Herb-drug interaction in the protective effect of Alpinia officinarum against gastric injury induced by indomethacin based on pharmacokinetic,tissue distribution and excretion studies in rats
Xuguang Zhang,Zhenrui Xie,Xun Chen,Junqiang Qiu,Yinfeng Tan,Xiaoliang Li,Hailong Li,Junqing Zhang
Key Laboratory of Tropical Translation Medicine of Ministry of Education;Hainan Provincial Key Laboratory for Research and Development of Tropical Herbs;School of Pharmacy,Hainan Medical University,Haikou,571199,China
Keywords:
Alpinia officinarum Hance
Indomethacin
Herb-drug interaction
Pharmacokinetics
Tissue distribution
Excretion
A B S T R A C T
Alpinia officinarum Hance of the Chinese traditional herb for the treatment of emesis,abdominal pain and diarrhea has been used to counteract gastric disease induced by indomethacin in rats without obvious side effects.However,the role of herb-drug interaction between indomethacin and A.officinarum based on pharmacokinetic,tissue distribution and excretion still remains unknown.In this study,an ultra-fast liquid-tandem mass spectrometry(UFLC-MS/MS)method was developed for simultaneous determination ofindomethacin and itsthree metabolites,O-desmethylindomethacin (ODI),deschlorobenzoylindomethacin(NDI)and indomethacin acyl-β-D-glucuronide(IDAβG)by oral administration of indomethacin solution with and without the ethanolic extract of A.officinarum and applied to comparative pharmacokinetic,tissue distribution and excretion studies.Our results clarified that oral administration of A.officinarum produced significant alterations in the pharmacokinetic parameters of indomethacin.And the pharmacokinetic interaction between indomethacin and A.officinarum reduced the systemic exposure of indomethacin and increased its elimination.Tissue distribution results demonstrated that co-administration of A.Of ficinarum could not reduce the accumulation of indomethacin in the target tissue of the stomach,but could accelerate the excretions of indomethacin and its three metabolites including ODI,NDI and IDAβG in the bile and feces of rats in the excretion study.Therefore,A.Of ficinarum might have a gastrointestinal protective effect through the interaction role with indomethacin based on the pharmacokinetics and excretion in rats.
1.Introduction
Non-steroidal anti-inflammatory drugs(NSAIDs)are widely applied worldwide and used by more than 30 million people every day,accounting forabout 60% of OTC marketshares of the painkiller in the United States[1].However,adverse reactions in the upper digestive tract,such as gastric ulcer,are always accompanied by therapeutic effects with markedly health risks,and thus the use of NSAIDs is frequently curtailed[2-4].Indomethacin,one of the most effective NSAIDs,is applied tothe treatment of reducing fever,pain and swelling but is also harmful to the gastrointestinal tract[5-7].Its plasma drug concentration and bioavailability are high and oral absorption is fast;however,cumulative medicinal doses and high plasma drug concentrations would also boost the gastrointestinal side effects of NSAIDs[8-10].Therefore,how to alleviate or eliminate the gastric injury caused by NSAIDs is a huge challenge we are facing.
So far,concomitant use of synthetic drugs,such as H2 receptor antagonists,mucosal protective agents and protonpump inhibitors,has been widely employed for relieving side effects of gastrointestinal injuries induced by NSAIDs,thereby reducing mortalityand morbidity rates[11-13].However,theyare not completelyeffective and these allopathic medicines may cause undesirable side effects.It has been reported that these agents may exacerbate NSAID-induced small intestine injury by dysbiosis and further research is needed to prove their safety and effectiveness[14,15].Safety and effectiveness concerns related to NSAIDs have prompted the development of plant-derived drugs.Traditional Chinese medicine from natural products of botanic origin is a promising therapeutic resource with fewer adverse effects in the treatment of the gastrointestinal damage[16-18].
Alpinia officinarum Hance of the Zingiberaceae family is a Chinese traditional herb that mainlygrows in the south of China.There is a long history of its use for counteracting emesis,abdominal pain and diarrhea without obvious side effects in Asia.Therefore,A.officinarum has been considered for the treatment of NSAIDsinduced gastric diseases[19-22].Furthermore,our previous studies have preliminarily demonstrated the antiulcer effect of A.officinarum extract in an ethanol-induced gastrointestinal injury rat modeland pharmacologicalmechanisms in treating indomethacin-induced gastric injury through cyclooxygenase and non-cyclooxygenase pathways[23,24].However,it is also plausible that the effect of reducing the gastric injury could be due to decreasing system exposure of indomethacin and increasing the excretion of indomethacin and its metabolites after oral administration of A.officinarum extract to rats.Researches on antiulcer effects and action mechanisms of A.officinarum induced by indomethacin in rats were previously conducted using molecular biology methods,and further research in terms of pharmacokinetics is necessary to understand the efficacy and safety of A.officinarum.Hence,comparative pharmacokinetic,tissue distribution and excretion studies of indomethacin and its three metabolites were conducted by the oraladministration of indomethacin solution with and without the ethanolic extract of A.officinarum to rats to clarify the gastrointestinal protective effect of A.officinarum.
2.Experimental
2.1.Chemicals and materials
A.officinarum Hance was collected from Xuwen County,Guangdong Province of China,in August 2016 and identified by Prof.Jianping Tian of Hainan Medical University,China.The plant was deposited at the School of Pharmacy of Hainan Medical University(No.20160716).The ethanol extract of A.officinarum was prepared based on our previous described methods[24].The pulverised rhizomes of A.officinarum(1 kg)wereweighed precisely and extracted 3 times under reflux with 8-fold 80% ethanol for 1 h.The combined ethanol extracts were concentrated to 40% and purified with AB-8 macroporous resin by 80% ethanol.Then,polysaccharides,monosaccharides,and phenolic acids were removed.
Indomethacin(purity 99%,CAS 53-86-1)was purchased from Dalian Meilun Biotechnology Co.,Ltd(China).The indomethacin metabolites of O-desmethyl indomethacin(ODI;purity 99%,CAS 2504-32-7)and N-deschlorodenzoyl indomethacin(NDI;purity 99%,CAS 2882-15-7),indomethacin acyl-β-D-glucuronide(IDAβG;purity 99%,CAS 75523-11-4)and internal standard(IS)of indobufen(purity 99%,CAS 63610-08-2)were supplied by Toronto Research Chemicals(Toronto,Canada).HPLC-grade acetonitrile and formic acid were imported from Swedish Oceanpak Co.(Goteborg,Sweden)and Aladdin Industrial Inc.(Shanghai,China),respectively.Deionized water was prepared using Lab Tower EDI 15 SYSTEM(Thermo Scientific,Langenselbold,Germany).
2.2.Animals
Male Sprague-Dawley (SD)rats (Tianqin Biotechnology,Changsha,China),weighing 220-250 g,were housed under a 12 h light/dark cycle at 25°C and allowed ad libitum access to water and food.All rat experiments were performed and approved by per International Guidelines for Care and Use of Laboratory Animals and the Animal Ethics Committee of Hainan Medical University(reg.no.201506017/HMU).
2.3.LC-MS/MS equipment and conditions
All biological samples were analyzed using a Shimadzu LC-20 AD UFLC system(Shimadzu Corp.,Tokyo,Japan)coupled to an AB-SCIEX API 4000 plus mass spectrometer(Toronto,Canada)with an electrospray ionization(ESI)source.
The ultra-fast liquid-tandem mass spectrometry(UFLC-MS/MS)system and data processing were controlled by AB-SCIEX Analyst software.Prepared biological samples were separated on a Kinetex XB-C18100A column(2.1 mm×50 mm,2.6μm,Phenomenex)with the column temperature of 40°C.The mobile phase system of 0.1‰formic acid aqueous solution(A)and acetonitrile containing 0.1‰formic acid(B)was programmed ona gradient as 0.01-0.50 min,5%B;0.50-0.51min,5%-45%B;0.51-4.00min,45%-60%B;4.00-4.01 min,60%-5%B;and 4.01-5.00 min,5%B,with a flow rate of 0.45 mL/min.
All analytes were measured with optimized multiple reaction monitoring(MRM)in the negative ion mode.The parameters of the ESI(-)mode were selected:curtain gas(CUR)at 50 psi;ion spray voltage at-4.5 kV;nebulizer N2gas(GS1)at 55 psi;and heater N2gas(GS2)at 55 psi at 550°C.The precursor-to-product ion pairs used for MRM of indomethacin,ODI,NDI,IDAβG and IS were m/z 356.0→312.0(DP,-46 V;CE,-12 V),342.1→297.9(DP,-45 V;CE,-12 V),218.0→159.0(DP,-50 V;CE,-19 V),532.2→193.1(DP,-60 V;CE,-20 V),and 294.0→250.1(DP,-40 V;CE,-10 V),respectively,with a scan time of 20 ms for each ion pair.
2.4.Preparation of calibration standards and quality control(QC)samples
Standard stock solutions of indomethacin,ODI,NDI and IDAβG were prepared in methanol at 1.2 mg/mL.The four stock solutions were pooled with the same volume to produce combined standard workingsolutionsatconcentrationsof0.3mg/mLforeverystandard.IS working solution was diluted with methanol to 4μg/mL.All preparedstockandworkingsolutionswerestoredat-20°Cpriortouse.
Calibration samples were freshly prepared by spiking the appropriate working solutions into the blank matrices(plasma/tissue homogenates/bile/urine/feces homogenates)to achieve the appropriate final concentrations for indomethacin and its three metabolites in different biological matrices.The QC sample concentrations were also prepared using the same method and stored at-20°C for whole analysis.
2.5.Sample preparation
A protein precipitation process was followed for extraction of indomethacin and its metabolites from rat samples(plasma/tissues/bile/urine/feces).An aliquot of 50μL plasma diluted 6-and 30-fold step by step using blank rat plasma,respectively,was precipitated with 290μL methanol-acetonitrile solution(1:1,V/V)after the addition of 10μL IS(indobufen,4μg/mL).The plasma diluted 6-fold was used to analyze the ODI and NDI,and 30-fold diluted plasma was analyzed for indomethacin.Then,the mixtures were vortex-mixed for 10 min at 2000 rpm and centrifuged at 13,000 rpm for 10 min.Finally,5μL of the resultant mixtures were applied for UFLC-MS/MS analysis.Tissue samples were homogenized in four parts of ice-cold 0.9% saline solution and then centrifuged at 13000 rpm for 10 min to obtain tissue homogenates.Tissue homogenates were treated by the same method as plasma sample preparation except dilution process.
And an aliquot of 10 μL urine was precipitated with 70 μL methanol-acetonitrile solution(1:1,V/V,including 5% formic acid and 80 ng/mL IS).After urine sample extraction,it was vortexmixed for 10 min at 2000 rpm and centrifuged at 13,000 rpm for 10 min.And then 5μL supernatants were injected into the UFLCMS/MS system to analyze.Besides,10μL of bile samples were mixed with the same volume of 0.5% formic-aqueous solution and then were precipitated with 60μL methanol-acetonitrile solution(1:1,V/V,including 0.5% formic acid and 40 ng/mL IS).After bile samples were extracted,the same treatment methods with urine samples were conducted and used for the bile analysis.Feces samples were homogenized in 0.9% saline and then centrifuged at 13,000 rpm for 15 min to gather the feces homogenates(4°C).The protein precipitation and subsequent experimental methods of feces homogenates were in the same way as those of urine and applied for the feces analysis.
2.6.Drug administration and sample collection
2.6.1.Grouping and drug administration
Seventy-two SD rats wereapplied to4 experiments bycollecting plasma,tissues,bile,urine and feces,respectively,to investigate the effects of A.Of ficinarum on systemic exposure,tissue distribution and excretion of indomethacin.Of them,eighteen rats were randomly divided into 3 groups(model,single-and multiple-dose groups of A.officinarum)with six rats in each group and applied to plasma experiment.The rat gastric ulcer model was induced by indomethacin as described preciously[25].Briefly,indomethacin(20 mg/kg of body weight)was orally administered to rats in the model group after 12 h of fasting.The single-dose group of A.officinarum(S-AOE)was pretreated by oral administration of A.officinarum ethanol extract(0.18 g/kg)once 30 min before receiving indomethacin(20 mg/kg).The multiple-dose group of A.officinarum (M-AOE) was pretreated once daily with A.officinarum extract(0.18 g/kg)for 15 days and then indomethacin at an oral dosage of 20 mg/kg body weight was given to rats 30 min after the end of the experimental period(15 days).And other experiments were divided into 2 groups:model group and M-AOE group,with six rats in each group.
2.6.2.Sample collection
Serial blood samples werewithdrawn from the orbital sinus into heparin-treated tubes under ether anaesthesia before dosing(0 min)and 0.167,0.5,1,1.5,2,3,5,7,9,11 and 24 h after dosing.Next,heparinized blood samples were centrifuged at 3000 rpm for 10 min at room temperature to obtain the plasma fractions.
Tissue experiment was performed by collecting the kidney,stomach,small intestine,large intestine,liver and spleen at 0.5,1,2,4 and 6 h after administration.These tissues were washed by cold 0.9% physiological saline(4°C)and excess water was drained with filter paper.Then tissue samples were accurately weighed and subjected to the processing described in the sample preparation section.The whole procedures for treating rat tissues were carried out on the ice surface.
The rats in bile experiment were fixed on wooden plates and anesthetized with 3% pentobarbital sodium(0.15 mL/100 g,i.p.).Then rats’abdomen was incised,and the common bile duct was cannulated with polyethylene tubing to collect the bile.The bile was collected during 0-1 h,1-2 h,2-3 h,3-4 h,4-5 h,5-6 h and 6-7 h after dosing.In the course of bile collection,1 mL of water was administered via the tail vein to the rats every 2 h.
The rats in urine and feces experiment were kept separate in stainless steel metabolic cages.Urine and feces were collected 0-6 h,6-18 h and 18-28 h after administration.Urine at each time point was centrifuged at 3000 rpm for 10 min and the supernatant was transferred into clean tubes.All biological samples were frozen at-80°C until assayed.
2.7.Method validation
The method was fully validated in compliancewith the U.S.Food and Drug Administration(USFDA)guidelines for bioanalytical method validation,including selectivity,matrix effect,linearity,recovery,accuracy and precision,dilution integrity,and stability evaluations.
2.8.Data processing
Non-compartmental pharmacokinetic parameters of all the analytes in rat biological samples were calculated by DAS 3.0 software package(BioGuider Co.,Shanghai,China).All data obtained from this study were assumed to describe a normal standard distribution and are expressed as the mean±standard deviation(SD),along with relative standard deviation(RSD).Statistical analysis was performed using a one-way analysis of variance.A value of P≤0.05 was deemed to be statistically significant.The cumulative excretion rates of indomethacin in bile and urine were calculated according to the following formula:cumulative excretion rate(%)=cumulative excretion/administered dose×100%.
3.Results and discussion
3.1.Method validation
All tested analytes and IS were well separated with no signi ficant endogenous or other interfering substances under the established UFLC-MS/MS conditions.Typical MS/MS ion spectra of the protonated molecules([M-H]-)of indomethacin,ODI,NDI,IDAβG and IS,as well as their chemical structures are shown in Fig.1.Typical MRM chromatograms of blank liver,blank liver spiked with four analytes and IS,as well as liver samples at 30 min after oral administration of indomethacin are shown in Fig.2.The retention time of indomethacin,ODI,NDI,IDAβG and IS was 2.53,1.92,1.53,1.81 and 1.86 min,respectively.The calibration curves of all analytes were established by an internal standard method and displayed good linearity(R>0.9911)over linearity ranges tested.The calibration curves,correlation coefficients,linearity ranges and LLOQs of four analytes in all matrices were sufficient for experimental studies shown in Table 1.The intra-and inter-day precision and accuracy data of QC samples in all of the matrices ranging from 1.30% to 9.53%,and from 89.98% to 110.80%,respectively,were within acceptable ranges,indicating that the method was reliable and reproducible(see Table S1).The stability for each analyte in all matrices under different storing and processing procedures was assessed by the QC samples.The results are summarized in Table S2.The storage of all samples at room temperature for 4 h(pretreatment)and auto-sampler room for 8 h(post-treatment)were stable and did not alter signal responses of four analytes.Also,three free-thaw cycles did not influence the stabilities of analytes.The extracted biological samples applied to LC-MS/MS analysis were not blow-dried with N2and re-dissolved with solvent due to very low extraction recovery efficiency.The matrix effects and extraction recoveries of four analytes in all matrices ranging from 87.93% to 107.40% and from 89.30% to 108.40% in different levels of QC samples are summarized in Table S3.The results manifested that the sample process method was suitable and endogenous substances had no significant effects on the determination for analytes.The dilution test results indicated that rat plasma samples were diluted 30 times by blank rat plasma without affecting the final concentrations and the accuracy and precision for 6 replicates of diluted samples were within the acceptable range(data not shown).Of note,IDAβG was not detected in plasma during metabolite screening,and therefore,method validation for it in plasma was not performed.
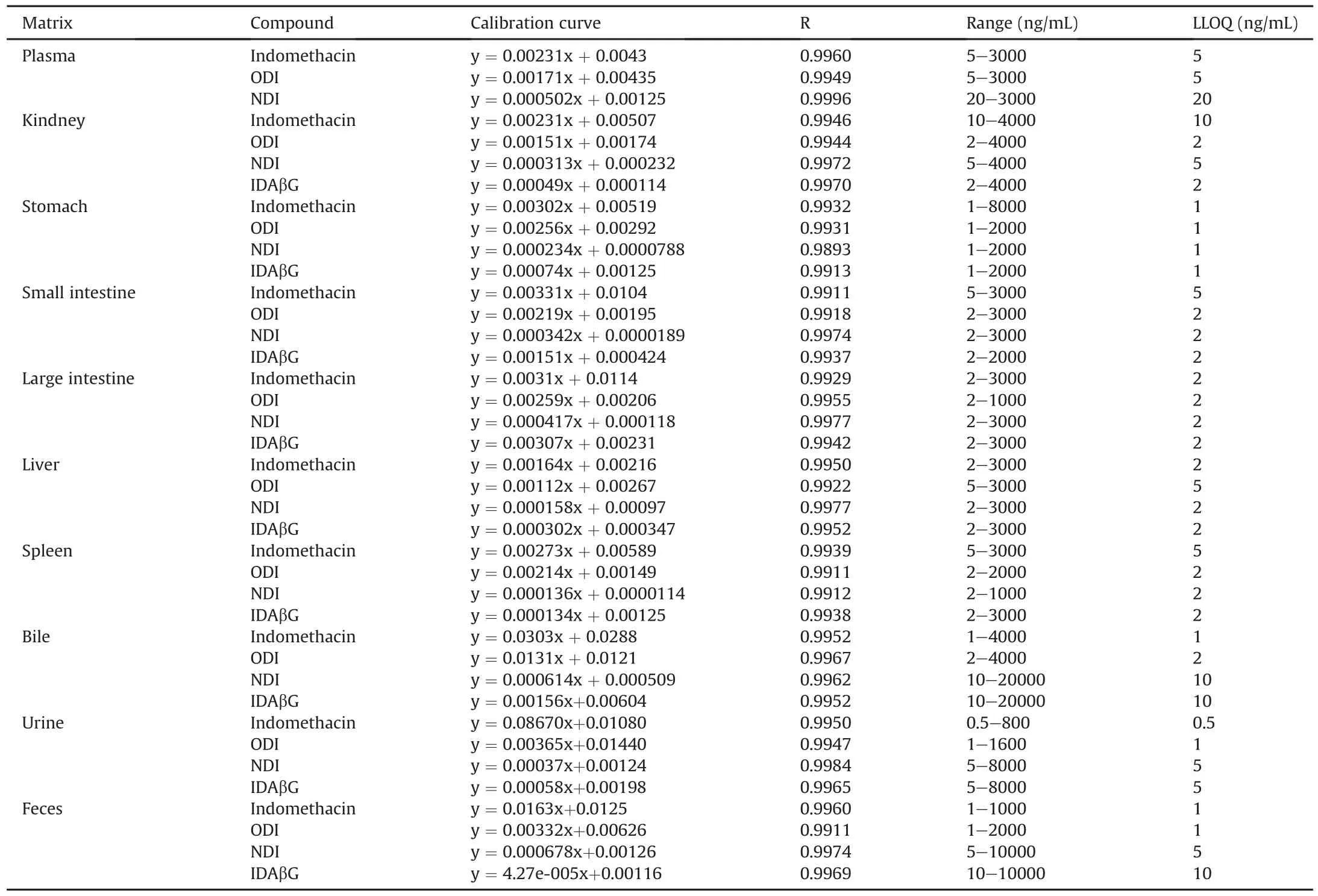
Table 1 Calibration curves and LLOQs of indomethacin and its three metabolites in rat biological samples.
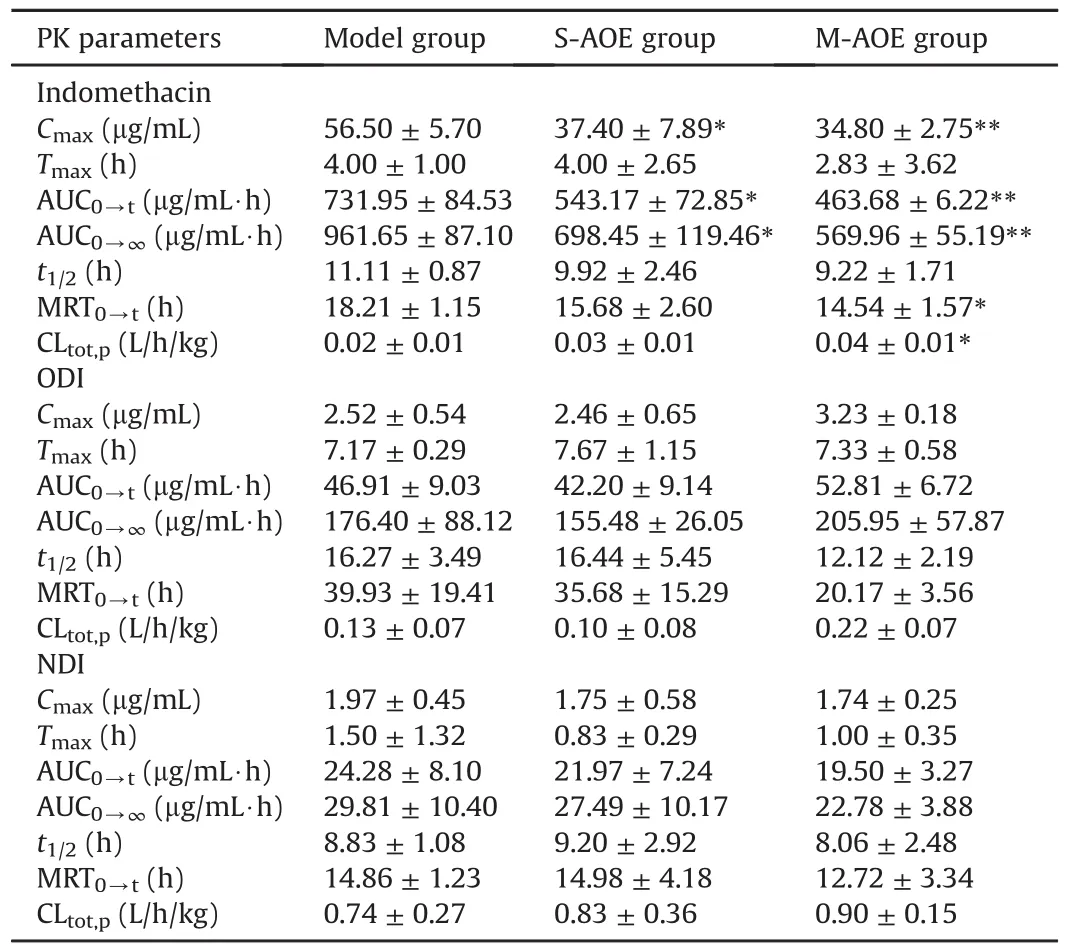
Table 2 Pharmacokinetic(PK)parameters of indomethacin and its two metabolites in the model,S-AOE and M-AOE groups in rat plasma.
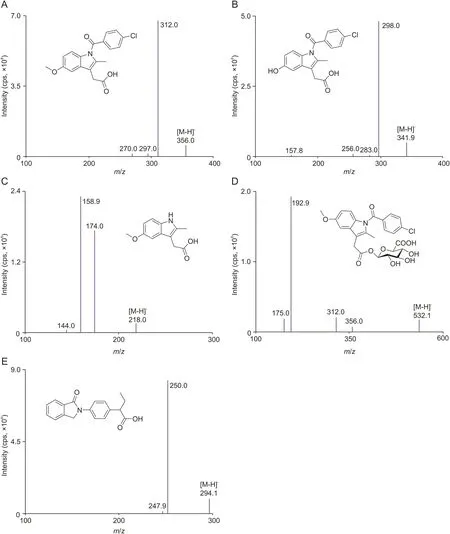
Fig.1.Typical MS/MS ion spectra of the protonated molecules([M-H]-)of(A)indomethacin,(B)ODI,(C)NDI,(D)IDAβG and(E)IS,as well as their chemical structures.
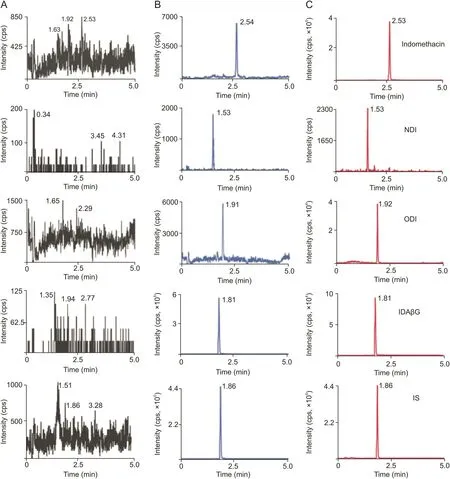
Fig.2.Representative MRM chromatograms of indomethacin,NDI,ODI,IDAβG and IS in rat liver.(A)Blank liver samples,(B)blank liver samples spiked with four analytes and IS,and(C)liver samples at 30 min after oral administration of indomethacin solution(20 mg/kg).
3.2.Pharmacokinetic study
The validated method described hereinwas successfully applied to the pharmacokinetic study of indomethacin and its metabolites in rat plasma after oral administration of indomethacin with and without A.officinarum ethanol extract.Plasma samples were diluted 6-fold for analysis of ODI and NDI and 30-fold for analysis of indomethacin by reason of the high exposure concentration of indomethacin and its metabolites in rat plasma.The plasma concentration-time curves of three analytes in model,S-AOE and M-AOE groups are presented in Fig.3,and main pharmacokinetic parameters are summarized in Table 2.
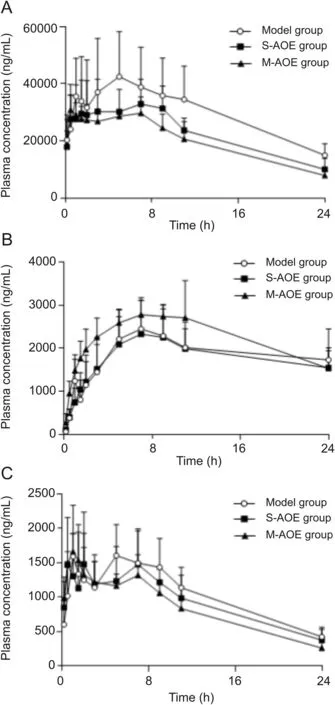
Fig.3.Mean(±SD,n=6)plasma concentration-time profiles of(A)indomethacin,(B)ODI and(C)NDI in the model,S-AOE and M-AOE groups in rat plasma.
Numerous studies have demonstrated that pharmacokinetic parameters,tissue distributions,and excretion could be altered by drug-drug,herb-drugorherb-herb interactions[8,9,26-29].Therefore,the protective effect of A.officinarum on indomethacininduced gastric injury might also be through the changes of pharmacokinetics for indomethacin and its metabolites to exert.In our study,thechanging trends in the plasma concentration-time curves of indomethacin in S-AOE and M-AOE groups were similar to those in model group shown in Fig.3,but significant differences between AOE groups and model group in the pharmacokinetic parameters of indomethacin can be observed in Table 2.Pharmacokinetic parameters including maximum plasma concentration(Cmax),area under the plasma concentration-time curve(AUC0-tand AUC0-∞),and mean residence time(MRT0-t)for indomethacin exhibited remarkable decreases in the S-AOE and M-AOE groups versus model group.And statistically data showed that the decreases in the M-AOE group(**P<0.01)were more significant than those in the S-AOE group(*P<0.05).The results demonstrated that oral administration of A.officinarum ethanolic extract before receiving indomethacin could significantly decrease the systemic exposure of indomethacin and reduce the retention time in plasma,and that multiple dosing was more effective.Moreover,the clearance(CLtot,p)of indomethacin was significantly increased in the M-AOE group versus model group(*P<0.05),while that in S-AOE group was not significantly changed.It suggested that multiple oral administration of A.officinarum before dosing indomethacin could signi ficantly increase the elimination of indomethacin.As for ODI,the values of Cmax,AUC0-tand AUC0-∞in the M-AOE groupwerehigher than those in model group,but no statistical difference existed.And therewerenot significant differences in all pharmacokinetic data of NDI in these experimental groups.In addition,IDAβG,the conjugated metabolite of indomethacin,was undetected in plasma samples but distributed in tissue,which was coincident with the previous report[30].Hence,A.officinarum appeared to be due to a pharmacokineticmechanism involvingin reducingsystemic exposure of indomethacin,retention in plasma and promoting its elimination for gastroprotection in indomethacin-induced rats.
3.3.Tissue distribution study
Since pharmacokinetic study results indicated that the method of multiple dosing was more effective,the tissue distribution and excretion experiments were not performed in a single-dose group.Tissue distribution profiles of indomethacin and its three metabolites in the model and M-AOE groups are depicted in Fig.4.
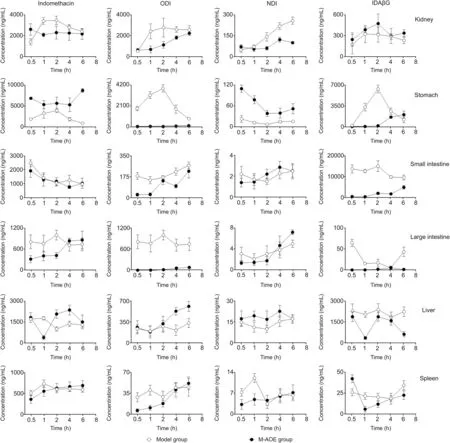
Fig.4.Tissue distribution profiles of indomethacin and its three metabolites in the model and M-AOE groups.
Tissue distribution results suggested that indomethacin was rapidly distributed into various tissues,including the kidney,stomach,small intestine,large intestine,liver and spleen,and mainly metabolized as the conjugated form,IDAβG,which principally existed in the small intestine following in the stomach,liver,kidney,spleen and large intestine in the model group.Interestingly,the concentration of indomethacin was almost unchanged in the small intestine in M-AOE group versus model group but the concentration of IDAβG was significantly decreased,which might be the cause that A.Of ficinarum promoted IDAβG metabolized into low molecular weight components by the gut flora or excreted in the bile.And A.Of ficinarum promoted the accumulation of indomethacin in the liver tissue 2 h after administration in the M-AOE group;thereby it benefited the metabolism of indomethacin by various metabolic enzymes.However,the concentration of indomethacin in the stomach tissue was not reduced in M-AOE group compared with model group but its metabolites of ODI and IDAβG were significantly inhibited,indicating that indomethacin was metabolized less in the stomach and A.Of ficinarum was not by means of reducing the accumulation of indomethacin in stomach to exert gastrointestinal protection.Meanwhile,the concentration of indomethacin in the kindney tissue was decreased after A.officinarum treatment,suggesting that A.Of ficinarum did not act by promoting the excretion pathway of indomethacin in the urine.In addition,the distributions of indomethacin and its metabolites in the large intestine tissue were all very little,and ODI in M-AOE group was significantly decreased,which might be also the effect of degradation of intestinal flora.The data in the spleen tissue were not changed statisticallyand the concentrations of all the analytes were very low.Therefore,the protective effect of A.Of ficinarum on the gastrointestinal tract induced by indomethacin did not occur by reducing the accumulation of indomethacin in the target tissue,but might be through the degradation of the gut flora in the small intestine.
3.4.Excretion study
The cumulative excretion profiles of indomethacin and its three metabolites in bile,urine and feces of rats after oral administration in the model and M-AOE groups are presented in Fig.5.

Fig.5.(A)Biliary,(B)urinary and(C)fecal cumulative excretion profiles of indomethacin and its three metabolites after oral administration of 20 mg/kg to rats in the model and MAOE groups(n=6).
Obviously,all analytes were excreted in the bile after 4 h of oral dosing in two experimental groups shown in Fig.5A and the biliary cumulative excretion of IDAβG was highest compared with the excretions of all the analytes in the bile,urine and feces(52.82% in model group and 75.86% in M-AOE group within 7 h),indicating that parent drug indomethacin was mainly metabolized to the phase II metabolite of IDAβG in the liver and excreted in the bile.Furthermore,we found that biliary excretions of indomethacin and its three metabolites in the M-AOE group were significantly more than that in model group,which suggested that co-administration of A.Of ficinarum contributed to increasing the excretions of indomethacin and its metabolites in the bile.Moreover,the consistent results in Figs.5B and C showed that urinary and fecal cumulative excretions of indomethacin and its three metabolites in M-AOE group were all larger than those in the model group except the excretion of indomethacin and ODI in the urine,which were coincident with tissue distribution results that indomethacin was less distributed in the kidney in M-AOE group versus model group.However,the urinary excretions of indomethacin and its metabolites in both experimental groups were very little(<2%),so that indomethacin was not primarily excreted in the urine.From the above,the excretion study results indicated that indomethacin was principally excreted in the bile in the form of phase II metabolite of IDAβG,and co-administration of A.Of ficinarum could facilitate the excretions of indomethacin and its three metabolites in the bile and feces of rats.Therefore,one of the reasons for the gastrointestinal protection of A.Of ficinarum might promote excretion effects of indomethacin and its metabolites in the excretory system of rats.
4.Conclusions
In summary,this study developed and validated a UFLC-MS/MS method for simultaneous determination of indomethacin and its three metabolites and successfully applied it to comparative pharmacokinetic,tissue distribution and excretion studies to expound protective effect of A.officinarum against gastric injury induced by indomethacin in rats.The results showed that co-administration of A.Of ficinarum produced significant alterations in the pharmacokinetic parameters of indomethacin and reduced the systemic exposure and the retention time of indomethacin,and increased its elimination.However,A.Of ficinarum did not act by reducing the accumulation of indomethacin in the targettissue of the stomach to exert gastrointestinal protection,but by promoting the excretions of indomethacin and its three metabolites including ODI,NDI and IDAβG in the bile and feces of rats,and thereby played a significant role in the gastroprotective effect.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China(No.81560721).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.05.009.
 Journal of Pharmaceutical Analysis2021年2期
Journal of Pharmaceutical Analysis2021年2期
- Journal of Pharmaceutical Analysis的其它文章
- Development of a rapid GC-FID method to simultaneously determine triethylamine,diisopropylamine,and 1,1,3,3-tetramethylguanidine residues in an active pharmaceutical ingredient
- Diversity,novelty,antimicrobial activity,and new antibiotics of cultivable endophytic actinobacteria isolated from psammophytes collected from Taklamakan Desert
- Taxifolin stability:In silico prediction and in vitro degradation with HPLC-UV/UPLC-ESI-MS monitoring
- Neuroprotective effects of Ginkgo biloba dropping pills in Parkinson’s disease
- Antimicrobial activity and mode of action of terpene linalyl anthranilate against carbapenemase-producing Klebsiella pneumoniae
- Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery
