Development of a rapid GC-FID method to simultaneously determine triethylamine,diisopropylamine,and 1,1,3,3-tetramethylguanidine residues in an active pharmaceutical ingredient
Minshan Shou,Haixiao Qiu
Analytical Research and Development,Abbvie Inc.,North Chicago,IL,60064,USA
Keywords:
GC-FID
Amines
API
Method development
Method qualification
A B S T R A C T
A rapid GC-FID method was developed to simultaneously determine residual levels of triethylamine(TEA),1,1,3,3-tetramethylguanidine(TMG),and diisopropylamine(DIPA)in the synthetic route of an active pharmaceutical ingredient(API).Due to the severe absorption of amines on GC stationary phases,GC columns with various stationary phases were evaluated for optimal peak shape and reproducibility.The final conditions used the Agilent CP-Volamine column to resolve the three amines in 12 min.Various inlet liners were also screened to further improve the sensitivity of the analysis.The Restek Siltek?liner was selected to achieve the desired detectability for the method.The quantitation limits were 4,3,and 4μg/mL for TEA,DIPA,and TMG in the presence of API,respectively.All three amines showed good linearity(r>0.999)and recoveries(>90%)over the concentration range of 3 to 16μg/mL.The testing of residual amines was initially performed at the penultimate stage of the synthesis.However,this work demonstrates that TMG can act as a proton sponge to react with salicylic acid,the counter ion of the penultimate,to form a volatile component that elutes at a different retention time.Consequently,in the final method,these three amines were monitored in the final API to circumvent the matrix interference.Key parameters of the method were qualified per method validation requirements in ICH guidelines.The method was successfully applied for batch testing during development and implemented as an inprocess control procedure at manufacturing sites.
1.Introduction
Volatile amines are widely applied reagents for chemical reactions in the pharmaceutical industry.They are frequently used as additives to alter a solution’s pH,control reaction rate,and improve product yield[1,2].Due to their inherent toxicity[3],volatile amines need to be effectively purged from intermediates or final products to ensure safety of the administered product.Their allowable residual levels are regulated by ICH guidelines[4,5],or are Specifically calculated based on the daily dosage of active pharmaceutical ingredient(API)[6,7]and the permitted daily exposure(PDE)limit for each substance.The purge and determination of residual amines in intermediates or APIs not only reflect the effectiveness of purification procedures but also ensure product quality.Therefore,analytical procedures that can quantitatively monitor residual levels of amines have become a critical component of the process control strategy.
A wide range of methods based on different analytical instruments have been developed to monitor amine residues in various sample matrices[8-10].Among them,gas chromatography(GC)is considered one of the most established techniques[11,12]due to its technical maturity,analysis time,and simultaneous quantitation of multiple analytes.Although GC methods exhibit these features,they frequently encounter sensitivity or reproducibility issues in amine analysis due to the highly polar nature of such analytes.Polar amines often display significant absorption and peak tailing in stationary phases.To overcome the problem,a variety of sample preparations[13-16]have been adopted toalleviate surface adsorption and improve sensitivity.However,the requirements of such unique procedures may limit their applicability.From the perspective of in-process control at manufacturing sites,a user-friendly GC method normally calls for common reagents,straightforward dissolve-and-inject procedures,and the use of standard detectors(e.g.,flame ionization detector(FID)).In order to provide a generally applicable quantitative GC method,development work would require a detailed optimization of stationary phases and instrumental parameters.In addition,the impact of the sample matrix and the selection of control stage are also critical aspects that need a thorough evaluation,since the presence of API or intermediate can introduce interference peaks[17],affect recovery[18],and cause side reactions.
In this study,we described a fast GC-FID method to determine residues of triethylamine(TEA),diisopropylamine(DIPA),and 1,1,3,3-tetramethylguanidine(TMG)(Fig.1)used in the synthetic route of an API.Although multiple generic GC-FID methods[19-21]have been reported for the analysis of amines and solvents in pharmaceutical samples,superbases such as TMG were typically not included as an analyte of interest.Based on our observation,TMG tends to be more severely adsorbed on the column surface than TEA and DIPA due to its stronger basicity,which introduces more obvious peak tailing and sensitivity issues.Therefore,different GC columns,inlet liners,and instrumental parameters were examined in this work to achieve the best sensitivity and reproducibility.The testing of amine residues was initially performed on the penultimate.Experimental evidence revealed that due to its strong basicity,TMG would react with salicylic acid,the counter ion of penultimate,to form a volatile component that eluted at different retention time than the target species.Consequently,the final API step was chosen to monitor amine residues in order to avoid the interference caused by the sample matrix.Key parameters of the method were qualified per Abbvie internal standard operating procedure(SOP)and ICH guidelines.Results indicated that the method is suitable to be applied as an in-process control procedure.The batch testing results demonstrated that these three amines have been effectively purged from the final API.
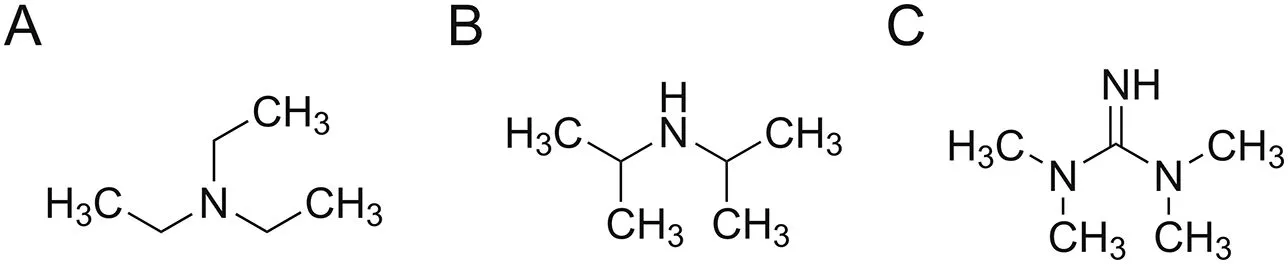
Fig.1.The chemical structures of(A)triethylamine(TEA),(B)diisopropylamine(DIPA),and(C)1,1,3,3-tetramethylguanidine(TMG).
2.Experimental
2.1.Chemicals and reagents
TEA,TMG,DIPA,acetonitrile,methanol,ethanol,isopropanol,N-methyl-2-pyrrolidinone(NMP),and dimethyl sulfoxide(DMSO)were purchased from Sigma-Aldrich(St.Louis,MO,USA)and Fisher Scientific(Milwaukee,WI,USA).The API batches,the penultimate,salicylicacid,and the freebase of the penultimate wereprovided by AbbVie Chemical Pilot Plant(North Chicago,IL,USA)and external contract manufacturing organizations(CMO).
2.2.Columns and chromatographic conditions
GC columns and inlet liners were purchased from Agilent(Santa Clara,CA,USA),Restek(Bellefonte,PA,USA),and Thermo Fisher Scientific(Milwaukee,WI,USA).The chromatographic system consists of an Agilent 7890 GC equipped with a FID detector and an Agilent G4513A auto sampler.Data acquisition and processing were performed on Thermo Scientific Atlas CDS software(Version 8.3).The Agilent CP-Volamine column(30 m ×0.32 mm,5μm film,Catalog#CP7447)and the Restek Siltek?liner(Catalog#20782-213.5)were selected to resolve the target amines.The detailed chromatographic parameters are summarized in Table 1.The injection needle in the GC system was rinsed three times with acetonitrile before and after each injection.
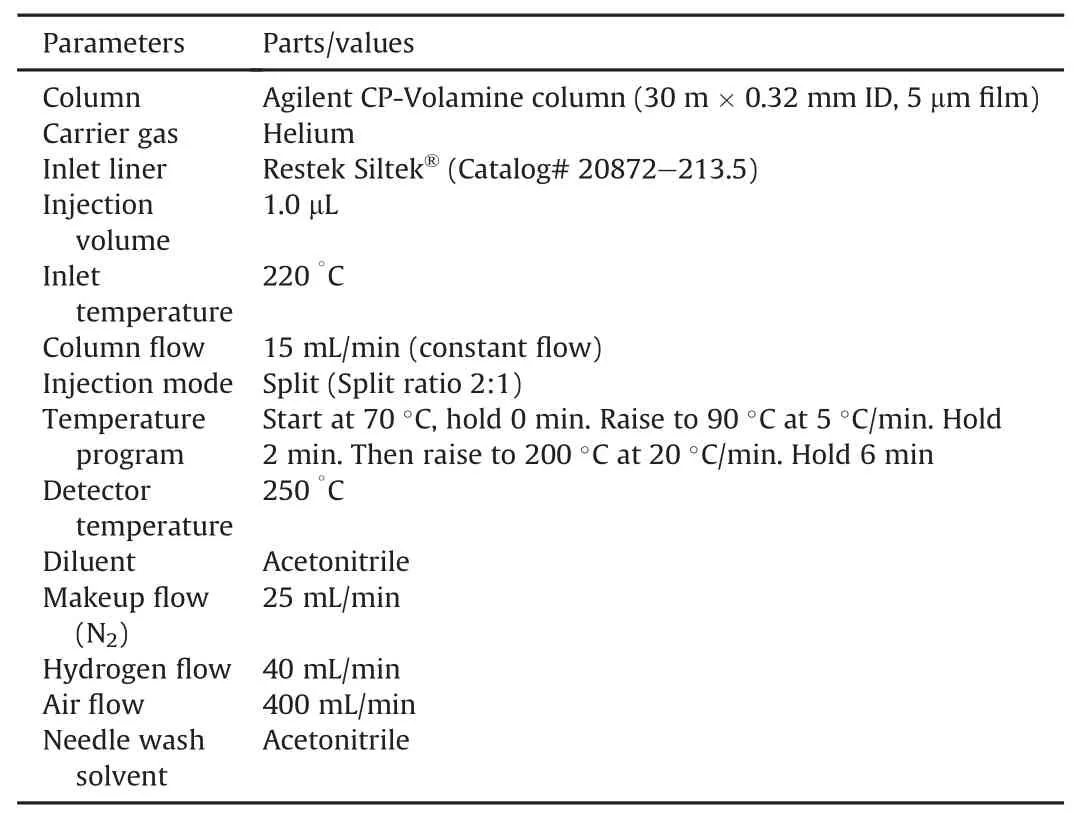
Table 1 The summary of finalized GC conditions and parameters.
2.3.Standard and sample preparations
The amine mixture stock solution was prepared by quantitatively weighing the appropriate amount of TEA,DIPA,and TMG into a 100-mL volumetric flask and diluting with acetonitrile to scale.The standard and linearity solutions were prepared by diluting the stock solution to the desired concentrations(8μg/mL for TEA,6μg/mL for DIPA,and 8μg/mL for TMG in the standard solution).The practical quantitation limit(QL)solution(4μg/mL for TEA,3μg/mL for DIPA,and 4μg/mL for TMG)was prepared by further dilution of the standard solution.The API sample solution(25 mg/mL)was prepared by dissolving an appropriate amount of API with acetonitrile.The standard solution is stable under ambient temperature for at least 5 days.The sample solution has at least 3 days of stability at 2-8°C storage condition.For the method accuracy study,25 mg of API was spiked into 1 mL of standard solution at different concentrations and mixed well.
3.Results and discussion
3.1.Development and optimization of GC conditions
In the API synthetic route,TEA,DIPA,and TMG were the only volatile amines that wereutilized in reactions.They wereapplied as additives in the synthesis of an intermediate prior to production of the penultimate.The reaction mixture of the intermediate step was subsequently taken directly into the penultimate step without further purification.Unreactedamines werenot purged and existed in the reaction mixture at high concentrations.Consequently,the quantitation of their levels is not meaningful at the intermediate stage.Therefore,an appropriate control point to monitor the three target amines would be at either the penultimate or API stage.
The API and penultimate have a higher solubility in acetonitrile than in other solvents examined.Therefore,acetonitrile was selected as the diluent of the method.The acceptance limits were determined to be 180 mg for DIPA,250 mg for TEA and TMG per day based on the dosage and permitted daily exposure(PDE)limit(150 mg/day)of the API.To strictly control the residual amines,the practical QL of the method was set to about one tenth of the values above.Accordingly,each amine should be preparedat 5 to10μg/mL in the practical QL solution after considering the common concentration(50 mg/mL or less)of API in sample solutions.The amine standard solution was prepared at 20 to 30μg/mL and tested on common types of GC columns ranging from nonpolar(dimethyl polysiloxane)to highly polar(polyethylene glycol)stationary phases.From the point of view of chemical structures(Fig.1),TEA and DIPA are common secondary and tertiary amines with pKa values around 11 while TMG,a guanidine related compound,has a pKa at 14-15[22,23].Due to its stronger basicity,TMG tends to be adsorbed on GC columns more severely than the two other amines.Results indicated that the tailing factor of TMG was significant and its signal was poor on all columns.For several stationary phase evaluated,TMG was not detected at 25μg/mL concentration,but TEA and DIPA exhibited sufficient signals at 5μg/mL level.Therefore,amine columns were subsequently examined since they were Specifically designed to analyze basic compounds.The performance evaluations of amine columns are summarized in Table 2.For most of the amine columns that were evaluated,TEA and DIPA showed symmetrical peak shapes and strong signals while the TMG peak eluted much later and still had a noticeable tailing factor.It was observed that the signals of TMG were similar at high concentrations(20μg/mL or higher)in most of the columns.However,as the TMG concentrations decreased,its peak area on some columns diminished drastically,leading to significant concerns regarding the sensitivity and linearity for TMG at these phases.These results suggested the detection of TMG wouldpresentunique challenges at low concentrations.Among the screened columns,the Agilent CPVolamine exhibited the least tailing for TMG.The peak response factor of TMG at low concentration was comparable to that of high concentrations.Although a discernable tailing still existed,TMG peak showed a better day-to-day reproducibility in tailing factor and peak height than when using other columns.

Table 2 The performance summary of amine columns.Standard solutions ranging from 10 to 50μg/mL of each amine were tested in the respective column.Column performance was compared by evaluating the resolution,peak height,and tailing factor of individual amine.
In addition to the GC column,another place where surface adsorption might be of concern is the inner wall of the glass inlet liners.Since the liners contain free silanol groups,these sites have been known to adsorb analytes during the sample vaporization step and affect sensitivity[24].Therefore,liners from different vendors were screened to further improve the sensitivity.Although selected liners had been treated with deactivation steps on both inlet surface and glass wool to minimize adsorption,they did exhibit noticeable variations on signal intensity of amines injected at trace levels(Fig.2).Among them the Restek Siltek?(catalog#20782-213.5)liner was chosen since the three amines consistently yielded the highest response.The detectability of the method with the use of Siltek?liner reached 1-1.5 μg/mL for each amine.Based on those results,the separation and sensitivity of chromatographic conditions were considered acceptable.The next step was to examine the effect of the sample matrix.
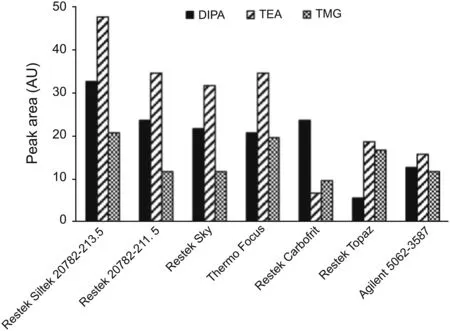
Fig.2.The impact of GC liners on the signal intensity of amines.Different GC liners that have an inert surface and base-deactivated wool were coupled with Agilent CPVolamine column and tested at the nominal GC conditions.The peak areas of TEA,DIPA,and TMG were plotted against different liners to select the best one that yielded the highest signals.
Due to the significant difference of boiling points between the three amines(84°C for DIPA,89°C for TEA,and 162°C for TMG),the column temperature was set to start at 70°C to elute diluent,DIPA,and TEA with a shallow ramp at 5°C/min.TEA and DIPA were baseline resolved and were not interfered with by the peak tailing of acetonitrile.The column temperature was then raised rapidly at 20°C/min to elute TMG.At this temperature gradient,the separation of three amines was achieved within 12 min(Fig.3).
3.2.Control stage and the impact of sample matrix
The penultimate was initially considered as the testing point since it was isolated as a high purity intermediate in the synthetic sequence by forming a salt with salicylic acid.The purified penultimate allowed the residual level of amines to be accurately determined.A solution of the penultimate material at different concentrations was mixed with standard solution to examine the impact of sample matrix.Although no interference components from the penultimate affected the detection of TEA and DIPA,both analytes showed significantly lower signals in comparison tothat of standard solution.These results indicated that the matrix has a noticeable impact on the sensitivity and recovery of both compounds.On the other hand,TMG was not clearly detected due to an interference peak that exhibited the same retention time(Fig.3).Additionally,a new peak that eluted about 2 min after TMG was reproducibly observed in all sample preparations.
As mentioned previously,TMG has a much stronger basicity than TEA and DIPA.It is considered one of the representative guanidine compounds classified as superbases or proton sponges to react rapidly with acidic compounds[22,25].Based on the experimental observation,it is possible that TMG might have formed a new compound by reacting with either the penultimate or salicylic acid,which is the counter ion of penultimate.To confirm the hypothesis,salicylic acid and the free base form of penultimate were dissolved in both neat diluent and the TMG standard solution,and then analyzed using the GC method.The free base form of penultimate did notintroduce interference peaks and the signal of spiked TMG was normal.However,the interference peak that eluted at the same retention time as TMG was observed in the salicylic acid solution(Fig.4).In addition,the signal of spiked TMG in salicylic acid was not observed and the new component peak was consistently seen.The signal of new component correlated very well with the level of the spiked TMG concentration(Fig.4).These results suggested that TMG has been converted to a new component by reacting with either salicylic acid or its impurities,which makes the simultaneous detection of three amines at the penultimate stage impractical.Therefore,the measurement was switched to the final API stage,where this matrix interference is not an issue.The chromatogram of API(Fig.5)illustrates that no sample components interfered with the detection of the three amines.To ensure sufficient sensitivity,the practical QL of standard solution was set at 3-4μg/mL.The API concentration in sample solutions was set at 25 mg/mL,accordingly.
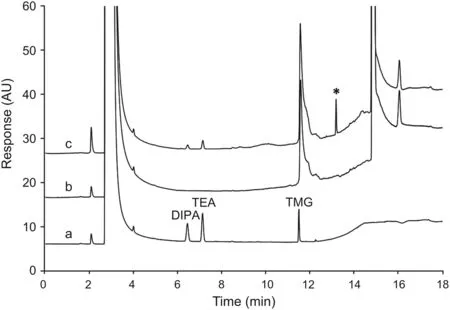
Fig.3.The impact of sample matrix on the detection of amines.Chromatograms of the standard mixture(a),the penultimate(b),and the penultimate spiked with amine standards(c)were overlaid to demonstrate that the detection of TMG is interfered by the sample matrix.An interference peak that had the same retention time as TMG was observed in penultimate.The TMG peak was not clearly detected in the standard spiked penultimate sample solution and a new peak(labelled with the asterisk symbol)was observed.
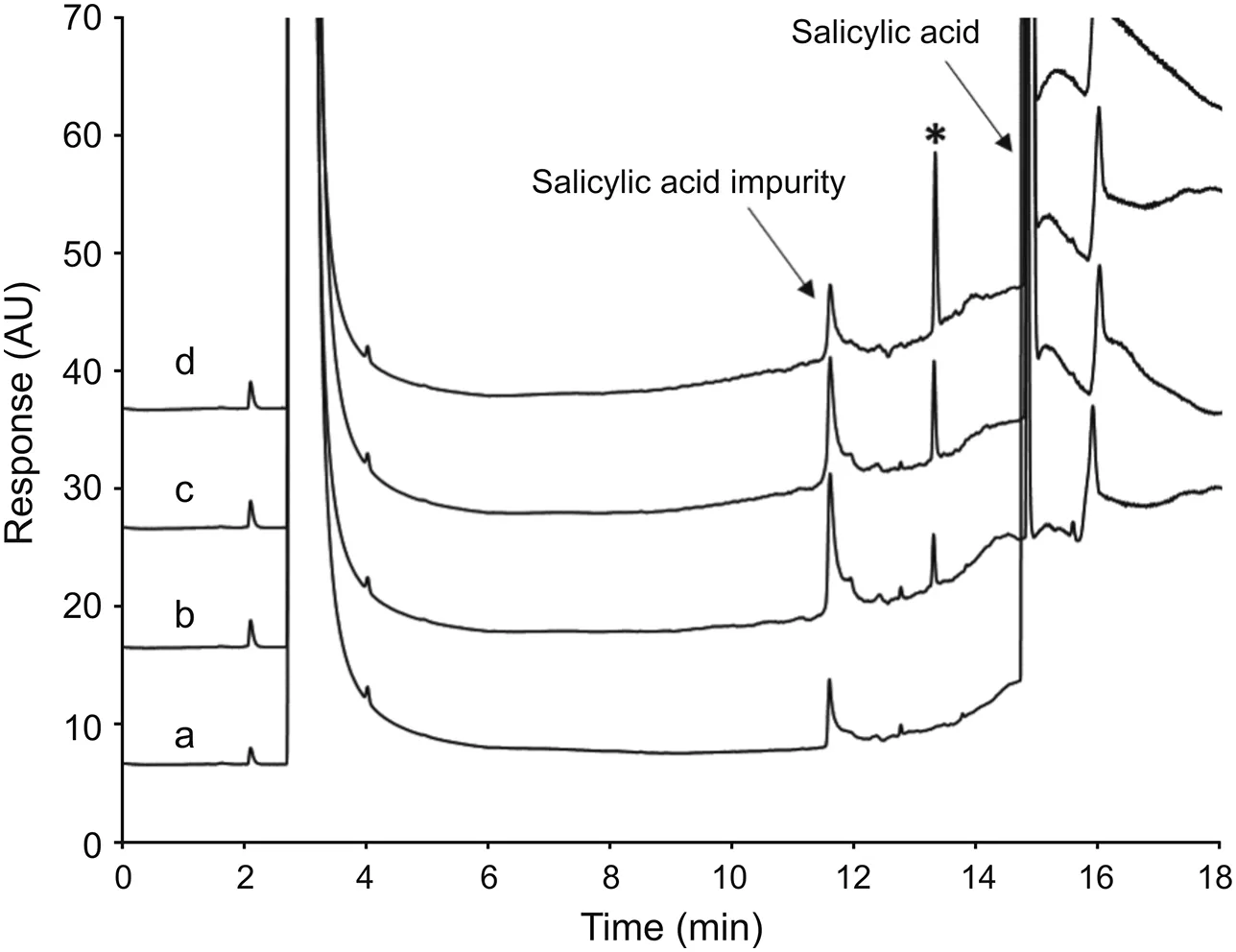
Fig.4.The reaction of TMG and salicylic acid.The chromatogram of salicylic acid(a)revealed that it contains the unknown peak that has the same retention time as TMG.About 5 mg salicylic acid was mixed off-line with 1 mL of TMG solutions at 10μg/mL(b),20 μg/mL(c),and 40 μg/mL(d),respectively.Sample were analyzed within 5 min after mixing.Overlaid chromatograms indicated that spiked TMG was not detected.However,the new peak(labelled with the asterisk symbol)appeared and increased correspondingly with the TMG concentration.The results indicated that TMG has formed a new compound with either salicylic acid or its impurities and eluted at a different retention time.
3.3.Method qualification
The GC method was qualified per requirements of AbbVie internalSOPand ICH method validation guidelinesafterall conditions were finalized.The scope of experiments was to characterize certain key parameters of the method and prove its suitability as an in-process controlprocedure,which allows appropriate adjustmentsofchromatographicconditionson different instruments.
3.3.1.Specificity
The Specificity of the method was evaluated by injecting the diluent,practical QL solution,individual amine standards,working standard solution,API sample solution,and a Specificity mixture(the API sample solution spiked with all three bases at their working concentrations),separately.No interfering peak was detected in the diluent injection at retention time corresponding to that of the individual base compounds.The presence of the API matrix was demonstrated to have no impact on the retention time and peak shapes of analytes.The three amines were well resolved from small unknown peaks(Fig.5)present in the API sample solution.
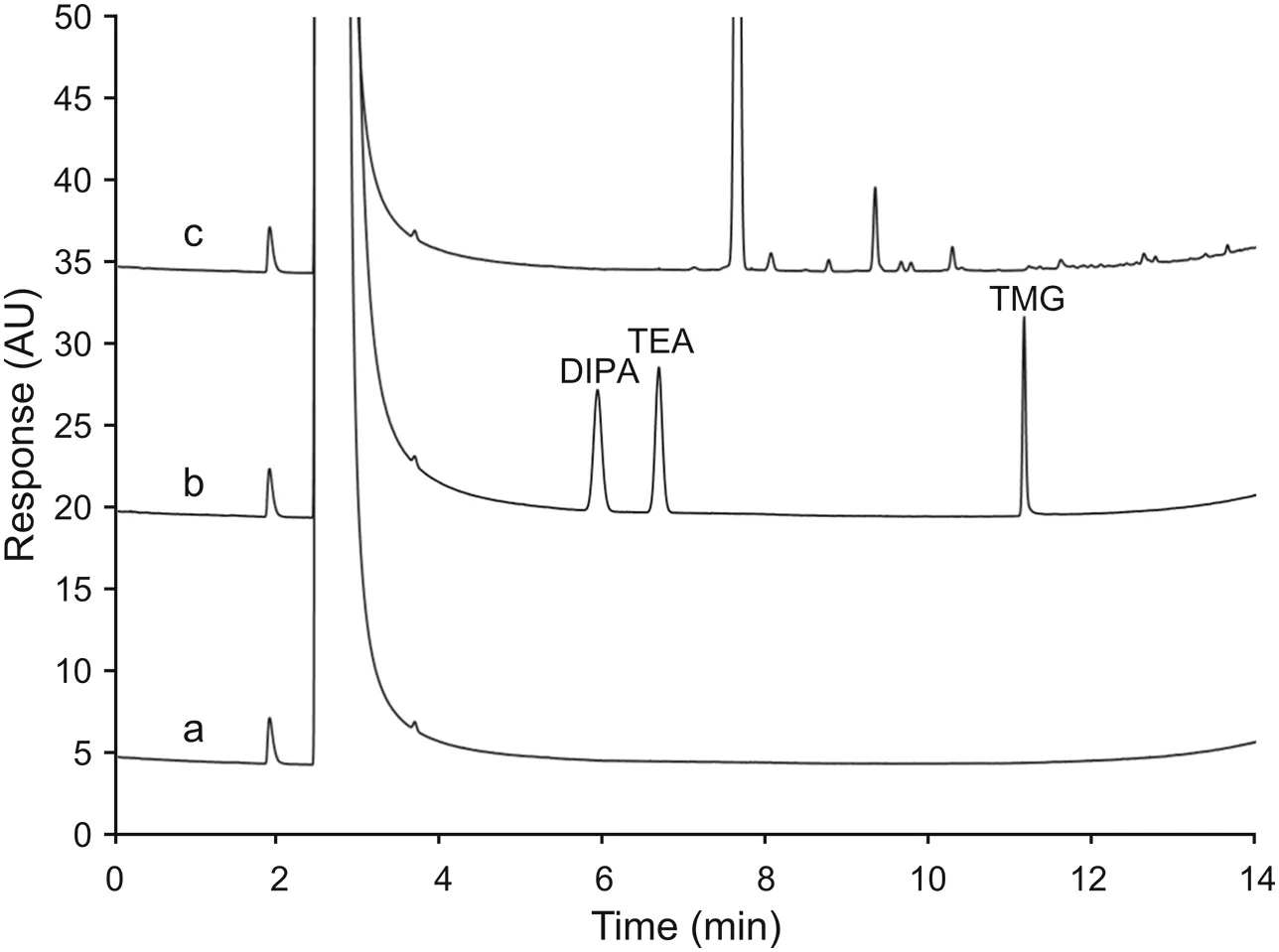
Fig.5.The overlaid chromatograms of diluent(a),amine standard mixture(b),and a representative API batch(c).No background peaks were observed in the Diluent injection.Small unknown peaks in the API sample solution did not interfere the detection of three amines.
3.3.2.Linearity,range,and accuracy
The linearity of the three amines was studied by preparing five concentration levels from practical QL(3-4μg/mL)to 200% of standard solution concentrations(12-16μg/mL).The linearity coefficients were 0.999 for TMG,and 1.000 for TEA and DIPA.Both the y and x-intercepts of the three amines were less than 25% of practical QL peak areas.The accuracy of the method was studied by spiking the mixture at practical QL and working standard level into the API matrix(25 mg/mL).The individual recovery was 89% for TEA,91% for DIPA,and 92% for TMG at practical QL level and 94% for TEA,93% for DIPA,and 95% for TMG at working standard level.
3.3.3.Practical QL,practical detection limit(DL),and precision
The signal/noise(S/N)ratio of practical QL injection of three amines was around 15-20.The practical QL solution was further diluted 2.5-fold to make the practical DL solution,which yielded S/N ratio of 6-10.The precision of the method was evaluated from repeatability(six injections of one practical QL solution)and sample reproducibility(injections of six standard spiked API solutions),respectively.The% RSD of peak areas for three amines ranged from 3% to 7% at the practical QL level and 1% to 4% at the standard level.
3.3.4.Method robustness
Different parameters of the chromatographic conditions were slightly altered to check the robustness of the method with the use of practical QL,standard,and standard spiked sample solutions.Results from altered parameters were compared to those of nominal conditions.Small alternations on injection volume(±0.1 μL),carrier gas flow rate(±1.0 mL/min),oven temperature(±2°C),and temperature ramp(±2°C/min)did not affect the quantitation of analytes.The retention time of three bases varied less than 0.2 min and the resolution of TEA and DIPA constantly remained at 3.2-3.4.The standard spiked sample solution indicated that bases were still adequately resolved from unknown peaks in sample matrix under moderate variations mentioned above.ThreeCP-Volamine columns from different lots were examined.For each base,the difference of retention time in two columns was less than 0.2 min and no discernable variations on peak shape were observed.The column durability was examined by making continuous injections of standard spiked sample solution.Over 300 injections did not deteriorate the column performance in terms of quantitation of the analytes,retention time,resolution,and tailing factor.
3.3.5.Batch testing
Four batches of API manufactured by the AbbVie Pilot Plant and another six batches from an external contract manufacturing organization were assessed under the nominal chromatographic conditions.The purposes of testing these ten batches were to 1)compare the API profile to ensure the detection of amines would not be interfered by components from sample matrix,and 2)quantitate amine levels across different manufacturing sites to evaluate the purge efficiency.Chromatograms of all batches exhibited a similar impurity profile.No components in these batches eluted at the retention time of three amines.TEA and DIPA were not detected across all tenbatches.TMG was only identified in one batch with a signal around one third of practical QL injection.These results demonstrated that purification steps in either penultimate or API stage had effectively purged all the three amines.Their residual levels were well below the predetermined PDE values to pass Specifications of the final API.
4.Conclusion
A rapid GC-FID method was developed to simultaneously monitor residual levels of TEA,DIPA,and TMG used in the synthetic route of an API.The method achieved sufficient practical quantitation limits of approximately 3-4μg/mL by optimizing GC columns and inlet liners.The sample preparation step was straightforward and all three amines exhibited good recoveries in the API.To the author’s knowledge,this article represents the first case inwhich TMG is reported as a residual amine and detected at a trace level in a commercial product.The results also highlight the importance of choosing an appropriate testing point,since the signal of analytes can be interfered by the presence of sample matrix.In this case,amines need to be monitored at the API stage due to the reaction of TMG with salicylic acid or its impurity in the penultimate to form a new compound,which elutes at a different retention time.The finding that carboxylic acids can interfere with the detection of strong bases such as TMG will be helpful to other GC method development efforts related to the analysis of volatile amines.This method was successfully qualified per ICH method validation guidelines and proven to be robust.Results demonstrated that the method can serve as a routine procedure in manufacturing environments for in-process-control purpose and it can be further implemented in quality control labs to release API batches.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors acknowledge Dr.Rong Xiang,Dr.Steve Doherty,and Dr.Steve Nowak for their helpful discussion on results of method development.All authors are employees of AbbVie Inc.and may own AbbVie Inc.stock.AbbVie Inc.sponsored and funded the study;contributed to the design;participated in the collection,analysis,and interpretation of data,and in writing,reviewing,and approval of the final publication.
 Journal of Pharmaceutical Analysis2021年2期
Journal of Pharmaceutical Analysis2021年2期
- Journal of Pharmaceutical Analysis的其它文章
- Diversity,novelty,antimicrobial activity,and new antibiotics of cultivable endophytic actinobacteria isolated from psammophytes collected from Taklamakan Desert
- Taxifolin stability:In silico prediction and in vitro degradation with HPLC-UV/UPLC-ESI-MS monitoring
- Neuroprotective effects of Ginkgo biloba dropping pills in Parkinson’s disease
- Antimicrobial activity and mode of action of terpene linalyl anthranilate against carbapenemase-producing Klebsiella pneumoniae
- Herb-drug interaction in the protective effect of Alpinia officinarum against gastric injury induced by indomethacin based on pharmacokinetic,tissue distribution and excretion studies in rats
- Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery
