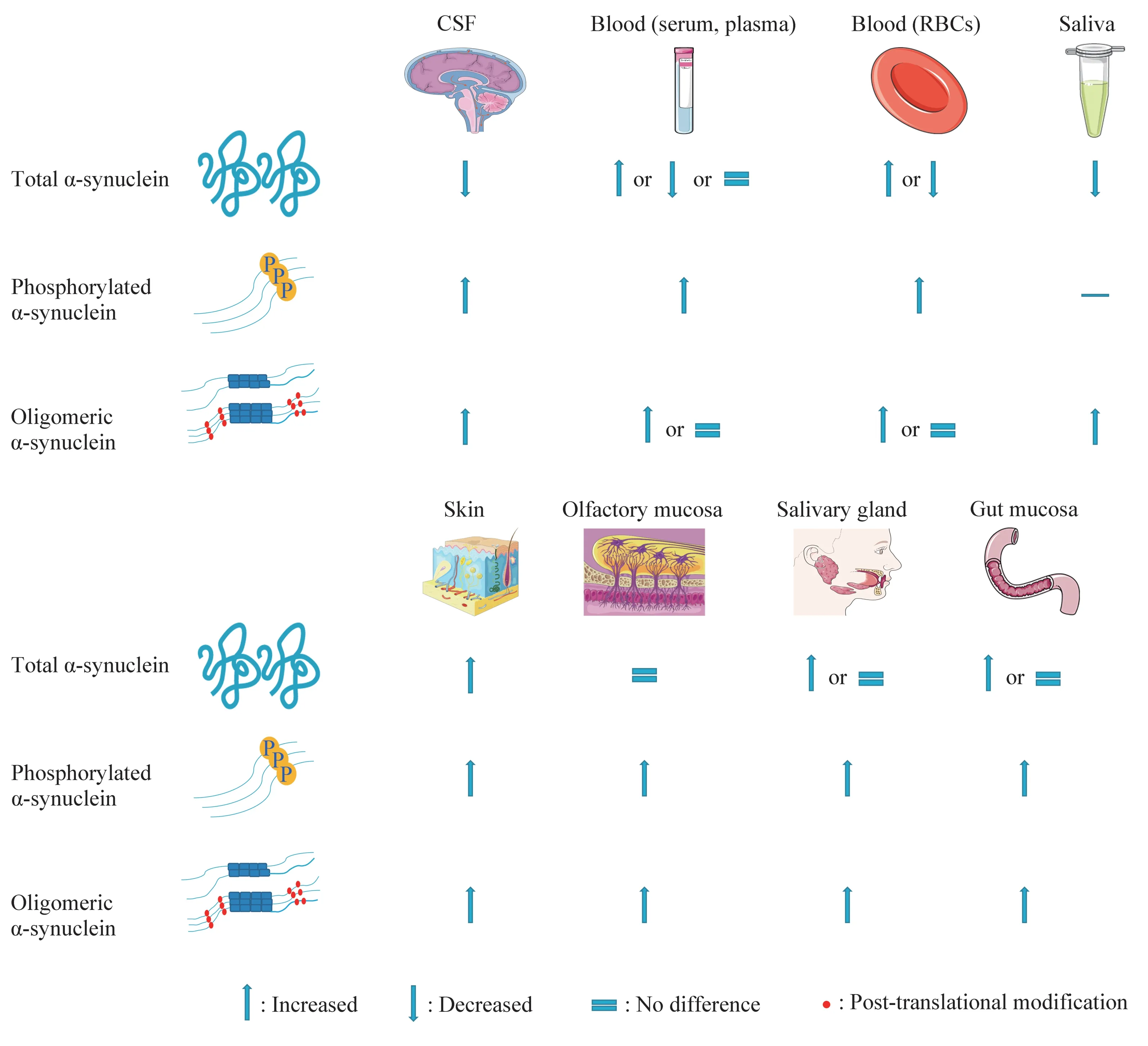
Fig.2 PD biomarkers based on the detection of α-synuclein from different samples compared with healthy controls
4 Therapeutic approaches that target α-syn
As α?syn deposition is the hallmark of PD pathology,therapeutic approaches that target it have been developed.α?Syn accumulation can contribute to a variety of pathogenic factors,such as mitochondrial dysfunction,autophagic dysfunction,oxidative stress,inflammation and so on[111].More and more studies have shown that targeting α?syn by gene therapy as well as adopting immunization therapy may help ameliorate PD symptoms by reducing α?syn production,suppressing α?syn aggregation,promoting the degradation of intracellular α?syn aggregate[111].RNA interference (RNAi) has been used to decrease α?syn production,which reduces α?syn mRNA levels through gene?silencing mechanisms[112].Since various DNA vectors (transcribing short RNA molecules that could enter the RNAi pathway) were developed[113],a study confirmed that delivered short RNA molecules(shRNA)viaa lentiviral vector in rats decreased 35%α?syn without causing toxicity[114].Antisense oligonucleotide (ASO) therapy is another method to reduce α?syn mRNA thereby decreasing α?syn production.A novel version of ASO named amido?bridged nucleic acid ASO (AmNA?ASO) down?regulated the expression ofSNCAmRNA in human cellsin vitroand it was efficiently delivered into the mouse brain and ameliorated neurological defects in a hSNCA?transgenic mouse model[115].To prevent the formation of α?syn aggregation,many small molecules that can cross the blood?brain barrier(BBB) have been reported.They include NPT200?11,NPT100?18A,NPT088,Anle138b,ENT?01[116?120].Recently,iron chelators have become a research hotspot as potential disease modifying agents in PD like deferiprone and PBT434[121?122].
Autophagy is one of the most critical ways to degrade α?syn[123],thus autophagy upregulation may be a possible therapeutic strategy in PD.Upstream signaling pathway mTORC1 plays an important role in autophagy,and reducing pyruvate transport into mitochondria is an ideal method to achieve mTOR inhibition.A novel mTOT modulating insulin sensitizer (MSDC?0160) is proved to cause mTOR inhibition and upregulate the autophagic process in neurons[124].Rapamycin is a mTORC1 inhibitor which has been reported reducing α?syn accumulation in SNCA transgenic mice[125]and relieving motor function in A53T α?syn transgenic mice[126].A 15?month double?blind,placebo?controlled study reveals that nilotinib is safe and tolerated in PD.However,it’s necessary to conduct a larger study to evaluate the efficacy of nilotinib 300 mg in the treatment of PD[127].
The mutations ofGBA(encoding the enzyme glucocerebrosidase (GBA))reduce glucocerebrosidase (GCase) activity in PD brains,which impairs α?syn degradation[128].Studies detected venglustat,a glucosylceramide synthase inhibitor,that delayed α?syn accumulation in GbaD409V/D409Vmice and improved cognitive function after 8 months of treatment[129].Using GBA stimulator LTI?291(NCGC?6078),the lysosomal function was restored to clear α?syn in human midbrain neurons from PD patients[130].In an open?label clinical trial of 17 PD patients,ambroxol was proved to cross the BBB and bind to β?glucocerebrosidase.It increased β?glucocerebrosidase protein levels as well as CSF α?syn levels without substantial deleterious effect in patients with or without GBA gene mutations[131].Leucine?rich repeat kinase 2 (LRRK2) inhibition is another target of PD therapy.A clinical trial has reported that DNL?201 which inhibits LRRK2 is well tolerated and is shown to achieve more than 90%inhibition ofLRRK2kinase activity at peak concentrations in the brain[132].Meanwhile,this group has announced that another LRRK2 inhibitor,DNL?151,is ongoing in a phase 1 dose escalation study in Netherlands[133].
According to the immunogenic epitopes within the C? and N?termini of α?syn[134],active immunization or passive immunotherapy has been tested to neutralize α?syn[135].One of active immunization vaccines is PD01A AFFITOPE(AFFiRiS),which has been reported that 55%patients generated serum antibodies against α?syn[136].Passive immune antibodies are being studied including PRX002[137],MEDI1341[111],BIIB054[138]and so on.Immunotherapy seems to be a promising intervention method.
Furthermore,nanobiology and bionics based on the biological nanostructures will make a difference on PD treatment such as injectable reagents and protein nanocage?based bioimaging[139].
Similar to the treatment strategies of AD,multi?target therapies that direct various aspects of PD pathogenesis just in time,will be a promising approach for slowing the progression of PD patients[140].
5 Conclusion and perspective
Recently,researchers make the point that the burden of neurological disorders (especially non?communicable disorders) is large and increasing,posing a challenge to the sustainability of health systems[141].Many experiments are being performed to explore promising biomarkers.A comprehensive meta?analysis concluded that α?syn decreased in the CSF of both MSA and PD patients,with more reduction in MSA[142].Synthesizing all the experimental conclusions,α?syn in CSF may be a potential and promising biomarker.At the same time,further studies are needed to improve the specificity of CSF biomarkers.After continuous attempts by researchers,many new laboratory testing techniques have been reported.RT?QuIC assay seems to be a highly sensitive and specific method when detecting biomarkers.But it’s difficult to master the best reaction conditions including pH,temperature,ion concentrationetc.Thus,in order to promote this technology,greater standardization is necessary to achieve the consistent results.A single biomarker is not enough to diagnose PD,and the combined application of several biomarkers may be more meaningful.Further studies of the role of α?syn in PD pathogenesis is incredibly important,which will provide fresh perspectives for disease diagnosis and therapy,especially at the early stages of PD.The PD biomarkers selected in the future will be able to achieve early diagnosis,simple operation,and condition prediction.We are confidence that off?the?shelf biomarkers will greatly increase the diagnosis rate of PD.With the improvement of the accuracy of diagnostic technology,the treatment of PD will be developed in the direction of eliminating the cause.Therapeutic research targeting α?syn is a good exploration,and more effective drugs may be discovered soon.

