Taxifolin stability:In silico prediction and in vitro degradation with HPLC-UV/UPLC-ESI-MS monitoring
Fernn Cristin Stenger Mour,Crmem Lúi os Sntos Mho,Fvero Reisorfer Pul,Ang′eli Gri Couto,Murizio Rii,Vlir Cehinel-Filho,Tigo J.Bonomini,Louis P.Snjo,Tni Mri Bell′e Bresolin,*
aPharmaceutical Sciences Graduate Program,School of Health Sciences,Course of Pharmacy,Universidade do Vale do Itajaí,Itajaí,SC,88302-202,Brazil
bLaboratory of Research and Development of Drugs,Pharmaceutical Sciences Graduate Program,School of Health Sciences,Course of Pharmacy,Universidade Federal do Pampa,Uruguaiana,RS,97500-970,Brazil
cDepartment of Pharmaceutical Science,Universit`a degli Studi di Perugia,Perugia,PG,06123,Italy
dChemistry Department,Universidade Federal de Santa Catarina,Trindade,Florian′opolis,SC,88040-900,Brazil
Keywords:
Dihydroquercetin
In silico stability prediction
Forced degradation
A B S T R A C T
Taxifolin has a plethora of therapeutic activities and is currently isolated from the stem bark of the tree Larix gmelinni(Dahurian larch).It is a flavonoid of high commercial interest for its use in supplements or in antioxidant-rich functional foods.However,its poor stability and low bioavailability hinder the use of flavonoid in nutritional or pharmaceutical formulations.In this work,taxifolin isolated from the seeds of Mimusops balata,was evaluated by in silico stability prediction studies and in vitro forced degradation studies(acid and alkaline hydrolysis,oxidation,visible/UV radiation,dry/humid heating)monitored by high performance liquid chromatography with ultraviolet detection(HPLC-UV)and ultrahigh performance liquid chromatography-electrospray ionization-mass spectrometry(UPLC-ESI-MS).The in silico stability prediction studies indicated the most susceptible regions in the molecule to nucleophilic and electrophilic attacks,as well as the sites susceptible to oxidation.The in vitro forced degradation tests were in agreement with the in silico stability prediction,indicating that taxifolin is extremely unstable(class 1)under alkaline hydrolysis.In addition,taxifolin thermal degradation was increased by humidity.On the other hand,with respect to photosensitivity,taxifolin can be classified as class 4(stable).Moreover,the alkaline degradation products were characterized by UPLC-ESI-MS/MS as dimers of taxifolin.These results enabled an understanding of the intrinsic lability of taxifolin,contributing to the development of stability-indicating methods,and of appropriate drug release systems,with the aims of preserving its stability and improving its bioavailability.
1.Introduction
Taxifolin,also called dihydroquercetin,was first isolated from Pseudotigusa taxifolia[1].It belongs to the class of dihydroflavonoids,which are present as a yellow pigment in many edible plants.Lavitol?,an enriched extract of the stem bark of Larix gmelinni,contains about 90% taxifolin.This natural product has been marketed and used as a food ingredient in the USA since 2009[2].It has been recently authorized as a novel food ingredient in Europe[3].Taxifolin has been reported in more than 700 articles published in the literature.
Taxifolin(3,5,7,3′,4′-pentahydroxyflavanone or dihydroquercetin)is a potent antioxidant,comparable to alpha-tocopherol,whose mechanism of action consists of lipid radical scavenging[4].Taxifolin presents several pharmacological properties,such as attenuating diabetic nephropathy,reducing sugar,uric acid and creatinine in human blood[5],decreasing the accumulation ofβ-amyloid and preventing memory deficits[6],protecting against oxidative stress[7],promoting osteogenic differentiation in human bone marrow mesenchymal stem cells[8],reducing blood viscosity and dilating the bloodvessels,andreducing arterialhypertension[9].Italsohelps prevent diabetic cardiomyopathy[10]and protects against alcoholic liver steatosis[11].Its in vivo gastroprotective effects were also demonstrated by our research group,with a similar effect to that of omeprazol,inhibiting 41% of the pump effect[12].Due to its relevant pharmacological activities,this potential phytodrug was previously incorporated into gastro adhesive microparticles for the treatmentof gastric disorders,by our research group[13].
However,despite its high clinical application potential,there is no sufficient data on its stability,a key quality statement for a drug.Moreover,there are few stability studies carried out with natural products[14].Previous studies revealed that taxifolin polymerizes when submitted to electrolysis in neutral solutions(pH 7.0)[15].This substance is also considered to be highly slightly soluble in water,and its absolute bioavailability after oral administration of lipid solution was only 36%[16].Some authors argue that the results for absorption profile and the parameters of taxifolin vary quite considerably,suggesting that the bioavailability of the compound depends onthe source[3].This compound also appears tobe degraded by the intestinal microflora[17].Based on the above,the investigation of taxifolin’s stability behavior is an important step in developing a new active pharmaceutical ingredient(API)and in stimulating the development of a potential new drug release system.Moreover,the detection of potential degradation products requires stability-indicating methods[18]and the application of forced degradation tests of the API candidate,in both solid and solution forms.The stress condition should be carefully selected,aiming to generate potential degradation products,which are likely to be formed under realistic storage conditions[19].
Therefore,taxifolin stability was investigated using in silico stability prediction studies and in vitro forced degradation studies,monitoring the formation of degradation products by high performance liquid chromatography with ultraviolet detection(HPLCUV)and ultra performance liquid chromatography coupled to electrospray ionization tandem high resolution mass spectrometry(UPLC-ESI-HRMS/MS).
2.Experimental
2.1.Chemicals and reagents
Methanol and acetonitrile of liquid chromatography with ultraviolet detection(HPLC-UV)and liquid chromatography-mass spectrometry(LC-MS)grade were obtained from PanReac,Castellar del Vall`es,Barcelona,Spain.Phosphoric acid of analytical grade was purchased from Din^amica,Sao Paulo,Brazil.Class 1 water was obtained by ultrapurification(Direct-Q?,Merck KGaA,Darmstadt,Germany).All the solutions were filtered through a regenerated cellulose 0.45 and 0.22 μm membranefilter(Macherey-Nagel,Düren,Germany)prior to injection into HPLC and UPLC,respectively.Taxifolin used in this experiment was isolated from the seed of Mimusops balata(from Itajaí,Santa Catarina,Brazil)with 99.4% purity(estimated by HPLC),and characterized by1H and13C NMR,infrared spectroscopy,HPLC and MS(Supplementary material).
2.2.In silico stability prediction studies
Computational analyses were performed using Spartan 08 version 116.2?for Windows(Wave function,Inc.,Irvine,CA,USA)and all initial structures were constructed using fragments of atoms and structural fragments by the molecular editor.Geometric optimization was carried out using the Merck Molecular Strength Field(MMFF94)followed by the Austin Model.The structure of taxifolin(Fig.1A and B)was subjected to conformational analysis.The increment of the torsion angle was 30°in a range of 0-360°,using systematic analysis,by the functional density theory(DFT)method to B3LYP/6,311G*(d,f).The lowest energy conformer calculated was re-optimized using the same method.This structure was used to determine the number of electrons at natural atomic population analysis(NPA)using single point energy at the same level of theory of geometry optimization.For these data,Fukui function(FF)derivatives values,positive(f-j,Eq.(1))for electrophilic attack,negative(f+j,Eq.(2))for nucleophilic attack and f0j(Eq.(3))for radical attack we calculated as follows:
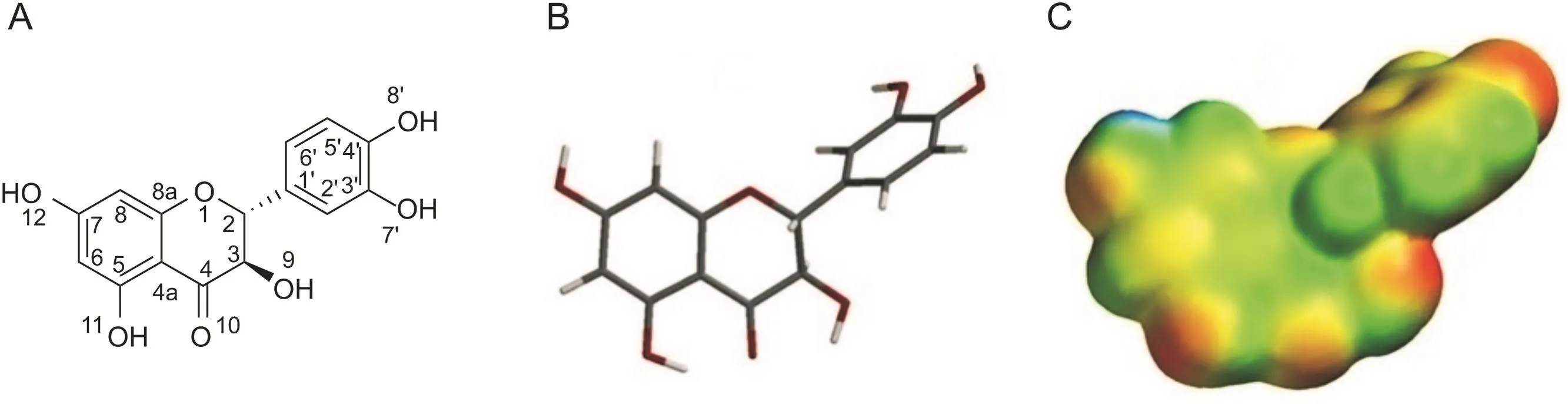
Fig.1.Chemical structure of taxifolin(A);3D visualization in tube(B);Molecular electronic potential(MEP)(C),beyond the van der Waals isosurface 0.002 eV using Spartan for Windows 08.Color scheme:blue positive to red negative electrostatic potentials values(-40.000-75.000 kcal/mol).
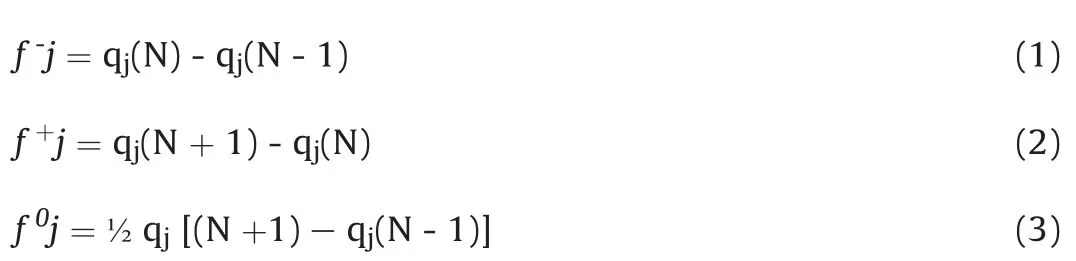
The possible regions of the molecule susceptible to degradation,under acid and alkaline conditions,were evaluated by the Fukui functions[20].These functions indicate the susceptibility of the electronic density to deform at a given position upon accepting or donating electrons[21].In this case,qjis the number of electrons(evaluated from NPA)at the j th atomic site in the neutral(N),anionic(N+1)or cationic(N-1)chemical species on the reference molecule.
The dual descriptorΔf(r)of local reactivity,which allows us to obtain a preferred locus for nucleophilic attacks(Δf(r)>0)and a preferred electrophilic attack site(Δf(r)<0)in the system at point r,was calculated using the Eq.(4)[22,23].

The main mechanism of auto-oxidation observed in the photolytic degradation reaction of the hydrogen atom abstraction energy(EHabstraction)was calculated for the bond of the hydrogen atom with the carbon atom,and bond dissociation energy(BDE)in the molecule,using the Eq.(5)[24,25].
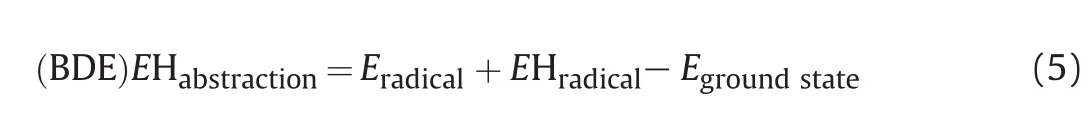
2.3.HPLC-UV methodology
The chromatographic profile,purityand extractive content were analyzed by HPLC(Prominence LC-20AT LC,Shimadzu,Tokyo,Japan)equipped with a binary pump,a photo diode array detector(SPD-M20A,Shimadzu,Tokyo,Japan),an auto-sampler(SIL-20AHT,Shimadzu,Tokyo,Japan)in-line degasser(DGU-20A5,Shimadzu,Tokyo,Japan),a column oven(CTO-10AS V,Shimadzu,Tokyo,Japan),a 20μL stainless steel loop and the software Class VP(version 6.14).
The analyses were carried out with a column Kinetex?F5(Phenomenex,Torrance,California,USA;150 mm×4.6 mm,2.6 μm)conditioned at 30°C,with detection at 288 nm.The best gradient elution(0.6 mL/min)was acidified water pH 3.0 with 0.1%(V/V)phosphoric acid(A),acetonitrile(B)and methanol(C)according to the following gradient A:B:C(V/V/V):85:10:5 to 80:15:5(0-5 min);80:15:5 to 70:25:5(5-15 min);70:25:5 to 50:45:5(15-25 min);and 50:45:5 to 85:10:5(25-30 min),maintaining these conditions until 35 min.
The HPLC-UV methodology was validated for linearity,limits of detection(LOD)and quantification(LOQ),robustness and selectivity.To access the linearity,taxifolin was dissolved in methanol in the range of 5.0-100.0μg/mL,in triplicate,followed by linear regression studies,using Excel software.The LOQ and LOD were determined based on the standard deviation of the response and the slope[26].Selectivity of the method for taxifolin was provenfirst by accessing the resolution(R)between the taxifolin peak and the nearest peak(impurity)in the original sample.Secondly,the methodology was applied to quantify taxifolin among the degradation products after the stress tests,measuring the purity peak of taxifolin through the photodiode array(PDA)detector.To check the robustness of the method,the flow(±0.06 mL/min),temperature(± 1°C)and storage time of the sample solution(3,8,12 and 24 h)were varied measuring the relative standard deviation(RSD)of the analyte content.The taxifolin content was calculated using the regression linear equation.
2.4.Forced degradation studies
Firstly,an aliquot of taxifolin(1 mg)was dissolved in 1 mL of methanol.Then,the volume was completed,to 5 mL,with the respective solvent of each forced degradation test.Hydrolytic stress studies were performed in acid medium(1 M HCl)and the mixture was maintained for 15,30,60,240 min and 24 h.Taxifolin was also treated with an alkaline condition,at 1mM NaOH,during 15,30,60,240 min and 24 h.Oxidative tests were carried out in 30% H2O2for 15,30,60,240 min and 24 h.All the stress experiments were carried out at 25°C.The final concentration of taxifolin in the respective degradation media was 200μg/mL.Acid and alkaline samples were neutralized with NaOH and HCl,respectively,and prior to HPLC analysis,the sample solutions were diluted with methanol to 100μg/mL.This concentration was chosen in order to improve the appearance of the degradation products in the HPLCUV chromatograms.For LC-MS analysis,taxifolin was submitted to 0.01 M NaOH degradation at room temperature,followed by immediate neutralizationwith equimolar HCl.The taxifolinpowder was stored in a photostability chamber(model 91423,ZEM,Minas Gerais,Brazil)under combined visible light(1.2 and 2.4 million lx·h)and ultraviolet-A(UVA)(200 and 400 Wh/m2irradiation).The negative control was protected with aluminium foil coating.The light intensity was monitored by a Luximeter(model MLM-1011,Minipa,S~ao Paulo,Brazil).The samples were also stored in an oven at 40°C and at 40°C/75% relative humidity(RH)(into a desiccator with saturated solution of NaCl)for 30 days[27].After exposure to light,heat and humidity,sample solutions of 100μg/mL in methanol were analyzed by HPLC.All stressed samples were assayed by comparison with non-degraded reference standards in the same concentration,in methanol,to calculate the percentage of degradation.
2.5.UPLC-ESI-HRMS/MS analysis
LC-MS analyses were performed on an Acquity UPLC system class H(Waters,St.Milford,MA,USA)composed of a PDA detector,sample manager and a quaternary solvent manager as well as an Acquity UPLC BEH C18column(St.Milford,MA,USA;130 ?,1.0 mm × 50 mm,particle size 1.7μm).Temperatures of 40°C and 20°C were set for the column and sample tray,respectively.A volume of 3μL was injected for each sample,and the separation was obtained in a gradient condition(0-5 min:95%A(water:formic acid,99.9:0.1,V/V)and 5%B(acetonitrile);5-8 min:20%A;8-10 min:50%A;10-13 min:55%A;13-16 min:90%A;and 16-20 min:95%A),at a flow rate of 0.3 mL/min.
Mass detection was conducted on a Xevo G2-S QTof(Waters,St.Milford,MA,USA)with an electrospray probe operating in negative ionization mode;nebulizer gas:nitrogen,cone gas flow 60 L/h;desolvation gas flow 900 L/h,sampling cone 40 V,source offset 80 V;collision gas,argon.Lockspray reference sample was leucine encephalin with reference mass at m/z 554.2615(ESI-).Temperatures of 300°C and 120°C were used for the desolvation and for the cone,respectively,while the capillary voltage was 3 kV.The collision energy was 30 eV.Data were acquired in a range of 100-1500 Da,at a scan time of 1.0 s during 20 min,and were processed with Mass Lynx V4.1(Waters,St.Milford,MA,USA).
3.Results and discussion
The isolated and purified taxifolin was characterized by Fourier transform infrared spectroscopy spectra(Fig.S1),mass spectrometry data(Fig.S2)and1H NMR spectral data(Fig.S3)and then,subjected to the stress tests.
3.1.LC-UV method validation
The method was linear in the range of 5.0-100.0μg/mL(y=113027x-67233,r2=0.9989).The LOD and LOQ were 4.6 and 15.9μg/mL,respectively.The chromatographic conditions showed selectivity with high resolution(R=4.2)between taxifolin and the nearest peak(impurity identified as a taxifolin isomer),showing a high purity peak index of 0.999973,through the PDA detector.The method was robust for changes in temperature(RSD of 0.25%),mobile phase flow(RSD of 0.47%),and storage time(RSD of 0.47%),with P>0.05,showing no statistical difference in the taxifolin assay in the small and deliberate modifications of the methodology,compared to the standard condition.
3.2.In silico stability prediction study
Taxifolin stability was first investigated by in silico prediction studies and after by in vitro forced degradation tests;the samples were monitored by HPLC-UV and UPLC-ESI-HRMS/MS.
In silico stability prediction studies were performed to further understand the chemical reactivity by predicting the most likely position of structural hydrolysis.The chemical structure(Fig.1A)in the tube model(Fig.1B)and the map of electrostatic potential charges(MEP)(Fig.1C)of taxifolin are shown in Fig.1.The MEP plot calculating on the van der Waals surface and focusing on the negative isopotential surfaces might be used to describe the occurrence of the electronic conjugation in the molecule.The most negative potential regions were demonstrated in red to oxygen atoms.The benzene ring showed no coplanarity with the chromen-4-ene ring,but the oxygen atoms from chromen-4-ene and hydroxyl(O10)moieties and benzene attached to position C2 showed a mesomeric effect.The center of the structure presented extended distribution of negative charges,which became more positive as the potential increased to hydrogen attached to oxygen atoms from hydroxyl moieties.
As shown in Table 1,the major values of the Fukui function(f(r))are those with higher reactivity[28],whose sites may be related to hydrolysis reactions during stress degradation studies.Table 1 also shows that positions C2,C4,and C7 were more susceptible to undergoing nucleophilic attack.According to the dual descriptorΔf(r)results,the following order was observed:C4>C7>O10>C8,indicating preferably the chromen-4-one ring and C2′(Fig.1)in benzene ring sites as the most reactive(Δf(r)>0)into the system at point r.All molecular regions,especially C4 and C7,were shown to be the most probable reactive sites to alkaline or acid hydrolysis(Table 1,Fig.1).
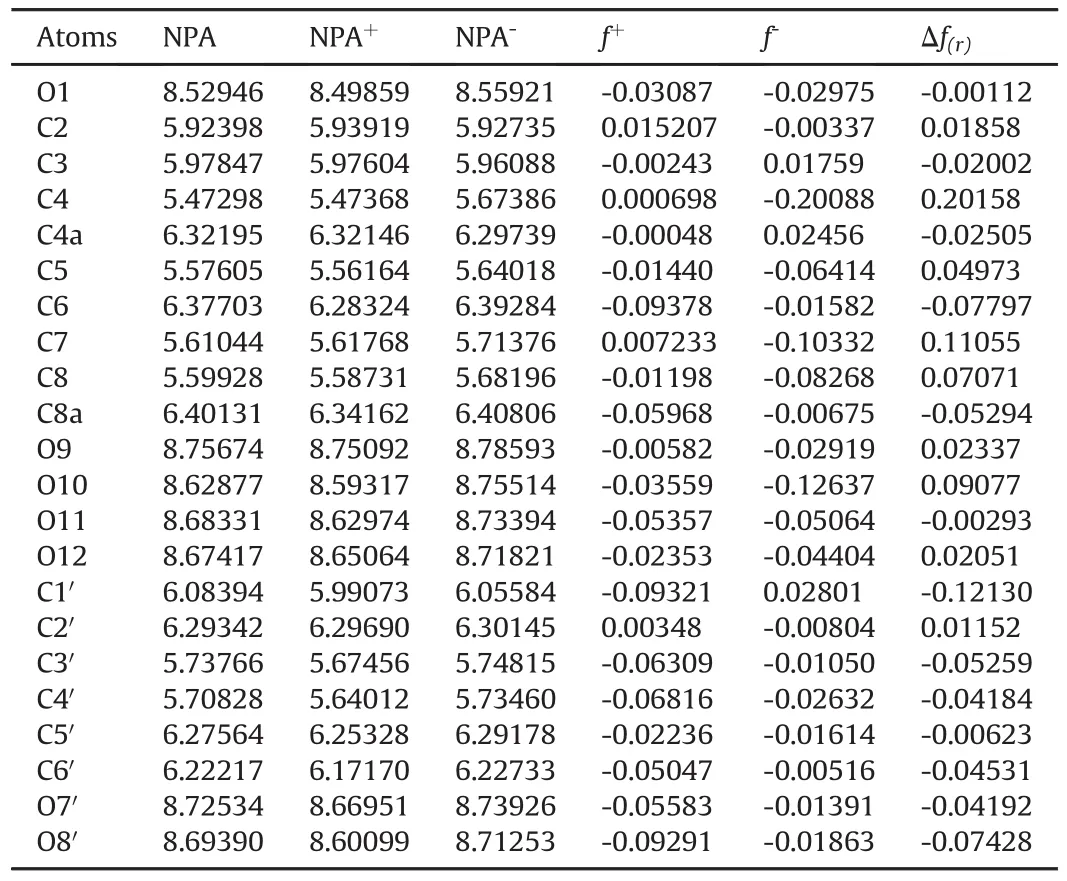
Table 1 Values of the neutral,positive and negative populational analysis(NPA),electrophilic f-and nucleophilic f+condensed Fukui functions andΔf(r)of the atoms of the taxifolin molecule calculated with the DFT/B3LYP and the 6.311G*(d,f)basis set considering Eqs.(1)-(3).
The Fukui function f0and BDE,used to estimate the hydrogen abstraction energies,indicate the auto-oxidation of taxifolin,as shown in Table 2.Fukui function showed a high value for the hydrogen atom of C2′in the chemical structure of taxifolin(Fig.1),indicating the main region of susceptibility in the oxidation process.However,this hydrogen atom is stabilized by the resonance effect,and may not easily receive an electron in auto-oxidation,which suggests that the mechanism of electron transfer is not related to the chemical structure oxidation of taxifolin.
BDE is a measure of the bond strength in a chemical bond,and may be defined as the standard energy(or even the enthalpy)change when a bond is broken by a reaction[29].BDE is an indicator of primary site(s)of the auto-oxidation of organic compounds and drugs[25].Low BDE values of hydrogen atoms from the taxifolin structure(Table 2)are more liable to be abstracted from the auto-oxidation reaction.The energies of hydrogen atoms attached to C2 and C3 from the chromen-4-ene ring and O7′and O8′from hydroxylmoieties attached tothe para-position of the benzene ring(Fig.1)reflect the high oxidative susceptibility that characterizes the instability of this region in the oxidative environment.On the other hand,the hydrogen atom from C8 showed a high BDE calculated value,indicating that this molecular position moiety is more stable in the studied conditions.The mesomeric effect observed in this structural region mayhinder the abstraction of this hydrogen.This procedure can be used for auto-oxidation and also for predicting photolytic degradation[30].
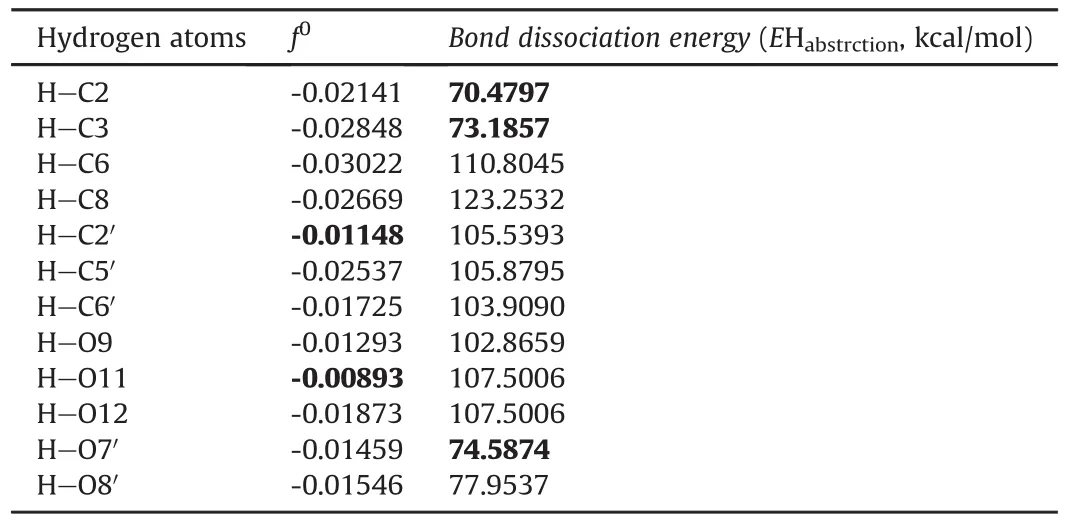
Table 2 Fukui function values for radical attack and bond dissociation energies of taxifolin hydrogen.
The Fukui function was previously used to estimate the antioxidant mechanism of taxifolin,compared to quercetin[31].However,the authors studied only the hydrogen atoms attached to oxygen atoms,and showed that in oxidant medium,taxifolin exhibited more susceptibility to hydrogen loss at O3′and O4′,which corresponds to the O7′and O8′position denominated in the present work(Fig.1A).Thus,these findings are in agreement with the results demonstrated here,considering the hydroxyl moieties.
On the other hand,forced degradation studies are recommended as part of stability studies for new drugs because the molecule is exposed to extreme conditions during its manufacturing,storage,and administration.Thus,to simulate the appearance of potential degradation products,stress tests are useful and allow us to establish the degradation pathways and the intrinsic stability of the molecule,and to validate a stabilityindicating method.The Guideline ICH Q1A(R2)[27]states that the molecule should be exposed to high temperatures,humidity,oxidation,photolysis,and susceptibility to hydrolysis across a range of pH values,in solution or suspension.
3.3.Forced degradation tests
Taxifolin was submitted to forced degradation studies,selecting the condition which provides about 10%-20% of degradation[27].
The taxifolin degradations,for each stress condition,were as follows:20.2%(1 M HCl,30 min),16.3%(1 mM NaOH,15 min),11.7%[30% H2O2,24 h],9.8%(dry heat,at 40°C,30 days),23.1%(humid heat,at 40°C and 75% RH,30 days)and 9.0%(photolysis at 2.4 million lx·h and at 400 Wh/m2of visible and UVA radiation,respectively).Taxifolin was shown to be extremely unstable(class 1)[32]under alkaline hydrolysis.Taxifolin degradation was increased by humidity,compared with dry heat,probably due to the hydrolysis process in the former condition.The stability of a semi-solid formulation containing taxifolinwas also evaluated[33].After 12 weeks,at 40°C,only 3% of the initial amount was found,which is in agreement with the results of the present study,reinforcing the thermolability of this compound,especially in the presence of humidity.Under oxidative conditions,taxifolin could be classified as class 4(stable),with a relative low photosensitivity[32].Thus,according to the above results,taxifolin needs to be protected against these stress conditions with a proper formulation,to avoid intestinal delivery in particular.
The chromatographic profile of taxifolin showed changes after being subjected to certain degradation conditions,with the appearance of supplementary peaks(Fig.2).Taxifolin used for the stress studies showed low levels of impurity(0.6%)(Fig.2A).In this original sample,four impurity peaks eluted after the major taxifolin peak(Fig.2A1).The acidic hydrolysis tests with 1 M HCl showed supplementary peaks with earlier elution,decreasing from the major peak,as well as the disappearance of impurity peaks,which probably suffered acid hydrolysis(Fig.2B).Alkaline tests were performed starting at 1 M NaOH,followed by 0.1 M NaOH and in both conditions;taxifolin was completely decomposed,as evidenced by the UV profile of the peak with a retention time of 18 min.After decreasing the concentration to 0.01 M NaOH,75% taxifolin degradation was observed,and as the contact time increased,this degradation was completed(Fig.S4).
Thus,the chosen conditions for LC-UV analysis,for alkaline degradation,were 1 mM NaOH for 15 min(Fig.2C).Oxidation with 30% H2O2resulted in a decrease in taxifolin and a change of chromatographic pro file,with many earlier eluted peaks(Fig.2D).Samples were also submitted to dry heat(40°C)(Fig.2E)and humid heat(40°C/75% RH)for 30 days(Fig.2F),without important chromatographic pro file changes.Finally,taxifolin was submitted to combined daylight fluorescent lamp and UVA(Fig.2G),showing photostability.
UPLC-ESI-HRMS-MS analysis was used to characterize the degradation products of taxifolin present in samples obtained from various conditions of degradation including alkaline medium(Fig.3).
The samples were more sensitive to negative ionization mode using the base peak ion as the mode of acquisition of the chromatograms[34].The first chromatogram obtained from the taxifolin sample(Fig.3A)displayed seven peaks,with the most intense one at 6.47 min,with a shoulder at 6.62 min,both with the base peak ion m/z 303.0515 corresponding to[C15H12O7-H]-calculated for m/z 303.0505(Δ3.37 ppm).The second peak at 6.62 min exhibited the same molecular ion,suggesting a taxifolin diastereomer.This suggestion was supported by fragment ions observed intheMS/MSspectraofbothcompoundsatm/z285.04[C15H12O7-H2O]-and 125.02(corresponding to phloroglucinol:ring A).The peak at 7.54 min with the pseudo-molecular ion m/z 301.0367[C15H10O7-H]-(Δ6.22 ppm)was assigned as quercetin based on the fragment ion observed at m/z 151.01 formed by the Retro-Diels-Alder rearrangement of ring C.This molecule is an oxidized form of taxifolin.The other peaks at 8.79,10.73,11.17,and 14.77 min did not give fragment ions that would enable their characterization.However,the last metabolite at 14.77 min(m/z 281.2484[C18H34O2-H]-Δ1.23 ppm)was assigned as oleic acid derivative.
In addition to the metabolites already present in the taxifolin sample,acid hydrolysis produced two new metabolites.The first was observed at 0.46 min(molecular ion m/z 197.79)and the second at 7.10 min(molecular ion m/z 723.51).The latter peak also appeared in the alkaline,oxidative and dry thermal degradation of taxifolin(Table 3).Diagnostic analysis of the MS/MS data of this peak was not conclusive.
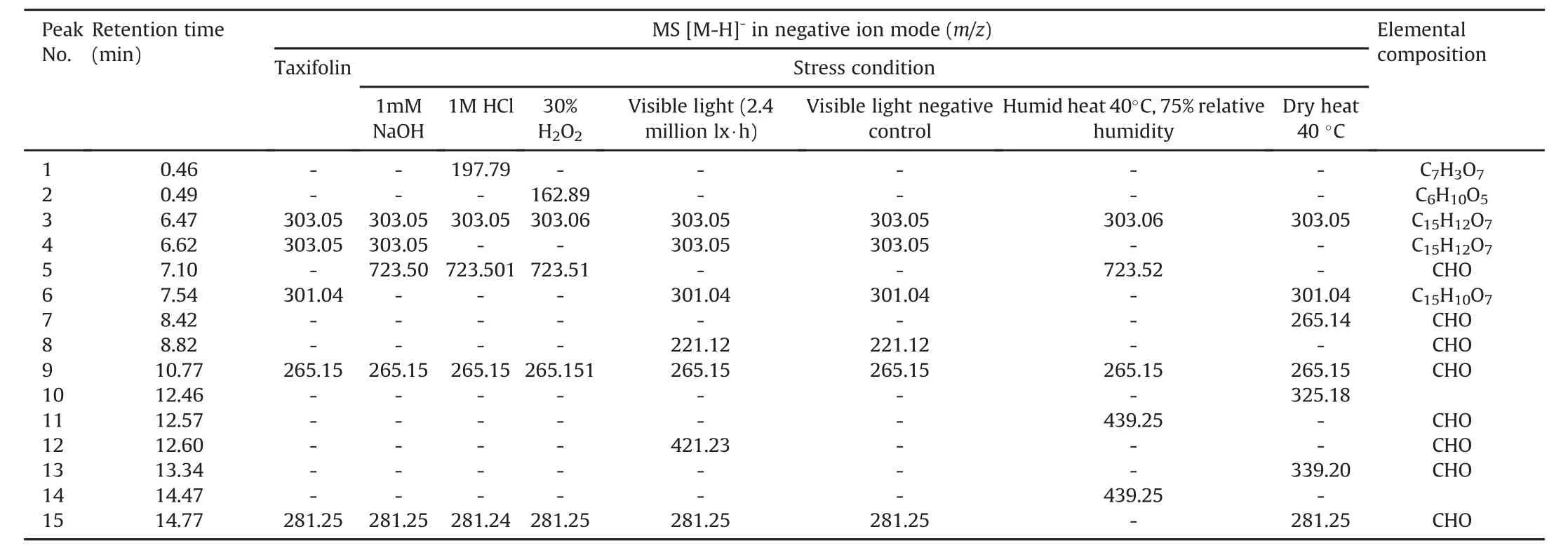
Table 3 Degradation products of taxifolin after exposition to different stress conditions,monitored by UPLC-ESI-MS.
Sample oxidative test showed in its LC-MS data features similar to those of acid degradation,with the exception of an additional peak at 0.49 min with m/z 162.88.The dry thermal test afforded a sample for which LC-MS analysis gave two peaks,at 12.46 min and 13.34 min,with m/z 325.18 and m/z 339.20,respectively.The structure of m/z 325.18 could not be assigned;whereas based on a literature search,m/z 339.20[C22H28O3-H]-(Δ4.66 ppm)is related to a steroid.These metabolites are probably contaminants of the starting material,and appear during the degradation process,which decreases the concentration of the main compounds.
The sample from humid thermal degradation afforded in its LCMS data peaks at 12.57 and 14.47 minwith the same mass value m/z 439.25.Because of the lack of fragment ions on the MS/MS spectrum,the structure of this metabolite could not be assigned.However,a literature search revealed that m/z 439.25 is related to a steroid.
In the UV photolysis sample,a peak was observed at 8.82 min with m/z 221.1180[C13H18O3-H]-(Δ1.04 ppm),like in visible photolysis.A search using this elemental composition led to a structure related to norsesquiterpene.An additional peak was also observed at 12.60 min(m/z 421.2301)with no relation to taxifolin.Some peaks that appear in the water conditions are probably degradation products of quercetin,because in acid,alkaline,oxidative,and dry thermic degradation,the peak at 7.54 min,for which the molecular ion is m/z 301.04,disappeared.In addition,these samples,when analyzed by HPLC,did not show a significant decrease in the taxifolin peak(Fig.2).
The purest taxifolin sample(99%)was submitted to alkaline hydrolysis,Specifically at 0.01 M NaOH for LC-MS analysis,and neutralized to afford,in its LC-MS analysis,a peak at 7.25 minflanked with m/z 603.0787[C30H20O14-H]-(calc.m/z 603.0775,Δ 2.02 ppm)(Fig.3B),suggesting the dimerization of taxifolin.The same chromatogram also showed the presence of a substance at 4.86 min with m/z 319.0456[C15H12O8-H]-(calc.m/z 319.0454,Δ 0.65 ppm),different from the taxifolin chemical composition(m/z 303[C15H12O7-H]-)by 16 Da.This substance may be a product formed from the oxidation of taxifolin by oxygen(O2)(Fig.4).
As ring B is more susceptible to oxidation than ring A[35],it is suggested that alkaline degradation involves the auto-oxidation promoted by the radical peroxide formation.After losing a molecule of water and forming a ketone group,the aromatic system of B ring was rebuilt,resulting in the ion m/z 319.0456(Fig.4A).UPLCESI-MS2data of this product showed fragment ions at m/z 193,195,153 and 163(Fig.S5)supported this hypothesis and indicated that oxidation occurred on B ring of the flavanone.
The second peak with m/z 337.0568[C15H14O9-H]-(calc.m/z 337.0560,Δ2.50 ppm),observed at 6.18 min(Fig.3B),differed from m/z 319.0456 by 18 Da,corresponding to an H2O molecule(Fig.4A).It was suggestedthat this compound was a resultof the oxidation of ring B of taxifolin and the opening of ring C.The MS/MS spectra of this second product showed a base peak ion of m/z 125 and a radical anion m/z 152.The fragmentation proposal in Fig.S5 revealed that m/z 125 could be related to A and B rings,whereas m/z 163 was produced by the sigma bond cleavage at theα-carbon atom.The latter fragmentation also indicated that ring C was opened.
The third and fourth peaks,with m/z 605.0964[C30H22O14-H]-(calc.m/z 605.0931,Δ5.40 ppm),at 6.99 and 7.43,min,respectively,were indicative of dimeric products of taxifolin.It was suggested that these dimers were formed by 2 mol of taxifolin in the alkaline medium,which reacted with one mol of O2to produce 2 mol of taxifolin anion radical and H2O2.Furthermore,radical delocalization led to the formationof two dimeric flavanones(Fig.4B).Similar oxidative dimerization was electrochemically produced by Chernikov et al.[15].The MS/MS spectrum of both compounds(m/z 605.0964)showed similar fragments at m/z 393,m/z 259 and m/z 217.The ion m/z 393 was obtained by the elimination of one chromenone moiety and H2O.While the loss of two chromenone units produced m/z 217,the product ion m/z 259 was obtained from the opening of C ring and the loss of a chromenone unit(Fig.S6).
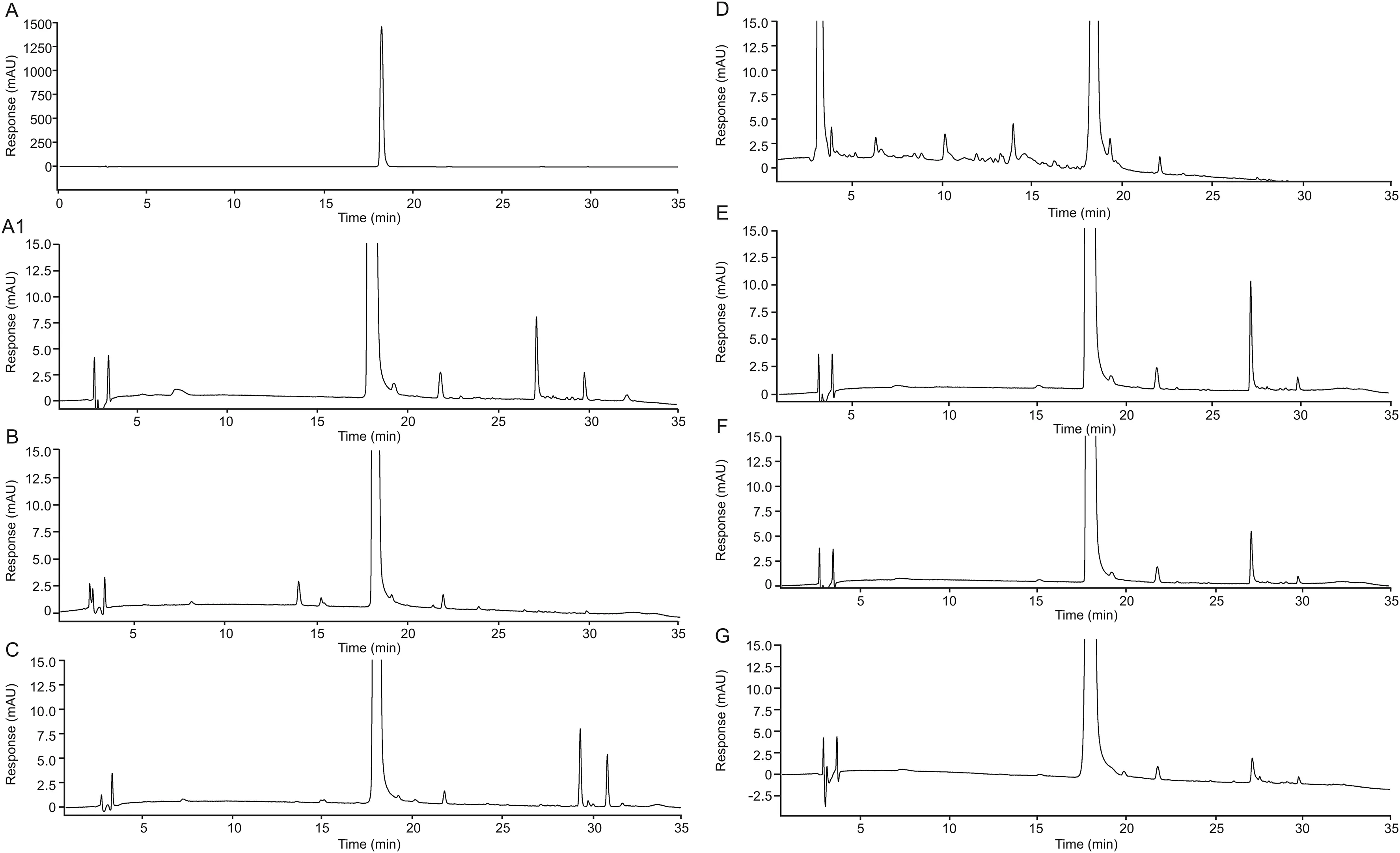
Fig.2.Chromatograms of pristine taxifolin(A and A1)and of taxifolin degraded with 1 M HCl,30 min(B),1 mM NaOH,15 min(C),30% H2O2,24 h(D),dry heat,30 days(E),humid heat,30 days(F)and photolysis,2.4 million lx?h(G).
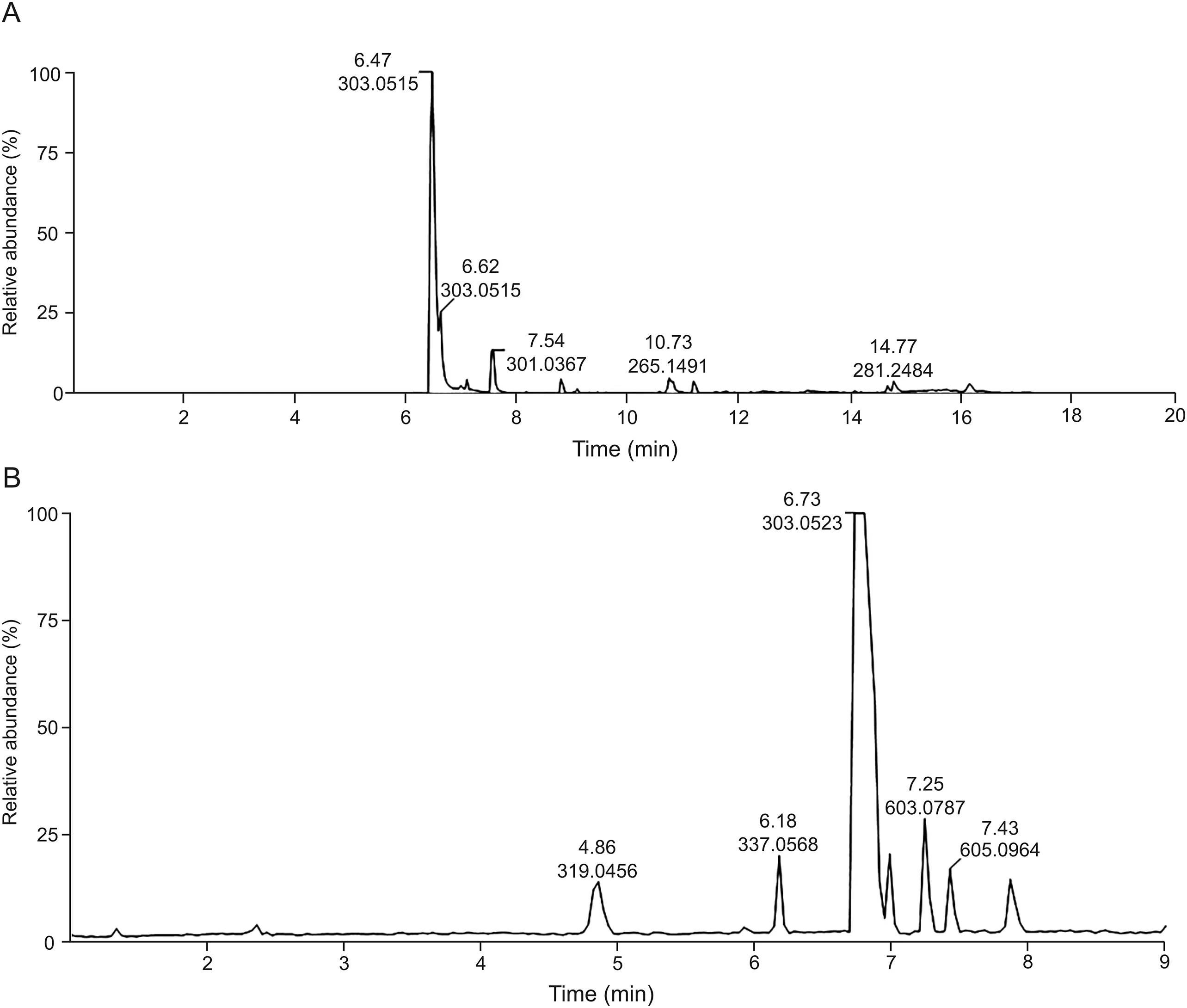
Fig.3.LC-MS of pristine taxifolin(A)and the alkaline forced degradation(0.01 M NaOH immediately neutralized with equimolar HCl)of taxifolin(B).
The product at m/z 603.0787 differs from at m/z 605.0964 by 2 amu,suggesting oxidation by O2(Fig.4C).In addition,the oxidation could produce an ortho-quinone dimeric flavanone,thus like a quercetin molecule,which has a similar structure to that of taxifolin,differing only in the double bound between C2 and C3.Quercitin also shows susceptibility to pH,temperature and storage conditions,generating the same ortho-quinone,which can interact with glutatione and may explain its high antioxidant effect[36].The latter,when analyzed by MS/MS,generated ions at m/z 409,391,381,193,and 177,demonstrating that oxidation occurred on the B ring but not on the C ring(Fig.S6).
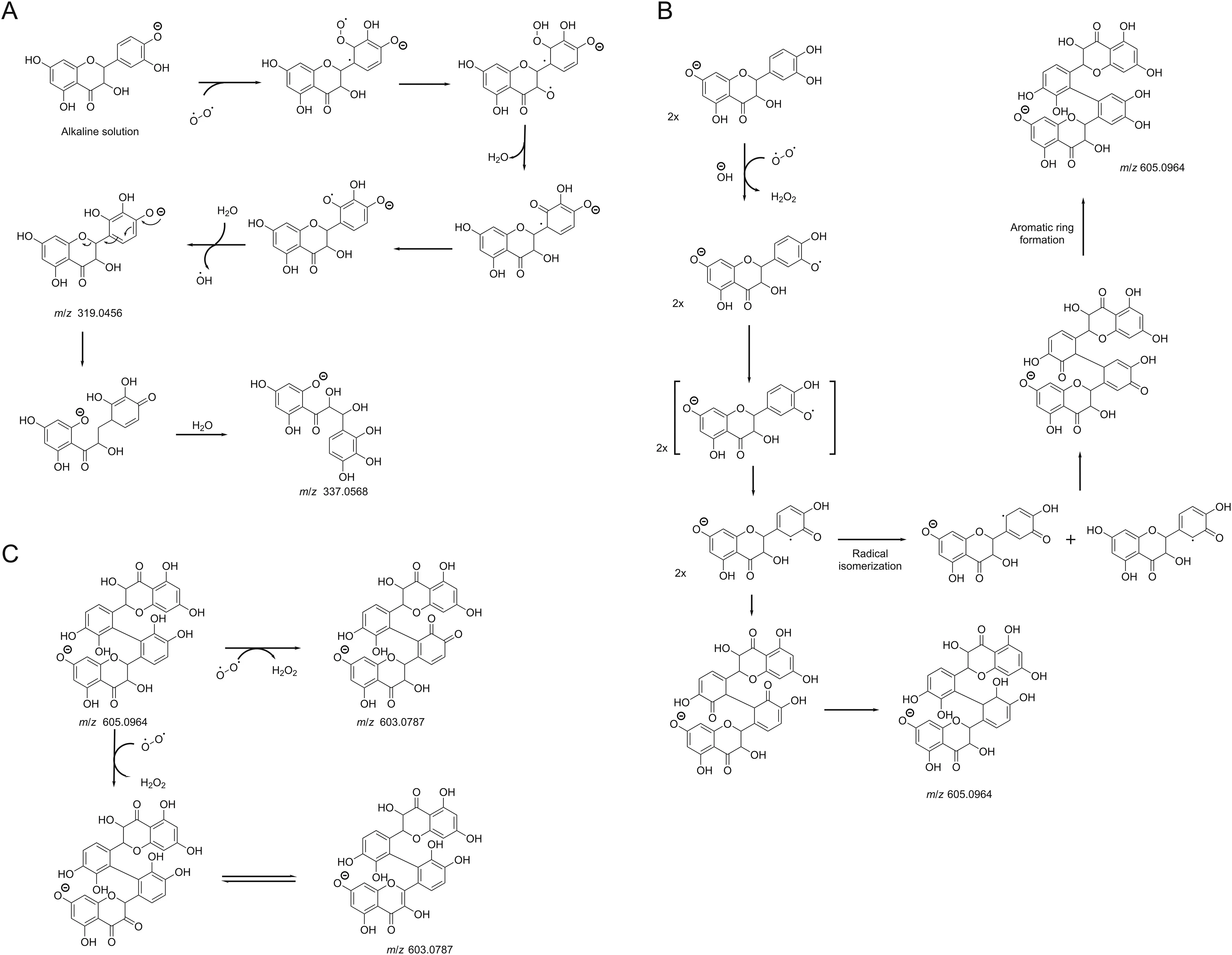
Fig.4.Hypothetical alkaline degradation mechanism of taxifolin with 0.01 M NaOH leading to m/z 319.0456 and m/z 337.0568(A);m/z 605.0964(B);m/z 603.0787(C).
4.Conclusions
Thus,in silico stability prediction analysis suggested that the taxifolin molecule is susceptible to nucleophilic attack in C2′,C4 and C7,as confirmed by the in vitro alkaline degradation,suggesting that the molecular ionwith m/z 603.0787 and m/z 605.0964 could be taxifolin dimers via nucleophilic attack in C2′for both molecules.The degradation products formed are also susceptible to nucleophilic attack.Other carbons are also previewed to be susceptible to nucleophilic attack,with less probability,but in vitro forced degradation studies proved that secondary degradation could occur.In the m/z 319 peak fragmentation,the nucleophilic attack occurred in C4 and O12(m/z 167),in C2,C3,and O12(m/z 153),in C2 and O12(m/z 193)and in C2 and O12(m/z 195),which corroborates the in silico stability prediction for susceptibility in C2,C4 and O12.In the m/z 337 fragmentations,the nucleophilic attack occurred in C2′,C4(m/z 125)and in C2,O12 and C4(m/z 125),in O1,O12(m/z 163),in C2 and C3(m/z 152),in agreement with in silico stability prediction for C2′,C2,C4,O12.Thus,the in silico stability prediction study should be predictive of the reactivitysusceptibility of taxifolin in the studied conditions,contributing to the understanding of taxifolin intrinsic lability and guiding the development of appropriate release systems for this phytodrug.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was partially supported by CAPES(PVE,Grant No.88887.116106/2016-00)(Coordena?~ao de Aperfei?oamento de Pessoal de Nível Superior),Brazil,which provided financial support in the form of a doctoral’s degree scholarship to Stenger,F.C.and financial support(Science Program Without Borders-Researcher Special Visitor-PVE),and CNPq(Conselho Nacional de Desenvolvimento Científico e Tecnolo′gico),Edital Universal(Grant No.88887.122964/2016-00).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.06.008.
 Journal of Pharmaceutical Analysis2021年2期
Journal of Pharmaceutical Analysis2021年2期
- Journal of Pharmaceutical Analysis的其它文章
- Development of a rapid GC-FID method to simultaneously determine triethylamine,diisopropylamine,and 1,1,3,3-tetramethylguanidine residues in an active pharmaceutical ingredient
- Diversity,novelty,antimicrobial activity,and new antibiotics of cultivable endophytic actinobacteria isolated from psammophytes collected from Taklamakan Desert
- Neuroprotective effects of Ginkgo biloba dropping pills in Parkinson’s disease
- Antimicrobial activity and mode of action of terpene linalyl anthranilate against carbapenemase-producing Klebsiella pneumoniae
- Herb-drug interaction in the protective effect of Alpinia officinarum against gastric injury induced by indomethacin based on pharmacokinetic,tissue distribution and excretion studies in rats
- Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery
